Abstract
The interspecific transfer of nitrogen (N) between white clover (Trifolium repens) and smooth meadow grass (Poa pratensis) in legume-based grasslands was assessed under North European field conditions using 15N individual plant leaf labelling. On average 50% of N in the grass was transferred from the white clover and about 6% of N in white clover was transferred from the grass. This corresponds to 2.5 and 0.3 g N m−2 being transferred over the growing season between the two species, respectively, and demonstrates that a significant part of the total N of the grass is coming through interspecific transfer. The majority of the 15N transferred was within a period of 20 days at relatively low soil temperatures. This implies that there is a need for a new focus on direct transfer pathways or exudation and transfer of organic N sources. Rhizodeposition in the top 10 cm of the soil was found to be 2.98 g N m−2 on average over the growing season for the grass and white clover mixture. Inclusion of adapted white clover varieties in the low-input grassland systems of northern Europe will lead to a substantial contribution of N.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grassland is a central component of European agriculture and covers 37% of the total agricultural area (Olesen and Bindi 2002). White clover (Trifolium repens L.) is an important pasture legume in many temperate regions of the world and significantly affects the N status in agricultural systems through N2 fixation. On average, 80% of the total N found in white clover grown in mixed grass swards is derived from the atmosphere, though this is influenced by such factors as the age of the sward, climate and cultivars (Carlsson and Huss-Danell 2003). This means that white clover is converting up to 545 kg of atmospheric N ha−1 year−1 into potentially available N for other organisms. However, considerably lower values have been presented in sub-arctic regions. Fagberg and Sundqvist (1994) reported up to 99 kg ha−1 of fixed N in Northern Sweden (65° N) and the amount of fixed N has been calculated to range from 40 to 169 kg N ha−1 in Iceland (Helgadóttir and Kristjánsdóttir 1993). Using legumes adapted to the northern regions of Europe may change the nutrient dynamics of the grass-dominated pastures in these regions. Little is, however, known about the potential impact of including such adapted varieties on the N status of the grassland.
In an N-limiting world, plants capable of N fixation have obtained well-earned attention. Legumes play an important role in enhancing the quality of the environment for other species. It has been generally assumed that N moves from N-rich legume plants to N-deficient grass plants but now it is acknowledged that N can also be transferred from non-legume plants to legumes (Høgh-Jensen and Schjoerring 2000). Nitrogen can be transferred both by direct and indirect pathways, below-ground and above-ground. Nitrogen can be transferred below-ground between species in a number of ways (Høgh-Jensen 2006). It can be released from living as well as decaying roots and nodules, and with the exudation of N compounds which are subsequently taken up by the receiver plant (Laidlaw et al. 1996; Paynel et al. 2001). Nitrogen can also be transferred through mycorrhizal fungi linking two plants (Bethlenfalvay et al. 1991; Johansen and Jensen 1996). Above-ground, N can be transferred via the atmosphere (Janzen and Gilbertson 1994), although volatilization of NH3 from agricultural systems mainly is related to high-N input conditions (Herrmann et al. 2001), or via grazing herbivores (Høgh-Jensen and Schjoerring 2000).
Results of measured N transfer vary between studies and depend on the method used and whether the measurements are of direct or indirect transfer or both. Values ranging from 6 to 80% of total N in the grass have been published for N transfer from the legume to the associated grass (Broadbent et al. 1982; Brophy et al. 1987; Haystead and Marriott 1979). Up to 52% N transfer from white clover to its grass companion and about 12% transfer from grass to clover has been reported, which corresponds to approximately 6 and 0.36 g m−2, respectively (Høgh-Jensen and Schjoerring 2000; Ledgard 1991).
It is difficult to investigate below-ground pathways, although over the years more precise methods have been developed (Høgh-Jensen 2006; Høgh-Jensen and Schjoerring 2000). Direct labelling of plants can be carried out by adding 15N to enclosed canopy or by immersing plant parts in a solution containing 15N. A direct 15N leaf feeding technique enables labelling of individual plants in plant communities and, more importantly, it allows the detection of transfer from the grass plant to the clover plant and vice versa, i.e. bi-directional transfer. In addition, it enables the detection of transfer within shorter periods of time.
Rhizodeposition makes N compounds accessible for mineralization and therefore is one of the main factors that affect N availability in the soil (Heal et al. 1999). Rhizodeposition is a transfer of root-derived organic and inorganic compounds into the soil that occurs via exudation as well as decomposition of root litter (Hertenberger and Wanek 2004). Such rhizodeposition can vary between plant communities as the proportion of N in the soil derived from rhizodeposition varies between plant species (Høgh-Jensen and Schjoerring 2001). Breakdown of organic compounds in the rhizosphere has major positive effects on the density and activity of microorganisms, thus greatly increasing their productivity near the roots.
There is a lack of knowledge on the importance of transfer and deposition of N in low-input systems in the northernmost parts of Europe. Soil temperatures are low in these regions and low rates of N mineralization could therefore be expected (Stanford et al. 1973). High amounts of mineralized N are, though, important properties of Andosols (Nanzyo et al. 1993), a soil type that is dominant in Iceland. N transfer has not been measured in the field in regions where there is a short growing season characterised by low temperatures and long photoperiods. It is therefore of interest to study the effects of such environmental conditions on N dynamics, in particular whether N transfer occurs over a short period of time.
The objective of the present experiment was to (1) quantify the bi-directional N transfer between white clover and smooth meadow grass in Iceland over one growing season using the direct 15N leaf feeding technique, (2) test whether leaves can represent the whole plant (roots, stolons, leaves) when N transfer is measured and (3) compare the rhizodeposition of N between white clover and smooth meadow grass over the experimental period.
Materials and methods
Experimental site
The experiment was carried out at Korpa Experimental Station in Iceland (64° 04′N, 21° 58′W, 30 m above sea level) in summer 2003. The growing conditions are characterized by long photoperiods and relatively long and cool summers. The soil at the experimental site is a Gleyic Andosol (Arnalds 2004, 2005) with a mean bulk density of 0.52 and 0.57 g cm−3 for the top 10 and 10–30 cm depth, respectively, and a total N of 0.9% in 0–30 cm depth (C/N ratio of 12; Guicharnaud and Björnsson 2004).
Experimental design
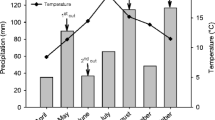
The experiment was established in a grass field that had been sown with a mixture of white clover (Trifolium repens L. cv. Norstar from Norway) and smooth meadow grass (Poa pratensis L. cv. Fylking from Sweden) in the spring of 1999. The site had received the equivalent of 50 kg N ha−1, 50 kg P ha−1 and 50 kg K ha−1 at sowing and 20 kg N ha−1, 30 kg P ha−1 and 50 kg K ha−1 each spring for the three subsequent years. In early May 2003 the site was fertilized with the equivalent of 20 kg N ha−1, 32 kg P ha−1 and 32 kg K ha−1 to prevent N deficiency in the plant community. Subsequently, 32 PVC cylinders with a diameter of 30 cm were inserted to a depth of 30 cm into the grass sward, thus making up the experimental plots. The experimental design was a randomised block with four replicates and a treatment consisting of four different times of first cut that cover the whole growing season (Fig. 1). For each cutting treatment, two cylinders were used to label either individual grass or white clover plants with 15N.
The first treatment was cut on 30 June (H1), and other times of cutting were on 14 July (H2), 28 July (H3) and 12 August (H4). At each sampling occasion, eight separate cylinders were cut, except on 12 August when one cylinder had to be omitted because of damage. At the last sampling date, the regrowth (H5) in those cylinders that had been cut on the 30 June (H1) was also taken.
15N leaf labelling
To detect bi-directional N transfer between white clover and smooth meadow grass either individual grass or clover plants within each cylinder were repeatedly labelled with a 0.5% (w/w) highly 15N enriched (99 atom%) urea solution (McNeill et al. 1997). One newly expanded clover leaf or a few young grass leaves of unspecified leaf area were submerged for a few days at a time into 1 ml of urea solution in Eppendorf tubes. The tubes were closed with inert plastic material (Sticky Tac, Henkel Consumer Adhesives, Winsford, UK) in order to limit evaporation and to prevent rainwater entering into the tubes. Care was taken to prevent contamination of 15N to the plots during the labelling.
The labelling was started in all cylinders on 10 June. The cylinders were repeatedly labelled over the growing season prior to sampling but the number of labelling occasions varied between cuts (Fig. 1). Thus, the cylinders sampled on 30 June (H1), 14 July (H2), 28 July (H3) and 12 August (H4) were labelled repeatedly, four, six, eight and ten times, respectively, and 15N was applied four times before the regrowth was cut on 12 August (H5). Leaves from different plants were used for each labelling occasion. The leaves were enclosed in the tubes between 6 and 9 days depending on weather conditions, influencing the evapotranspiration of the plants, the driving force in the uptake of the 15N. At the end of each labelling period, the tubes were removed from the cylinders in order to give sufficient time for the 15N to diffuse in the plant before each cut. The plots were cut 4, 6, 6 and 7 days after the labelling was completed for the four different sampling dates, respectively.
Sampling and analysis of above-ground and below-ground material
The cylinders were cut with hand shears to a stubble height of 5 cm and the herbage was separated into white clover, smooth meadow grass and unsown species. Each fraction was dried at 80°C for 60 h and dry matter determined. All fractions were ground using a ball mill (Retsch, Germany). After each cut, the cylinders, containing soil cores, were removed from the experimental site. The following day the soil cores were separated into two fractions; 0–10 and 10–30 cm. Cylinders originally cut on 30 June were not dug up until after the regrowth had been taken on 12 August.
Roots from the top 10 cm were carefully separated into clover and grass roots by hand and washed, and clover stolons and grass crowns were removed from three sub samples of turf taken by auger with a diameter of 10 cm. Utmost care was taken in separating the roots and roots of unsown species were discarded. All plant parts were washed and dried at 80°C for 60 h and ground by a ball mill.
All soil particles were carefully removed from roots by hand and mixed with the rest of the soil in the top 10 cm. The two soil fractions were weighted and homogenized and a subsample from each fraction was subsequently dried at 80°C for 60 h.
All three plant parts of clover and grass, leaves, stolons/crowns and roots, from all cuts as well as soil samples from all cylinders were analyzed for total-N and 15N using a ANCA-SL Elemental Analyser coupled to a 20–20 Tracer Mass Spectrometer (Europa Scientific, Crewe, UK).
Identical samples were also collected and analyzed from an unlabelled area on 12 August to provide information on the natural 15N concentration on the background or for each plant part and the soil.
Calculation of N transfer
The calculated N transfer between the species is presented as percentage of transferred N (%Ntrans) of the total N of the receiver plant. The %N transferred between white clover and grass and vice versa was calculated using a modification of an equation from Høgh-Jensen and Schjoerring (2000). Transfer from white clover labelled with 15N to unlabelled grass was calculated as:
where Ngrass and Nclover denote N content of smooth meadow grass and white clover, respectively, G1 is the atom% of grass and G0 is the natural enrichment of grass growing under unlabelled field conditions next to the experimental plots, C1 is the atom% of labelled clover and C0 is the natural enrichment of clover growing under unlabelled field conditions. Calculations were carried out for each plant fraction, i.e. leaves, stolons/crowns and roots, separately as well as all plant parts together using the weighted atom% and thus transfer between different plants parts could be calculated. When calculating the N transfer from grass to clover Eq. 1 was modified accordingly. In these calculations it is assumed that 15N enrichment of the donor plant stays constant over time and that the absorbed 15N in all plant N pools subject to N transfer is equally distributed within the plant.
To determine the quantity of N transferred, the %Ntrans value obtained by Eq. 1 was subsequently multiplied by the total N content of leaves of the receiver plants within the cylinder.
Calculation of N rhizodeposition
The N rhizodeposition (%Ndfr) derived from the two species, white clover and grass, was calculated using an equation described in Schmidtke (2005) of excess atom% for each part:
Atom% 15N soil is measured 15N abundance in soil. Natural abundance of soil samples measured outside the experimental plots were used as atom% 15N background soil. Atom% 15N roots is measured 15N abundance in roots of labelled white clover or labelled smooth meadow grass. The natural abundance of unlabelled white clover and grass roots growing outside the experimental plots was used as atom% 15N background roots. The rhizodeposition was calculated separately for cylinders with labelled white clover and for cylinders where grass was labelled, only taking into account roots from species that were labelled in each cylinder.
To determine the quantity of N in soil derived from rhizodeposition (Ndfr) the %Ndfr was multiplied by total N in top 10 cm of soil. In order to quantify the proportion of plant-derived N in the soil, Ndfr was divided by total N in white clover and grass, respectively (Schmidtke 2005).
Statistical analysis
The data were analyzed using standard ANOVA, t-test and correlation calculations in Genstat Version 7.1 (Lawes Agricultural Trust 2003). Missing value was estimated for the damaged plot for the cut on 12 August. Results for the transfer and rhizodeposition were analysed separately for each species component. For the leaf fractions, results from all five cuts (H1–H5) were included in the same ANOVA.
Results
Dry matter and N accumulation
The dry matter yield increased significantly from H1 to H3 with an overall mean of 484 g m−2 over the whole growing season (Table 1). The proportion of the clover in the sampled herbage did not change significantly over the growing season (Table 1), averaging 28% of the total dry matter.
The N content was calculated in the herbage as well as in the samples collected by the auger thus allowing determination of the total N of the plant (Table 1). For H1 values are only presented for N in the herbage as no soil cores were taken at this stage. The difference between the total N and N in the herbage is therefore the N content in stolons/crowns and roots of the plants. The N yield of the plant community generally increased from H1 to H3 reflecting increased dry matter yield.
Nitrogen concentration (%N) was higher for white clover than smooth meadow grass (Table 2) in all plant parts although both the amount of N in the herbage as well as total N was higher for the grass (Table 1). For both species %N in the leaves was highest at H1 and H5.
Ranking the %N for different plant parts at each cut separately (H2 to H5) showed that the grass leaves had higher %N than other grass organs at all cuts (P < 0.001) and the %N was lower in roots than the crowns (H2, H4, H5, P < 0.001; H3, P = 0.007). For the white clover the %N was always highest in the leaves (P < 0.001) but no significant difference was found between the stolons and the roots.
Labelling
The 15N labelling of white clover and grass resulted in elevated 15N atom% compared to natural abundance of samples taken outside the experimental plots, both in grass and white clover during the whole growing season (Table 3). This shows that the direct leaf feeding technique resulted in sufficient enrichment of 15N in the donor plant for 15N to be detected in the receiver plant. Further, the 15N atom% exceeded the natural enrichment of the air for both white clover and smooth meadow grass grown under unlabelled field conditions at the experimental site.
The grass component generally had higher 15N atom% than the clover component at all cuts (Table 3). For both species, the lowest atom% appeared in the roots and the highest in the stolons of the clover and crowns of the grass though this trend was not significant at all cuts. It is important to bear in mind that it was not possible to compare atom% of different plant parts in H1 as no soil cores were taken on that occasion.
The plants received 15N either by direct labelling or by transfer from associated companion species. For white clover, the 15N atom% was highest in the stolons and lowest in the leaves when the 15N derived from transfer whereas it was highest in the leaves and lowest in the roots when the 15N derived from direct leaf labelling. For the grass the atom% was lowest in the roots and highest in the crowns independent of the origin of the 15N.
Relative and absolute transfer
Around half of the N in grass was derived from the associated white clover over the whole growing season, whereas only about 6% of the N in clover was derived from the grass companion (Table 4). The calculated %Ntrans generally did not vary between cuts except for the following; transfer from clover leaves to grass leaves was significantly lower at H1 compared to later cuts (P = 0.031), and transfer from grass leaves to clover leaves decreased between H1 and H2 (P = 0.008).
The highest calculated %Ntrans (82.1%) was found for transfer from clover roots to grass roots but lowest transfer (23.2%) was found from clover stolons to grass crowns (Table 4). In contrast, higher %Ntrans values were found for transfer from grass crowns to clover stolons compared to transfer from leaves and roots. The values for %Ntrans based on calculations for the whole plant, on one hand, and the leaves, on the other, were however highly correlated both for white clover (r = 0.924) and grass (r = 0.966). The %Ntrans values from clover to grass were overestimated up to 8% (P = 0.078) when leaf values were used compared to whole plant values and this overestimation increased as the growing season progressed. However, transfer from grass to clover was underestimated up to 20% by basing the calculations on leaf values instead of whole plant parts (P < 0.001).
The observed N transfer is reflected in the N yield of the plant community. The amount of N transferred from clover to grass is considerably higher than the corresponding value for transfer from grass to clover (Table 4). The amount of N transferred from clover to grass increased from H1 to H3 but the same did not apply for transfer in the other direction.
Soil and rhizodeposition
Analyses of soil samples revealed no significant differences of the total N in the soil, between neither cuts nor different soil depths. The soil samples indicate a higher 15N atom% in the soil than the natural enrichment of the atmosphere and the atom% was significantly higher in the top 10 cm compared to the lower soil layer (10–30 cm) in the experimental plots (P < 0.001; Fig. 2). However, no significant difference in the natural abundance of 15N was found at different depths for soil samples taken outside the experimental plots.
When the plant community and the top 10 cm of soil are considered as an isolated system and the contribution of each component of the system to total N and total 15N, including all plant parts and the soil, is calculated it becomes evident that almost all (97%) of the N is found in the top 10 cm of soil (Table 5). However, the proportion of 15N found in the soil is not as high as for the total N.
Rhizodeposition in the top 10 cm of soil was calculated according to equation (2). A significantly higher percentage of N in the soil was derived from white clover deposition (%Ndfr) than its grass companion, or 0.46 and 0.18%, respectively, averaged over cuts (P = 0.022). Similarly, the quantity of the N deposition (Ndfr) was significantly higher for white clover compared to the grass, or 2.16 and 0.82 g m−2, respectively, averaged over cuts (P = 0.029).
Further, the proportion of plant-derived N found in the top 10 cm of soil was significantly higher for white clover compared to grass, i.e. 47 and 10%, respectively, of the total N of the species (P = 0.021).
Discussion
In the present work, transfer of N between white clover and smooth meadow grass as well as rhizodeposition was quantified over a full growing season in Iceland using direct 15N leaf labelling technique under field conditions. Most grassland areas in northern Europe are characterised by a short growing season, low mineralization rates of the soil organic N and relatively low productivity. Thus, introducing adapted clover varieties into such areas may have important consequences for the whole N dynamics in the system (Nesheim and Oyen 1994).
Assumptions for the 15N direct leaf labelling technique
The direct leaf feeding technique is straight forward, effective and more accurate than soil 15N isotope dilution techniques. The use of 0.5% urea solution for labelling as used here has been shown to cause negligible damage to the labelled plant tissue (Khan et al. 2002). It has also been shown that the direct leaf feeding technique is valid for examining below-ground N deposition (Hertenberger and Wanek 2004; Høgh-Jensen and Schjoerring 2001; McNeill et al. 1997).
The 15N leaf labelling technique relies on the assumptions of (1) constant 15N enrichment of the donor plant over time, and (2) equal distribution of the absorbed 15N in all plant N pools subject to N transfer. The current study complied with the first assumption by applying frequent labelling during the experimental period that maintained a relatively high 15N over the whole growing season (Table 3). Further, the study complied with the second assumption by having comparable enrichments of the N pools in the various organs, although the 15N atom% was always lowest in the roots of both clover and grass (Table 3). This agrees with previous studies showing the highest atom% in the shoot material of labelled plants (Ledgard et al. 1985; McNeill et al. 1997) although previous studies have mostly not reported enrichments of leaves, stolon and crown separately (Hertenberger and Wanek 2004; Ledgard et al. 1985; McNeill et al. 1997; Zebarth et al. 1991).
Senescence may influence the 15N distribution in the plant organs. It has been reported that mature lupine roots can have somewhat lower concentration of 15N than younger tissue and it is also known that plant parts are not all equally susceptible to the reduction of 15N by age, with leaves and stems being less susceptible than roots (Russell and Fillery 1996). Further, nodules of legumes are not as highly enriched as roots. This can lead to a distortion in the calculation of below-ground N of legume systems as the assumption, that the atom% of recovered roots is representative of enrichment of all root derived N, may not hold (Khan et al. 2002). The distribution of 15N is also influenced by the growth stage of the plant as N moves internally within the plant depending on the N need at a particular time (Table 2; Farrington et al. 1977). Høgh-Jensen and Schjoerring (2000) reported a decrease in atom% with increasing rooting depth. However, this should have negligible effect on the present calculations as the main root mass was in the top 10 cm.
The calculated N transfer based on leaves or the whole plants was comparable although there was a tendency to overestimate the N transfer from white clover to grass using only samples from leaves compared to all plant parts (Table 4). Similarly the data showed a small underestimation of N transfer from grass to clover using samples from leaves instead of samples from the whole plant.
N transfer
Bi-directional transfer of N between white clover and smooth meadow grass was confirmed in the present study (Table 4). Almost 50% of the N found in the grass derived from white clover and 6% of the N in the clover derived from the grass companion. This amounts to a mean transfer of 2.5 and 0.3 g N m−2 from clover and grass, respectively, over the growing season (Table 4). There was no significant difference in the amount transferred between different cuts, except that transfer from white clover leaves to grass leaves was significantly lower at H1 compared to later cuts.
There are only a few studies that have measured bi-directional transfer between legumes and grasses. The proportion of transferred N found in this study is comparable with previous results although the amount of N transferred is somewhat lower (Høgh-Jensen 2006; Høgh-Jensen and Schjoerring 2000; Ledgard 1991). Other studies have reported between 6 and 80% N transfer from legumes to grass under diverse conditions and using different methods (Broadbent et al. 1982; Brophy et al. 1987; Haystead and Marriott 1978) or failed to detect N transfer from legumes to their grass companions (Trannin et al. 2000; McNeill and Wood 1990).
Considering the northern growing conditions in Iceland, the above-mentioned difference in the amount of N transferred can be explained by lower growth rate and lower dry matter yields than in more southern regions. The average yield over the growing season in Iceland was 484 (Table 1) compared to 780 g DM m−2 on average in the experiment of Høgh-Jensen and Schjoerring (2000). Further, new nodules have to be formed every spring in temperate legumes (Bordeleau and Prevost 1994) possibly limiting the N transfer to the companion grass early in the growing season (Table 2).
It is surprising how quickly the 15N tracer appeared in substantial amounts in the associated species (Table 4) as the soil temperature at 10 cm was only around 13°C during this period. Mineralization rates can be expected to be low at such soil temperatures and normal hierarchical mineralization concepts (Christensen 2001) therefore seem unable to explain such high rates of N transfer. Thus attention is drawn to direct transfer pathways and/or release and uptake of organic N compounds as possible transfer mechanisms. Transfer values based on indirect 15N isotope dilution methodologies (Brophy et al. 1987; Høgh-Jensen and Schjoerring 1997; Ledgard et al. 1985) must thus be used with caution.
Soil deposition
The largest amount of N in the plant–soil system was found in the soil (Table 5). Decomposition is a very important process in the nutrient cycles of most terrestrial agroecosystems (Wachendorf et al. 1999). In grasslands, where the above-ground biomass is removed, the root turnover provides most of the dead organic matter in the soil (van der Krift et al. 2001). In the current study, clover had a higher (P < 0.05) proportion (%Ndfr) and amount of N (Ndfr) rhizodeposited than grass.
The Andosols in Iceland normally have high contents of organic carbon and N (Guicharnaud and Björnsson 2004; Satio 1990) but these soils do not supply the grass with more than 0.3–0.4 kg N ha−1 d−1 over the growing season (Björnsson 2004). However, rhizodeposition and mineralization are interacting soil pathways. In the current study rhizodeposition amounted to approx. 3.0 g N m−2 (Table 5; Fig. 2) demonstrating how the inclusion of appropriate legumes in these swards may lead to much more dynamic and short-term N fluxes (Tables 4 and 5; Høgh-Jensen 2006). The deposition amounted though to less than what was observed for pastures under more favourable growing conditions (Høgh-Jensen and Schjoerring 2001; McNeill et al. 1997).
Pathways for N transfer in light of the results
In the present study 1.1 g N m−2 were rapidly transferred from white clover to grass over the first period of 20 days in early summer. Mineralization can only explain a small part of this because of the limited time that was available for the 15N to move from labelled clover leaves to the companion grass particularly under conditions with low soil temperatures. This contrasts at least partly with the perception that N transfer from clover to grass occurs mostly by decomposition of roots and nodules (Ledgard 1991). More rapid pathways for the N transfer must therefore be considered, such as direct uptake of organic N compounds (Chapin et al. 1993; Jones and Darrah 1993, 1994; Kielland 1994). Such organic N compounds may come from degradation of fresh organic materials or from exudations, in particular from clover (Paynel et al. 2001; Rasmussen et al. 2006). Transfer through mycorrhiza is another possibility which could explain the rapid transfer observed (Moyer-Henry et al. 2006; Zhu et al. 2000).
The applied labelling approach opens for the possibility that the newly labelled leaves, during periods of rain, could leach 15N from the leaf surfaces to the soil and thus create artefacts. It cannot be excluded that such leaching has taken place and thus influenced the data for N transfer as well as deposition. However, such an effect is considered minor due to the fact that the top soil was only slightly more enriched with 15N than the subsoil (Fig. 2). Further, such an effect should have been detectable in the grass component at H5 as grass is the strongest competitor for inorganic N (Høgh-Jensen and Schjoerring 1997) and therefore for any leached 15N from the first labelling event (H1). This was, however, not observed in the current study (Table 5). Such potential leaching of 15N needs nevertheless to be addressed in future studies.
In conclusion, the current study demonstrates a considerable bi-directional N transfer between legume and non-legume species showing that a significant part of the total N of the plant is coming through interspecific transfer. Basing calculations of N transfer on the N pools in different plant organs gave comparable results. The transfer took place within a period of 20 days indicating that transfer following mineralization may not be the major pathway. On average, 2.98 g N m−2 were deposited in the soil by white clover-based grassland. It may be expected that these results will encourage additional use of legumes in agriculture in the northern regions.
References
Arnalds Ó (2004) Volcanic soils of Iceland. Catena 56:3–20
Arnalds Ó, Óladóttir BA, Guicharnaud R (2005) Aðferðir við að lýsa jarðvegssniðu (Methods to describe soil profiles). Rit LbhÍ nr. 5 (in Icelandic)
Bethlenfalvay GJ, Reyessolis MG, Camel SB, Ferreracerrato R (1991) Nutrient transfer between the root zones of soybean and maize plants connected by a common mycorrhizal mycelium. Physiol Plant 82:423–432
Björnsson H (2004) Mineralization of nitrogen in relation to climatic variation and soil. In: Hatch D, Chadwick DR, Jarvis SC, Roger JA (eds) Controlling nitrogen flows and losses. Wageningen Academic Publisher, Wageningen, The Netherlands, pp 140–142
Bordeleau LM, Prevost D (1994) Nodulation and nitrogen-fixation in extreme environments. Plant Soil 161:115–125
Broadbent FE, Nakashima T, Chang GY (1982) Estimation of nitrogen fixation by isotope dilution in field and greenhouse experiments. Agron J 74:625–628
Brophy LS, Heichel GH, Russelle MP (1987) Nitrogen transfer from forage legumes to grass in a systematic planting design. Crop Sci 27:753–758
Carlsson G, Huss-Danell K (2003) Nitrogen fixation in perennial forage legumes in the field. Plant Soil 253:353–372
Chapin FS, Moilanen L, Kielland K (1993) Preferential use of organic nitrogen for growth by a nonmycorrhizal arctic sedge. Nature 361:150–153
Christensen BT (2001) Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur J Soil Sci 52:345–353
Fagberg B, Sundqvist U (1994) Öjebynprojektet Vallarnas botanisk sammansättning 1992–93 samt symbiotiska kvävefixering 1990–93. Röbäcksdalen meddelar 9. Svergies Lantbruksuniversitet, Umeå, p 41
Farrington P, Greenwood EAN, Titmanis ZV, Trinick MJ, Smith DW (1977) Fixation, accumulation, and distribution of nitrogen in a crop of Lupinus-Angustifolius cv Unicrop. Aust J Agr Res 28:237–248
Guicharnaud R, Björnsson H (2004) C and N mineralization rates of cultivated Icelandic Andosols. In: Óskarsson H, Arnalds Ó (eds) Rala report no. 214. Volcanic soil resources in Europe. COST Action 622 final meeting, pp 88–89
Haystead A, Marriott C (1978) Fixation and transfer in a clover-grass sward under hill conditions. Ann Appl Biol 88:453–457
Haystead A, Marriott C (1979) Transfer of legume nitrogen to associated grass. Soil Biol Biochem 11:99–104
Heal O, Anderson JM, Swift MJ (1999) Plant litter quality and decomposition: an historical overview. In: G Cadisch, KE Giller (eds) Driven by nature – plant litter quality and decomposition. CAB International, Oxon, UK, pp 3–30
Helgadóttir Á, Kristjánsdóttir TA (1993) Rætkun hvítsmára (Cultivation of white clover). Ráðunautafundur (in Icelandic), pp 188–197
Herrmann B, Jones SK, Fuhrer J, Feller U, Neftel A (2001) N budget and NH3 exchange of a grass/clover crop at two levels of N application. Plant Soil 235:243–252
Hertenberger G, Wanek W (2004) Evaluation of methods to measure differential 15N labeling of soil and root N pools for studies of root exudation. Rapid Commun Mass Spectrom 18:2415–2425
Høgh-Jensen H (2006) The nitrogen transfer between plants: an important but difficult flux to quantify. Plant Soil 282:1–5
Høgh-Jensen H, Schjoerring JK (1997) Interactions between white clover and ryegrass under contrasting nitrogen availability: N2 fixation, N fertilizer recovery, N transfer and water use efficiency. Plant Soil 197:187–199
Høgh-Jensen H, Schjoerring JK (2000) Below-ground nitrogen transfer between different grassland species: direct quantification by 15N leaf feeding compared with indirect dilution of soil 15N. Plant Soil 227:171–183
Høgh-Jensen H, Schjoerring JK (2001) Rhizodeposition of nitrogen by red clover, white clover and ryegrass leys. Soil Biol Biochem 33:439–448
Janzen HH, Gilbertson C (1994) Exchange of 15N among plants in controlled environment studies. Can J Soil Sci 74:109–110
Johansen A, Jensen ES (1996) Transfer of N and P from intact or decomposing roots of pea to barley interconnected by an arbuscular mycorrhizal fungus. Soil Biol Biochem 28:73–81
Jones DL, Darrah PR (1993) Influx and efflux of amino acids from Zea Mays L roots and their implications for N nutrition and the rhizosphere. Plant Soil 156:87–90
Jones DL, Darrah PR (1994) Amino-acid influx at the soil–root interface of Zea mays L and its implications in the rhizosphere. Plant Soil 163:1–12
Khan WDF, Peoples MB, Herridge DF (2002) Quantifying below-ground nitrogen of legumes 1. Optimising procedures for 15N shoot-labelling. Plant Soil 245:327–334
Kielland K (1994) Amino acid absorption by arctic plants – implications for plant nutrition and nitrogen cycling. Ecology 75:2373–2383
Laidlaw AS, Christie P, Lee HW (1996) Effect of white clover cultivar on apparent transfer of nitrogen from clover to grass and estimation of relative turnover rates of nitrogen in roots. Plant Soil 179:243–253
Ledgard SF (1991) Transfer of fixed nitrogen from white clover to associated grasses in swards grazed by dairy cows, estimated using 15N methods. Plant Soil 131:215–223
Ledgard SF, Freney JR, Simpson JR (1985) Assessing nitrogen transfer from legumes to associated grasses. Soil Biol Biochem 17:575–577
McNeill AM, Zhu CY, Fillery IRP (1997) Use of in situ 15N-labeling to estimate the total below-ground nitrogen of pasture legumes in intact soil–plant systems. Aust J Agr Res 48:295–304
McNeill AM, Wood M (1990) Fixation and transfer of nitrogen by white clover to ryegrass. Soil Use Manage 6:84–86
Moyer-Henry KA, Burton JW, Israel D, Rufty T (2006) Nitrogen transfer between plants: a 15N natural abundance study with crop and weed species. Plant Soil 282:7–20
Nanzyo M, Dahlgren R, Shoji S (1993) Chemical characteristics of volcanic soils. In: S Shoji, M Nanzyo, R Dahlgren (eds) Volcanic ash soils: genesis, properties and utilization. Elsevier, Amsterdam, The Netherlands, pp 145–187
Nesheim L, Oyen J (1994) Nitrogen fixation by red clover (Trifolium pratense L) grown in mixtures with Timothy (Phleum pratense L) at different levels of nitrogen fertilization. Acta Agric Scand B Soil Plant Sci 44:28–34
Olesen JE, Bindi M (2002) Consequences of climate change for European agricultural productivity, land use and policy. Eur J Agron 16:239–262
Paynel F, Murray PJ, Cliquet JB (2001) Root exudates: a pathway for short-term N transfer from clover and ryegrass. Plant Soil 229:235–243
Rasmussen J, Eriksen J, Jensen ES, Esbensen KH, Høgh-Jensen H (2006) In situ carbon and nitrogen dynamics in ryegrass–clover mixtures: transfer, deposition and leaching. Soil Biol Biochem 39:804–815
Russell CA, Fillery IRP (1996) In situ 15N labelling of lupin below-ground biomass. Aust J Agr Res 47:1035–1046
Satio M (1990) Nitrogen mineralization parameters and its availability indices of soils in Tohuku district, their relationship. Japanese Journal of Soil Science and Plant Nutrition 61:265–272
Schmidtke K (2005) How to calculate nitrogen rhizodeposition: a case study in estimating N rhizodeposition in the pea (Pisum sativum L.) and grasspea (Lathyrus sativus L.) using a continuous 15N labelling split-root technique. Soil Biol Biochem 37:1893–1897
Stanford G, Frere MH, Schwanin DH (1973) Temperature coefficient of soil nitrogen mineralization. Soil Sci 115:321–323
Trannin WS, Urquiaga S, Guerra G, Ibijbijen J, Cadisch G (2000) Interspecies competition and N transfer in a tropical grass–legume mixture. Biol Fertil Soils 32:441–448
van der Krift TAJ, Gioacchini P, Kuikman PJ, Berendse F (2001) Effects of high and low fertility plant species on dead root decomposition and nitrogen mineralisation. Soil Biol Biochem 33:2115–2124
Wachendorf C, Irmler U, Blume HP (1999) Relationship between litter fauna and chemical changes of litter during decomposition under different moisture conditions. In: G Cadisch, KE Giller (eds) Driven by nature – plant litter quality and decomposition. CAB International, Oxon, UK, pp 135–144
Zebarth BJ, Alder V, Sheard RW (1991) In situ labeling of legume residues with a foliar application of a 15N-enriched urea solution. Commun Soil Sci Plant Anal 22:437–447
Zhu YG, Laidlaw AS, Christie P, Hammond MER (2000) The specificity of arbuscular mycorrhizal fungi in perennial ryegrass–white clover pasture. Agric Ecosyst Environ 77:211–218
Acknowledgement
Financial support from the Agricultural University of Iceland is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Euan K. James.
Rights and permissions
About this article
Cite this article
Gylfadóttir, T., Helgadóttir, Á. & Høgh-Jensen, H. Consequences of including adapted white clover in northern European grassland: transfer and deposition of nitrogen. Plant Soil 297, 93–104 (2007). https://doi.org/10.1007/s11104-007-9323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9323-4