Abstract
Aims
More sustainable agricultural systems, which contribute to C sequestration and biological N fixation, require accurate quantification of plant C and N inputs into soils. This has to be conducted under field conditions, as there are serious shortcomings to pot-based experiments, which have dominated studies on rhizodeposition estimation in the past. Therefore, amounts of below-ground plant C, N, and especially the rhizodeposition of peas were quantified in the field more accurately, with particular emphasis on their transfer into different soil compartments during vegetation.
Methods
Pea (Pisum sativum L.) plants were labelled with multiple pulses of 13C-glucose and 15N urea using the cotton wick method. Plants were harvested at four dates depending on plant development. Representative soil samples were taken at harvest with a special drilling tool, considering the spatial distribution of pea roots.
Results
Our results indicate that the quantity of C and N rhizodeposition did not change between flowering and maturity. About one third of the C and N rhizodeposits were present in microbial biomass and extractable C or the inorganic N pool of soil. When comparing this field study to a former pot experiment, a higher root-to-shoot ratio was detected; also the rhizodeposition-to-root ratio was altered in the field, questioning the assumption that results from pot experiments can be transferred to field conditions.
Conclusions
Due to a higher below-ground C (BGC) and N (BGN) input compared to pot experiments, studies aiming at quantifying BGC and BGN input will have to be conducted under field conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Roots are an important part of plant carbon (C) and nitrogen (N) input, in particular in arable systems, where they are often the main input source (Rasse et al. 2005; Wichern et al. 2007a, b), affecting virtually all soil processes, especially those mediated by soil microorganisms (Wichern et al. 2007a, b; de Graaff et al. 2013). The rhizosphere of living and decaying roots is a hot spot of microbial activity (Kuzyakov and Blagodatskaya 2015). Especially in organic farming systems, legume roots are a crucial source of N input into cropping systems (Rasmussen et al. 2012). This requires the exact assessment of the N2 fixed by rhizobia, the N stored in plant biomass and the N left on the field as an N source for subsequent crops (Askegaard and Eriksen 2007). Therefore, below-ground N (BGN) quantification is important, as it harbors a substantial part of total plant N not accounted for when estimating the aboveground biomass only (Peoples et al. 2009).
Below-ground C (BGC) and BGN are often quantified as root biomass by sieving out roots from a defined soil volume in pot or microcosm experiments. Under field conditions, root biomass is assessed from a representative volumetric soil sample and calculated on a hectare basis (Johnson and Morgan 2010). Roots are separated from soil by hand picking and/or wet sieving using different mesh sizes (Bolinder et al. 1997; Pierret et al. 2005; Kanders et al. 2017). This makes quantitative root estimation subjective to a certain extent. Furthermore, all root sampling and sieving cannot account for smaller root fragments and root debris from decaying roots, as well as root exudates and smaller molecular compounds or even ions released from plants during the vegetation period. The process of release of all of this is often referred to as rhizodeposition and assessed as C or N released from roots during the vegetation period (Uren 2001; Wichern et al. 2008).
Quantitative assessment of this C and N released from roots into the soil is usually done by labelling plants with stable (13C, 15N) or radioactive (14C) isotopes and tracing the isotopes in the soil. The assumption that both, light (12C, 14N) and heavy (13C, 15N) isotopes of C and N are similarly distributed within the plant allows assessment of BGC and BGN, including the rhizodeposits (Wichern et al. 2008; Hupe et al. 2016). Multiple pulse labelling often showed reasonably good tracer distribution in plants, when added by the cotton wick method (Hupe et al. 2016; Kanders et al. 2017). The rather slow solution uptake via a cotton wick resulted in a linear initial release of 14CO2 as rhizorespiration, indicating a continuous metabolization of the labelled glucose by the plant, which reflects the more continuous tracer solution uptake using the wick method (Wichern et al. 2010, 2011). Usually, these isotopic studies are conducted under controlled conditions in pots in disturbed soils with restricted root space to facilitate accurate estimation of root biomass and isotopes (Mayer et al. 2003; Hupe et al. 2018) or rarely also in small (Pausch et al. 2013) or large undisturbed soil columns (Wichern et al. 2007a, b).
In pot experiments, rhizodeposits accounted for between 10 and 30% of total plant C and N, depending on plant species (Kuzyakov 2001; Nguyen 2003; Wichern et al. 2008). Most investigations for C were conducted on wheat (Triticum aestivum L.) and mainly at early growth stages and for N mainly on peas (Pisum sativum L.) and wheat and usually for a longer growth period (Jones et al. 2009). The contribution of pea rhizodeposits to total plant C and N varied in a large range from 4 to 74% (Wichern et al. 2007b; Jones et al. 2009; Fustec et al. 2010; Hupe et al. 2016, 2018). As rhizodeposition is closely related to root biomass (Shamoot et al. 1968; de Graaff et al. 2007), the lower root-to-shoot ratio often observed in pot experiments compared with field conditions (Poorter et al. 2012; Kanders et al. 2017) most likely reduces total rhizodeposition under these conditions. Moreover, soil moisture and temperature usually vary more strongly under field conditions. As plant roots have a high plasticity, they respond to these changes with altered root growth and decay (Poorter et al. 2012) as well as exudation patterns, which results in quantitative and qualitative changes of the rhizodeposition (Wichern et al. 2008). However, it has to be acknowledged that controlled pot experiments are still a valid tool for studying processes of rhizodeposition to improve our mechanistic understanding plant roots responses, e.g. to abiotic stress. Nevertheless, BGC and BGN estimation by solely assessing root biomass in pot experiments not only disregards rhizodeposits as an important C and N pool but in particular does not consider the temporal and spatial variability of BGC and BGN under field conditions with the ever changing abiotic conditions in the field as described above. The approach of Pausch et al. (2013) who proposed transferring the rhizodeposition C-to-root C ratio from pot experiments to field conditions may not be valid, as root growth restriction may affect also the rhizodeposition-to-root ratio, although it may work as an approximation (Kanders et al. 2017). Therefore, accurate estimation of the C sequestration potential of crops and quantification of the N input by legumes in cropping systems requires experiments that investigate rhizodeposition under field conditions without root growth restriction.
Root exudates of small molecules are an easily available energy source for soil microorganisms (Kuzyakov 2002; Paterson 2003; Dennis et al. 2010). However, according to the definition of Uren (2001), rhizodeposits consist to a large extent of root debris and root fragments (Wichern et al. 2008), which most likely do not contribute much to the C and N transfer from roots into the microbial biomass in the short term (Mayer et al. 2003; Hupe et al. 2018), leaving a substantial proportion unrecovered (Wichern et al. 2007a, b). Under field conditions, the extended root growth enhances in particular this C and N transfer pathway (Wichern et al. 2007a, b, 2008).
For this reason, we conducted a field experiment with the objectives (i) to quantify below-ground plant C and N of peas (Pisum sativum L.) under field conditions without any restriction of root growth, (ii) to quantify C and N rhizodeposition during plant growth and (iii) to estimate the particular distribution of plant derived C and N in the soil, especially the transfer from plant roots into the soil microbial biomass.
Materials and methods
Study site
The experiment was carried out at the research station of the University of Kassel in Neu-Eichenberg, Germany (51°23’N, 9° 55′E 220 m asl), which has been managed organically since 1998. In 2013, the total annual rainfall was 711 mm. During the study time period (May–August 2013), rainfall was 240 mm and mean temperature was 15.3 °C (min 5.6 °C; max 25.0 °C). The silt loam was classified as a Haplic Luvisol (FAO-WRB 2014), with a pH of 6.1, 1.2% soil organic C and 0.13% total N. At 0–30 cm soil depth, the soil contained 20 μg Ca-acetate lactate extractable P g−1 soil, 54 μg K g−1 soil, and 90 μg Mg g−1 soil. The preceding crops before the experiment started were wheat (Triticum aestivum L.) in 2012 and oilseed rape (Brassica napus L.) in 2011.
Experimental design and labelling
In April 2013, semi-leafless peas (Pisum sativum L. “Santana”) were sown manually in 40 microplots (0.50 m × 0.38 m). The microplots were arranged in a completely randomized block design on an area of 33 × 18 m. In each microplot, 12 pea plants were cultivated at a distance of 12.5 cm between seedlings and rows to achieve an optimal seeding rate (64 plants m−2). Plants were harvested four times, depending on plant development according to the BBCH (Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie) code (Lancashire et al. 1991; Meier 2018): (1) 62 days after sowing (DAS), first petals visible, flowers still closed (BBCH 59), (2) 68 DAS, flowering (BBCH 69), (3) 82 DAS, pods have reached typical size, green ripe (BBCH 79) and (4) 103 DAS, fully ripe, all pods are dry and brown, seeds are dry and hard (BBCH 89). To figure out the best method for labelling plants in the field, a leaf feeding and a stem feeding method (cotton wick method) were compared in the greenhouse regarding their handling and applicability in the field. To approximate continuous labelling, the leaf feeding method requires the destructive use of one new leaf at least every week, which strongly affected plant growth and thus reduced plant biomass.
Therefore, the cotton wick method (Russell and Fillery 1996; Wichern et al. 2007a) was used in half of the microplots (20) and twelve plants were labelled with a 13C-glucose (99 atom%) and 15N-urea (95 atom%) solution. For this technique, the stem of each pea plant was drilled with a 0.5 mm drill, approximately 3–4 cm above the ground. Then, a small cotton wick was passed through the hole. To prevent evaporation losses and contamination of the tracer solution, the cotton wick was put through small silicon tubes. These silicon tubes (and the cotton wick) were passed through two holes in the lid of a 2 ml vial, which contained the labelling solution. A kneading mass (Teroson, Henkel, Germany) was placed between stem or lid and silicon tubes to prevent evaporation losses of the labelling solution (supplementary material Fig. S1). Plants usually took up most of the solution within 48 h. The labelling solution was produced by mixing deionized water and the stable 13C-glucose and 15N-urea and then sterile filtered (< 0.2 μm). All material used for producing the labelling solution was steam sterilized for 20 min at 121 °C in advance.
Continuous isotope uptake is important for homogeneous enrichment of 13C and 15N of the plant. For this reason, plants were labelled starting at 41 DAS (BBCH 13; 3 leaves unfolded) until 93 DAS with 0.5 ml labelling solution. Solution uptake was documented every second day. The vials were refilled with 0.5 ml labelling solution after complete solution uptake by the plants. A new sterile labelling system was installed if the labelling system showed contamination by fungal or algae growth. All pea plants, independent of sampling day, had labelling solution available for their disposal until harvest.
The labelling procedure was done using an adapted multicarrier manual weeding device (supplementary material Fig. S2). This multicarrier consisted of a flatbed with a hole for the head, installed on a large wooden board with four tires, having an appropriate width to cross the microplot without damaging the plants. This multicarrier was height-adjustable for vial refilling or replacement of the labelling system during later plant growth stages. The soil of the microplots was covered with a 1 mm mesh to prevent soil contamination from falling leaves, which were collected regularly. The described labelling approach approximated continuous labelling by application of multiple pulses at high frequency.
Sampling and analyses
At each harvest date, the labelling system was carefully removed from the plants and frozen for analysis of the remaining labelling solution. Then, one pea plant in the middle of the microplot was completely harvested for exact shoot and root biomass determination, separating the above-ground biomass into flower/grain, stem, and leaves. After this, the remaining 11 pea plants were similarly harvested for isotope determination. For calculating root biomass and measuring microbial biomass, soil samples were taken at 0–30 and 30–60 cm depth with a motorized HUMAX (Burch, Rothenburg, Switzerland) soil corer (diameter 80 mm) that automatically transferred the soil samples into 1.5 l PVC tubes (supplementary material Fig. S3). This prevented a cross-contamination of labelled soil from one microplot to the next. A sampling pattern according to Anthes (2005) was used (Fig. 1): (α) directly at the position of the single harvested pea, (β) exactly between two peas within the row, and (γ) in the middle of four peas between two rows of peas. Solely for analyzing 13C/12C and 15N/14N ratios, the remaining pea roots of the 11 peas were completely excavated with a spade and soil samples were taken at 0–30, 30–60, and 60–90 cm depth. This was done at every harvest date for five labelled and five non-labelled control microplots.
Soil sampling schemes: (a) Soil samples were taken at three defined points in each microplot around one pea plant (α: directly on one single pea; β: between two peas in the row; γ: in the middle of 4 peas between two rows). (b) Theoretical scheme for calculating the complete root DM of one pea plant
All plant parts were dried for 48 h at 60 °C for estimating dry matter (DM). With a ball mill, dry plant parts were ground to a fine powder for analyzing total C, total N and isotope ratios. Subsamples of soil were sieved (< 2 mm) to remove all visible root fragments (> 2 mm), supported by hand collecting with tweezers. Then, the soil was dried for 24 h at 105 °C for DM estimation and ground with a ball mill. Total C and N of all samples were determined using an elemental analyzer (Fisons, Milano, Italy). All 13C/12C and 15N/14N ratios were determined by isotope ratio mass spectrometry (IRMS) (Finnigan MAT, Bremen, Germany). For estimating isotope recovery, vials and labelling systems were extracted with 200 ml 0.05 M K2SO4, followed by determination of extractable organic C and total N as described below. The recovered 13C and 15N in the extracts of the labelling systems were calculated, assuming that the C and N were solely derived from the isotope solution.
Microbial biomass C (MBC) and N (MBN) were estimated using fumigation extraction (Brookes et al. 1985; Vance et al. 1987), including pre-extraction to remove living roots (Mueller et al. 1992). For pre-extraction, 30 g of field moist soil was horizontally shaken for 30 min with 70 ml 0.05 M K2SO4 at 200 rev min−1. Then, the soil suspension was centrifuged for 10 min at 4000 g and 6 °C. The supernatant was filtered and frozen for measuring organic C, total N, inorganic N, and the isotope ratios in the extracts. After pre-extraction, 15 g of the 0.05 M K2SO4 saturated soil was fumigated for 24 h at 25 °C with ethanol-free CHCl3. After CHCl3 removal, the fumigated and 15 g of the non-fumigated pre-extracted soil were further extracted with 60 ml 0.05 M K2SO4 and the extract kept frozen until further analyses. Organic C and total N in all extracts were analyzed using a CN Analyzer (Multi N/C 2100S, Analytik Jena, Germany). After freeze-drying of extracts, the 13C/12C and 15N/14N isotope ratios were determined by IRMS.
Calculation and statistical analyses
Total root DM was calculated as the sum of root DM found in the three sectors α, β and γ. To prevent over- or underestimation of root DM, because of the theoretical overlapping of these sectors, each sector was weighted differently, depending on microplot size and the diameter of the drilling tool. The area of the theoretical overlapping of the three sectors was evenly distributed on each sector (Fig. 1). More detailed information on the calculation of the rooted area per pea plant is given in the supplementary material Fig. S4.
MBC was calculated as EC / kEC, where EC = (organic C extracted from fumigated soils) – (organic C extracted from non-fumigated soils) and kEC = 0.45 (Wu et al. 1990). MBN was calculated as EN / kEN, where EN = (total N extracted from fumigated soils) - (total N extracted from non-fumigated soils) and kEN = 0.54 (Joergensen and Mueller 1996). 13Ctracer derived from rhizodeposition (= 13CtracerdfR) and 15NtracerdfR were calculated using the mass balance approach (eqs. 1 and 2) as described in Hupe et al. (2016).
It was assumed that the distribution of 13Ctracer and 15Ntracer in the plant corresponds to the distribution of total plant C and total plant N, consequently 13CtracerdfR (%) = CdfR (%) and 15NtracerdfR (%) = NdfR (%). CdfR and NdfR in the soil were separately calculated for MBC, MBN, SOC, total N and inorganic N (Hupe et al. 2016).
Results in tables are given as arithmetic means in boxplots as medians and expressed on an oven-dry basis. Normality was tested by the Shapiro-Wilk test and equal variance by the Levene test. Differences between DAS and soil depths were determined by repeated measures or one-way analysis of variance (ANOVA), using the Scheffé post-hoc test. Differences between pot and field experiments were analyzed using a BBCH-specific t-test. All statistical analyses were performed by IBM SPSS Statistics 22.
Results
13C and 15N enrichment, uptake and recovery
The cotton wick method achieved a relatively similar homogeneous enrichment with 13C and 15N during the complete vegetation period among the different plant parts of peas analyzed (Table 1). Highest 13C enrichment was achieved at 103 DAS in leaves and stem (0.57 atom% excess), whereas enrichment of soil was constant during plant growth at all three soil depths. Similarly to 13C, highest 15N enrichment was achieved at 103 DAS in leaves and stem (4.94 atom% excess). However, differences were observed for 15N over time in the subsoil: There was a higher 15N enrichment 82 DAS at 30–60 cm depth as compared with 60–90 cm depth, whereas the enrichment was higher at 60–90 cm depth 68 DAS and 103 DAS.
During plant growth, one pea plant took up between 11 and 37 mg 13C and between 5 and 14 mg 15N (Table 2). Isotope recovery increased during plant growth, peaked at 82 DAS, and declined again thereafter. Between 32 and 89% of the applied 13C and between 59 and 117% of the applied 15N were recovered in stem, leaves, grain and roots. The recovery of isotopes in soil decreased from 11% (13C) and 17% (15N) at 62 DAS to 5% and 8%, respectively, at 103 DAS. Between 45 and 94% of the applied 13C and between 72 and 127% of the applied 15N were recovered in total. Approximately 2% of the applied isotopes remained in the wick system.
C and N derived from rhizodeposition
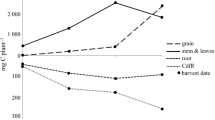
Root C and N increased to 382 mg and 23 mg plant−1, respectively, until the end of flowering (68 DAS), followed by a 87% decrease until green ripe (82 DAS) (Table 3). Total plant C and N increased to 10.5 g and 0.44 g plant−1, respectively, until green ripe, followed by a 37% decrease. CdfR varied between 426 and 879 mg plant−1 and NdfR between 20 and 35 mg plant−1 without significant changes between the beginning of flowering and maturity. Furthermore, similar amounts of CdfR and NdfR were found at all three soil depths.
Transfer of rhizodeposits into soil
The CdfR proportion in MBC and the percentage of CdfR incorporated into MBC were constant over time and soil depths (Table 4). The CdfR proportion ranged from 2 to 5% of MBC at 0–30 cm depth and from 4 to 10% at 30–60 cm depth, whereas MBC incorporated approximately one quarter of CdfR at both depths. At 0–30 cm depth, extractable C increased during flowering from 81 to 136 μg g−1 soil. At 30–60 cm depth, significantly more extractable C was found at the end of flowering and green ripe compared with the beginning of flowering. The CdfR proportion in extractable C and the percentage of CdfR present as extractable C was constant over time and soil depth. The CdfR proportion ranged from 2 to 5% of extractable C at 0–30 cm depth and from 3 to 7% at 30–60 cm depth. Extractable C varied between 6 and 19% of CdfR at both depths.
At 0–30 cm depth, MBN increased until green ripe from 27 to 41 μg g−1 soil and decreased to 29 μg g−1 soil until maturity (Table 4). At 30–60 cm depth, MBN increased from 7 to 14 μg g−1 soil until green ripe and decreased to 8 μg g−1 soil until maturity. The NdfR proportion of MBN was constant at about 1% during plant development. Similar to CdfR, the percentage of NdfR incorporated into MBN was on average 22% at 0–30 cm depth, whereas it was only between 7 and 11% at 30–60 cm depth. The inorganic N content in soil was stable during plant development at 0–30 cm depth, but decreased from 4 μg g−1 soil at beginning of flowering to 2 μg g−1 soil at maturity at 30–60 cm depth. The NdfR proportion of inorganic N and the percentage of NdfR present as inorganic N were constant over time and soil depth. The NdfR proportion ranged from 6 to 8% of inorganic N at 0–30 cm depth and from 2 to 3% at 30–60 cm depth. The percentage of NdfR present as inorganic N varied between 8 and 22% at 0–30 cm depth and between 3 and 9% at 30–60 cm depth.
Discussion
Labelling plants under field conditions
The approach used in the present study (cotton wick method, multicarrier, HUMAX soil sampler) resulted in homogeneous and high enrichment of plants with 13C and 15N. Until now the cotton wick method has mainly been used in experiments where plants were grown in pots or columns with restricted root growth. The advantage of the cotton wick method is the possibility of a more continuous labeling as compared with leaf labelling, where solution uptake is faster. As shown earlier (Wichern et al. 2011), the cotton wick allows continuous solution uptake from a vial (which can also be monitored), delivering the tracer solution continuously to the plant organs. Enrichment and tracer recovery in the current field study are similar to other labelling experiments under controlled conditions (Mayer et al. 2003; Mahieu et al. 2007; Arcand et al. 2013; Hupe et al. 2018) or under field conditions with restricted root growth (Wichern et al. 2007a). However, at green ripe, isotope recovery in plants was excessively high, due to error accumulation of single fractions, e.g., a total 13C recovery of 94% is impossible, as shoot and root respiration results in 13C losses as 13CO2. The possible contribution of 13C enrichment from one plant to the next with 13CO2 to this error, e.g. by photosynthesis, is unlikely as shown in a previous pot experiment (Hupe et al. 2018).
In spite of using the multicarrier system to minimize plant and soil disturbance during labelling events, the labelling procedure affected plant development for unknown reasons. Non-labelled peas formed slightly but significantly more above-ground DM as compared with labelled peas at maturity, whereas no differences were detected for root DM or the other harvest dates. In contrast, Wichern et al. (2007a, b, 2010) and Mahieu et al. (2009) found no differences in below-ground, above-ground and total DM, when using the cotton wick technique. Differences in DM may have been caused by mechanical damage during plant labelling or documentation of plant development and solution uptake. The labelling system was renewed whenever plants stopped taking up solution due to possible contamination of the labelling system or the formation of callus on the plant stem. However, in a second field experiment conducted at the same time under similar conditions and investigating the transfer of C and N from peas to triticale, no effect of labelling on the biomass of peas was observed. This suggests that soil inhomogeneity between the small microplots may have contributed to the current DM difference at maturity.
The exact quantification of root DM under field conditions is another challenge when estimating below-ground biomass. In the present study, analyzing the whole microplot for root biomass was not an option due to the excessive amount of soil to be sieved and washed for root recovery. Moreover, the almost transparent fine roots are difficult to detect due to their small size, often leading to serious underestimation of root biomass (Pierret et al. 2005). Consequently, root fragments and fine roots not recovered by sieving are accounted for as rhizodeposits (Uren 2001; Wichern et al. 2008). Therefore, a sampling scheme according to Anthes (2005) was developed and used and soil samples were taken down to 60 cm depth. No pea roots were detected below 60 cm soil depth at harvest, although most likely some reached deeper depths during flowering, as indicated by tracer presence below 60 cm. Gan et al. (2009) found 95% of the root biomass at 0–60 cm depth for field peas at maturity, which is in line with Cutforth et al. (2013), who measured 95% of pea root length density at 0–60 cm in the field at 77 DAS, although some pea roots reached depths below 100 cm. Armstrong et al. (1994) found pea roots even at 160 cm depth. The detection of pea roots below 60 cm is additionally hampered by the strongly decreasing diameter of pea roots with depth (Fan et al. 2016), making their detection by sieving almost impossible.
Rhizodeposition in time and space
CdfR and NdfR did not change between flowering and maturity of pea plants, although differences could be expected as C and N are differently transported in plants: C is solely transferred in the phloem, mainly as sucrose from source to sink (Kühn and Grof 2010); N is transported in the phloem as urea (Witte 2011) and amino acids (Lalonde et al. 2003) as well as in the xylem, mainly as NO3− but also as NH4+. In a pot experiment, it has already been shown that CdfR constantly increased during vegetation and that NdfR increased until flowering and remained constant thereafter (Hupe et al. 2018). However, no differences between CdfR and NdfR were detected under field conditions in the present investigation. The development of the pea root system is at its maximum during flowering (Thorup-Kristensen 1998; Gavito et al. 2001; Arcand et al. 2013). This implies that, until flowering, root fragments are a smaller component of rhizodeposition as compared with later growth stages. After flowering, 40–50% of roots die (Gavito et al. 2001; Arcand et al. 2013) so that an increasing amount of decaying root fragments contribute to rhizodeposition. However, under field conditions, where changing abiotic conditions trigger root death, decaying roots may be a bigger part of the rhizodeposits.
The absence of more studies dealing with rhizodeposition over time, using a similar experimental setup, makes it difficult to compare the current results with other data. Arcand et al. (2013) investigated pea rhizodeposition using the same labelling method and a similar labelling intensity, but focused solely on N rhizodeposition. Wichern et al. (2007a, b) investigated C and N rhizodeposition with 13C and 15N double labelling. However, their approach considerably differed in frequency and duration of labelling and time of harvest. Such differences in experimental setup are most likely an important reason for the large range in values of rhizodeposition reported in literature (Wichern et al. 2008; Jones et al. 2009; Arcand et al. 2013).
Regardless of plant development, similar amounts of CdfR and NdfR were found at 0–30, 30–60, and 60–90 cm depth. Leaching of rhizodeposition from topsoil to subsoil can be excluded, due to the absence of downward water movement from May to August. This indicates that some roots must have reached depths down to 90 cm during maximum development at flowering, which are decayed to a large degree thereafter until maturity (Thorup-Kristensen 1998; Gavito et al. 2001; Kleikamp and Joergensen 2006; Arcand et al. 2013). Such small decaying root fragments and root debris might be detectable as particulate organic matter (Magid and Kjærgaard 2001; Joergensen and Wichern 2018), which needs to be considered in future studies. The even distribution of rhizodeposition in α, β, and γ sectors indicates an even horizontal root distribution around the pea stem. The absence of vertical differences in CdfR and NdfR at different soil depths despite a decreasing root biomass is presumably largely due to the strong small scale spatial variation of roots. Another important reason might be a reduction in microbial turnover of CdfR and NdfR with increasing depth, although this relationship still lacks experimental evidence (Sanaullah et al. 2011; Struecker et al. 2016).
Inhomogeneous soil conditions caused significant differences in MBC contents between different sampling days. Constant MBC/SOC ratios during plant development (on average 2.0% at 0–30 cm and 1.7% at 30–60 cm) indicate that microbial biomass changes due to growth and death processes can be excluded. At 0–30 cm depth, between 21 and 42% of C and between 15 and 33% of N released by pea roots were incorporated into MBC and MBN, respectively, in the current study, depending on plant development stage. At 30–60 cm depth, the respective values were lower. Wichern et al. (2007b) recovered between 11 and 21% of CdfR in MBC and between 1 and 23% of NdfR in MBN, depending on the time of plant labelling and growth stage at harvest. Mayer et al. (2003) found 18% of NdfR incorporated into MBN at pea maturity. Under field conditions, the higher production of root DM presumably causes a higher C and N transfer from plants into the microbial biomass but also into the microbial necromass, which is the sum of all non-biomass metabolites, such as extracellular enzymes, extracellular polymeric substances, secondary metabolites, and dead microbial tissue (Joergensen and Wichern 2018). To better understand the influence of rhizodeposition on soil microbial biomass, future studies have to evaluate the transfer of plant derived C and N into microbial necromass.
Pot versus field conditions
The current results can be compared with a pot experiment conducted earlier (Hupe et al. 2018), cultivating leafy pea plants (Pisum sativum cv. Frisson) in soil taken from the site of the current field experiment. Both, the double labelling method and the growth stages at the time of harvest were similar in that study.
During flowering, root DM was significantly lower under pot than under field conditions (Fig. 2), before developing to similar levels at maturity. After flowering, CdfR of pot grown peas varied constantly around 1.66 g g−1 root DM (Table 5), whereas that of field grown peas increased to 4.45 g g−1 root DM at maturity. At begin of flowering and at maturity, NdfR was significantly higher under field than under pot conditions, whereas the reverse was true at the end of flowering. At this time, the BGC/AGC and BGN/AGN ratios were twice as high under field as under pot conditions (Fig. 3ab), leading to higher amounts of CdfR and NdfR in the field. This supports the view that rhizodeposition is closely related to root biomass and must be higher in the field (Shamoot et al. 1968; de Graaff et al. 2007).
However, most studies on rhizodeposition were conducted in pot experiments with restricted root growth, because the estimation of rhizodeposition under field conditions is very labor intensive, due to repeated plant labelling and sampling. Consequently, Pausch et al. (2013) proposed to use the CdfR/root C ratio of maize derived from a pot experiment to estimate the CdfR under field conditions by solely assessing root DM, assuming a constant CdfR/root C ratio. This approach was adapted by Kanders et al. (2017), showing a substantial contribution of N rhizodeposition to total plant N in cover crops. However, the largest uncertainty of this approach is that the fine roots decomposed during the vegetation period are not included (Pausch and Kuzyakov 2018). Also the results of the current study indicate that the approach proposed by Pausch et al. (2013) may not be valid.
A shift in the root/shoot ratio is evident by comparing pot- and field-grown peas. More root C and N as well as rhizodeposition than AGC and AGN were formed under field conditions. Consequently, CdfR/root C (Fig. 3c) and NdfR/root N (Fig. 3d) ratios were more than twice (P < 0.05, t-test) as high at maturity under field than under pot conditions, neglecting the bias of comparing two different pea varieties. Very few experiments have been performed to investigate the genetic variation in root establishment of pea varieties (Bourion et al. 2007). Veitenheimer and Gritton (1984) observed similar relative differences in root DM at flowering, comparing four pea varieties in the field and under controlled pot conditions. Pea varieties certainly differ in their root growth pattern to some extent (Baigorri et al. 1999; Bourrion et al. 2007). However, the different plant growth pattern of the leafy variety ‘Frisson’ and semi-leafless ‘Santana’ has been considered by comparing similar BBCH stages of plant development and not simply DAS.
In pot experiments with sieved soils (Mayer et al. 2003; Pausch et al. 2013; Kanders et al. 2017), the loose soil structure facilitates root growth and reduces abrasion of root cells, with largely unknown effects on the quantity and quality of rhizodeposits. With increasing higher penetration resistance, e.g. under water limiting conditions, plants have to excrete more mucilage (Carminati et al. 2017; Ahmed et al. 2018), which qualitatively changes rhizodeposition (Brimecombe et al. 2007) and most likely also increases the CdfR/root ratio. Constant soil moisture, close to optimum for plant growth and for microbial activity, often combined with elevated soil temperature in pot experiments leads to high nutrient availability to plants (Audet and Charest 2010). This usually reduces the demand of plants in pot experiments to invest in root growth, additionally to the spatial restriction in comparison with field experiments and consequently affects rhizodeposition.
After flowering, the rapid decay of roots increases the contribution of small particulate root fragments and root debris as well as sloughed cells to rhizodeposition, which are both important fractions of rhizodeposition (Uren 2001; Wichern et al. 2008). The larger amounts of decaying roots during maturation inevitably shifts the CdfR/root C and NdfR/root N ratios of field experiments to a much higher level than those of pot experiments. Further, this highlights the fact that studies aiming at quantifying rhizodeposition of C or N have to consider the whole growth period, including the generative phase in annual plants. Consequently, most of the studies conducted on C rhizodeposition are likely to be wrong, as indicated in the review by Jones et al. (2009), showing that most studies on C rhizodeposition were only investigating the vegetative growth stage of plants. Consequently, the contribution of rhizodeposits to the C sequestration potential of plants is most likely underestimated and needs more attention in future studies. Moreover, these findings indicate the strong demand, along with quantification of rhizodeposition, to gather more information on the quality of rhizodeposits in the future, which will make it possible to separate the contribution of mucilage-like components from particulate root fragments and root debris.
Conclusions
The present study has clearly shown that, depending on plant development, the C and N rhizodeposition of peas differs between pot and field experiments. Reasons can be found in the restricted root growth in pot experiments. Moreover, biotic and abiotic factors under field conditions can influence the amount and quality of rhizodeposition. For estimating rhizodeposition, a continuous labelling is required, which in peas can be achieved using multiple pulses and a wick labelling approach. For the first time, pea plants were successfully labelled with the cotton wick method under field conditions without restricted root growth. This allowed a more accurate estimation of plant rhizodeposition. Future studies will help to give more exact information about the C sequestration potential of plants or to develop more exact N balances by quantifying the N input more precisely. A transfer of results from pot experiments to field conditions is not recommended. Therefore, for estimating rhizodeposition, we recommend carrying out more experiments under field conditions, without restricted root growth. Further, as the composition of rhizodeposition changes during plant growth from exudates to small particulate root fragments and root debris, future experiments must investigate these two fractions during the whole growth period if aiming at quantifying C or N rhizodeposition.
References
Ahmed MA, Banfield CC, Sanaullah M, Gunina A, Dippold MA (2018) Utilisation of mucilage C by microbial communities under drought. Biol Fertil Soils 54:83–94
Anthes J (2005) Beitrag von Ackerbohne (Vicia faba L.), Luzerne (Medicago sativa L.) und Saatwicke (Vicia sativa L.) zur Selbstregelung der N-Zufuhr in leguminosenbasierten Fruchtfolgen. Dissertation, Universität Göttingen
Arcand MM, Knight JD, Farrell RE (2013) Temporal dynamics of nitrogen rhizodeposition in field pea as determined by 15N labeling. Can J Plant Sci 93:941–950
Armstrong EL, Pate JS, Tennant D (1994) The field pea in south western Australia. Patterns of water use and root growth in geno-types of contrasting morphology and growth habit. Funct Plant Biol 21:517–532
Askegaard M, Eriksen J (2007) Growth of legume and nonlegume catch crops and residual-N effects in spring barley on coarse sand. J Plant Nutr Soil Sci 170:773–780
Audet P, Charest C (2010) Identification of constraining experimental-design factors in mycorrhizal pot-growth studies. J Bot 2010:718013
Baigorri H, Antolìn MC, Sánchez-Díaz M (1999) Reproductive response of two morphologically different pea cultivars to drought. Eur J Agron 10:119–128
Bolinder MA, Angers DA, Dubuc JP (1997) Estimating shoot to root ratios and annual carbon inputs in soils for cereal crops. Agric Ecosyst Environ 63:61–66
Bourion V, Lagguerre G, Depret G, Voisin AS, Salon C, Duc G (2007) Genetic variability in nodulation and root growth affects nitrogen fixation and accumulation in pea. Ann Bot 100:589–598
Brimecombe M, De Leij FAAM, Lynch J (2007) Rhizodeposition and microbial populations in: Pinton R, Varanini Z, Nannipieri P (eds) the rhizosphere: biochemistry and organic substances at the soil-plant interface. Marcel Dekker, New York, pp 73–109
Brookes PC, Landman A, Pruden G, Jenkinson D (1985) Chloroform fumigation and the release of soil nitrogen : a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Carminati A, Benard P, Ahmed MA, Zarebanadkouki M (2017) Liquid bridges at the root-soil interface. Plant Soil 417:1–15
Cutforth HW, Angadi SV, McConkey BG, Miller PR, Ulrich D, Gulden R, Volkmar KM, Entz MH, Brandt SA (2013) Comparing rooting characteristic sand soil water withdrawal patterns of wheat with alternative oilseed and pulse crops grown in the semiarid Canadian prairie. Can J Soil Sci 93:147–160
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72:313–327
Fan J, McConkey B, Wang H, Janzen H (2016) Root distribution by depth for temperate agricultural crops. Field Crop Res 189:68–74
FAO-WRB (2014) World Reference Base for Soil Resources. World Soil Resources Reports No. 103. FAO, Rome
Fustec J, Lesuffleur F, Mahieu S, Cliquet J-B (2010) Nitrogen rhizodeposition of legumes. A review. Agron Sustain Dev 30:57–66
Gan YT, Campbell CA, Janzen HH, Lemke R, Liu LP, Basnyat P, McDonald CL (2009) Root mass for oilseed and pulse crops: growth and distribution in the soil profile. Can J Plant Sci 89:883–893
Gavito ME, Curtis PS, Jakobsen I (2001) Neither mycorrhizal inoculation nor atmospheric CO2 concentration has strong effects on pea root production and root loss. New Phytol 149:283–290
de Graaff MA, Six J, Jastrow JD, Schadt CW, Wullschleger SD (2013) Variation in root architecture among switchgrass cultivars impacts root decomposition rates. Soil Biol Biochem 58:198–206
de Graaff MA, Six J, van Kessel C (2007) Elevated CO2 increases nitrogen rhizodeposition and microbial immobilization of root-derived nitrogen. New Phytol 173:778–786
Hupe A, Schulz H, Bruns C, Joergensen RG, Wichern F (2016) Digging in the dirt – inadequacy of belowground plant biomass quantification. Soil Biol Biochem 96:137–144
Hupe A, Schulz H, Naether F, Bruns C, Haase T, Heß J, Joergensen RG, Wichern F (2018) Even flow? Changes of carbon and nitrogen release from pea roots over time. Plant Soil
Joergensen RG, Mueller T (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the k EN value. Soil Biol Biochem 28:33–37
Joergensen RG, Wichern F (2018) Alive and kicking: why dormant soil microorganisms matter. Soil Biol Biochem 116:419–430
Johnson JMF, Morgan J (2010) Plant sampling guidelines. In: Follett RF (ed) sampling protocols, pp 2-1 - 2-10
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33
Kanders MJ, Berendonk C, Fritz C, Watson C, Wichern F (2017) Catch crops store more nitrogen below-ground when considering rhizodeposits. Plant Soil 417:287–299
Kleikamp B, Joergensen RG (2006) Evaluation of arbuscular mycorrhiza with symbiotic and nonsymbiotic pea isolines at three sites in the Alentejo, Portugal. J Plant Nutr Soil Sci 169:661–669
Kuzyakov Y (2001) Tracer studies of carbon translocation by plants from the atmosphere into the soil (a review). Eurasian Soil Sci 34:28–42
Kuzyakov Y (2002) Review : factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396
Kuzyakov Y, Blagodatskaya E (2015) Microbial hotspots and hot moments in soil: concept & review. Soil Biol Biochem 83:184–199
Kühn C, Grof CP (2010) Sucrose transporters of higher plants. Curr Opin Plant Biol 13:287–297
Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26:37–56
Lancashire PD, Bleiholder H, Boom TVD, Langelüddeke P, Stauss R, Weber E, Witzenberger A (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol 119:561–601
Magid J, Kjærgaard C (2001) Recovering decomposing plant residues from the particulate soil organic matter fraction: size versus density separation. Biol Fertil Soils 33:252–257
Mahieu S, Fustec J, Faure M-L, Corre-Hellou G, Crozat Y (2007) Comparison of two 15N labelling methods for assessing nitrogen rhizodeposition of pea. Plant Soil 295:193–205
Mahieu S, Fustec J, Jensen ES, Crozat Y (2009) Does labelling frequency affect N rhizodeposition assessment using the cotton-wick method? Soil Biol Biochem 41:2236–2243
Mayer J, Buegger F, Jensen ES, Schloter M, Heß J (2003) Estimating N rhizodeposition of grain legumes using a 15N in situ stem labelling method. Soil Biol Biochem 35:21–28
Meier U (2018) Entwicklungsstadien mono- und dikotyler Pflanzen. BBCH Monografie, Quedlinburg, Open Agrar Repositorium. https://doi.org/10.5073/20180906-075119
Mueller T, Joergensen RG, Meyer B (1992) Estimation of soil microbial biomass C in the presence of living roots by fumigation-extraction. Soil Biol Biochem 24:179–181
Nguyen C (2003) Rhizodeposition of organic C by plants : mechanisms and controls. Agronomie 23:375–396
Paterson E (2003) Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur J Soil Sci 54:741–750
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob Change Biol 24:1–12
Pausch J, Tian J, Riederer M, Kuzyakov Y (2013) Estimation of rhizodeposition at field scale: upscaling of a 14C labeling study. Plant Soil 364:273–285
Peoples MB, Brockwell J, Herridge DF, Rochester IJ, Alves BJR, Urquiaga S, Boddey RM, Dakora FD, Bhattarai S, Maskey SL, Sampet C, Rerkasem B, Khan DF, Hauggaard-Nielsen H, Jensen ES (2009) The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 48:1–17
Pierret A, Moran CJ, Doussan C (2005) Conventional detection methodology is limiting our ability to understand the roles and functions of fine roots. New Phytol 166:967–980
Poorter H, Bühler J, van Dusschoten D, Climent J, Postma JA (2012) Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Funct Plant Biol 39:839–850
Rasmussen J, Søegaard K, Pirhofer-Walzl K, Eriksen J (2012) N2-fixation and residual N effect of four legume species and four companion grass species. Eur J Agron 36:66–74
Rasse D, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356
Russell CA, Fillery IRP (1996) Estimates of lupin below-ground biomass nitrogen, drymatter, and nitrogen turnover to wheat. Crop Pasture Sci 47:1047–1059
Sanaullah M, Chabbi A, Leifeld J, Bardoux G, Billou D, Rumpel C (2011) Decomposition and stabilization of root litter in top- and subsoil horizons: what is the difference? Plant Soil 338:127–141
Shamoot S, McDonald I, Bartholomew WV (1968) Rhizodeposition of organic debris in soil. Soil Sci Soc Am Pro 32:817–820
Struecker J, Kaiser M, Dyckmans J, Joergensen RG (2016) Maize root decomposition in subsoil horizons of two silt loams differing in soil organic C accumulation due to colluvial processes. Geoderma 283:101–109
Thorup-Kristensen K (1998) Root growth of green pea (Pisum sativum L.) genotypes. Crop Sci 38:1445–1451
Uren NC (2001) Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere –biogeochemistry and organic substances at the soil-plant interface. Marcel Dekker, New York, pp 19–40
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Veitenheimer EE, Gritton ET (1984) Preliminary root studies of Pisum sativum. Pisum Newsletter 16:73–74
Wichern F, Mayer J, Joergensen RG, Müller T (2007a) Rhizodeposition of C and N in peas and oats after 13C–15N double labelling under field conditions. Soil Biol Biochem 39:2527–2537
Wichern F, Mayer J, Joergensen RG, Müller T (2007b) Release of C and N from roots of peas and oats and their availability to soil microorganisms. Soil Biol Biochem 39:2829–2839
Wichern F, Eberhardt E, Mayer J, Joergensen RG, Müller T (2008) Nitrogen rhizodeposition in agricultural crops: methods, estimates and future prospects. Soil Biol Biochem 40:30–48
Wichern F, Mayer J, Joergensen RG, Müller T (2010) Evaluation of the wick method for in situ 13C and 15N labelling of annual plants using sugar-urea mixtures. Plant Soil 329:105–115
Wichern F, Andreeva D, Joergensen RG, Kuzyakov Y (2011) Stem labelling results in different patterns of 14C rhizorespiration and 15N distribution in plants compared to natural assimilation pathways. J Plant Nutr Soil Sci 174:732–741
Witte CP (2011) Urea metabolism in plants. Plant Sci 180:431–438
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction - an automated procedure. Soil Biol Biochem 22:1167–1169
Acknowledgements
We are very grateful to Lydia Hans, Tino Hupe, Franziska Naether and Gabi Dormann. Special thanks to Rainer Wedemeyer and all the student workers, for many hours together in the field, the laboratory and in front of the precision balance. We also thank Reinhard Langel and Lars Szwec for isotope analyses and Mick Locke for carefully correcting our English. This study was funded by the German Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric Paterson.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2471 kb)
Rights and permissions
About this article
Cite this article
Hupe, A., Schulz, H., Bruns, C. et al. Get on your boots: estimating root biomass and rhizodeposition of peas under field conditions reveals the necessity of field experiments. Plant Soil 443, 449–462 (2019). https://doi.org/10.1007/s11104-019-04238-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04238-z