Abstract
Aims
Elevated levels of metals reduce plant growth, including contaminated, acid, and saline soils, but much remains unknown regarding their mechanisms of toxicity. In this regard, it is important to understand the kinetics of changes in root elongation rate (RER) and root morphology.
Methods
Seedlings of soybean (Glycine max) were grown in solutions containing toxic levels of one of seven metals that differed markedly in their properties (Ag, Al, Ca, Cu, Hg, Na, and Sr), with mannitol and ‘mixed salts’ treatments also included.
Results
Despite their widely differing properties, all treatments caused similar symptoms, with roots swelling radially within the elongation zone, possibly associated with ethylene or auxin. In addition, Ag, Al, Cu, and Hg caused a rupturing of the outer root tissues likely associated with inhibition of wall loosening. Finally, using kinematic analyses to examine the effects of Hg in 5 min intervals, it was found that RER decreased by 50% after only 40 min, primarily associated with a decrease in the rate at which individual cells were elongating.
Conclusions
The information provided here will assist in understanding the mechanisms by which toxic levels of metals reduce root elongation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals, such as Al, Cu, and Hg, are natural components of the environment but are toxic at elevated concentrations. The importance of elevated metals in the environment has long been recognised (for example, see Veitch 1904). Acid soils, in which soluble Al is elevated, comprise ca. 4 billion ha of the global ice-free land or ca. 40% of the world’s total arable land (Eswaran et al. 1997). In Australia alone, acid soils cost AU$1.5 billion per year in lost productivity (NLWRA 2002). Elevated levels of other trace metals (such as Hg and Cu) occur in sites contaminated by mining, industry, and transport. Similarly, some metals such as Na, reduce plant growth when present at elevated concentrations in the 20% of all irrigated land that is salt-affected (Ghassemi et al. 1995).
The presence of high concentrations of soluble metals in soils is of concern due to the associated yield losses, and in some cases this risk is compounded as toxic levels of metals can enter the food chain via plant uptake. That elevated concentrations of metals are toxic to plants has been known for decades, and in some cases for more than a century, but for many metals the underlying reasons for their toxic effects have remained elusive. For metals such as Na, it is known that plant growth is reduced due to osmotic effects, direct toxicity, and indirect nutritional effects (Munns and Termaat 1986; Parida and Das 2005). In contrast, for Al, root elongation is reduced rapidly due to the binding of Al to the cell wall which inhibits its loosening within the elongation zone as required for growth (Jones et al. 2006; Kopittke et al. 2015). Yet, despite these marked differences between Na and Al toxicity (for example), it is also known that there are also some commonalities in the toxic effects of various metals. For example, it has been proposed that a range of metals are toxic in a similar manner to Al through a ‘common’ (non-specific) mechanism (Kinraide and Yermiyahu 2007; Kopittke et al. 2014).
By understanding both the commonalities and the differences in the effects of various metals, this provides information regarding their underlying mode of toxicity. Although some metals are better understood than others, much remains unknown regarding the kinetics (speed) with which various metals reduce growth, the nature of this growth reduction, and the changes in root morphology (symptoms) associated with short-term exposure. For example, considering changes in root morphology, a range of metals have been found to cause the rupturing (tearing) of the outer tissues of the root cylinder in a variety of plant species, including Ag, Al, Cu, La, Ga, Gd, Hg, In, Ru, and Sc (Blamey et al. 2010; Kopittke et al. 2009; Matsumoto and Motoda 2012; Osawa et al. 2011). In addition, Al toxicity has been reported cause swelling of epidermal cells in wheat (Triticum aestivum) (Kinraide 1988). Finally, a number of stresses (including Al toxicity, Cd toxicity, and salt stress) have also been reported to result in a radial swelling of the root cylinder (Burssens et al. 2000; Jones et al. 2006; Kopittke et al. 2015; Sasaki et al. 1997; Valentovičová et al. 2012; Zelinova et al. 2011). Such information is important in identifying how metals exert their toxic effects and reduce root elongation.
The aim of the present study was to use a single plant species to examine the kinetics with which various metals reduce growth, the nature of this growth reduction, and the changes in root morphology during short-term exposure. Given that it has been proposed that the toxic effects of metals are related to the strength with which they bind to hard ligands (Kinraide and Yermiyahu 2007; Kopittke et al. 2014), we examined seven different metals that differed widely in their properties. Furthermore, two additional treatments were included that reduced root elongation rate (RER) through osmotic stress, being mannitol and ‘mixed salts’ treatments. Using light microscopy, the symptoms of toxicity were examined and compared between treatments. We also examined the speed with which these treatments reduced RER. Finally, for one metal (Hg), kinematic analyses were used to examine changes in RER within 5 min intervals following exposure. This kinematic analysis also allowed an assessment as to whether the comparatively rapid decrease in RER was due to a decrease in the length of the elongation zone (LEZ) or due to a decrease in the rate at which individual cells were elongating (i.e. the maximum elemental elongation rate, MEER) (Baskin 2013). It is hoped that the information obtained in the present study will assist in understanding how various metals reduce root growth, with this being important for improving growth in degraded soils.
Materials and methods
General experimental procedures
Seeds of soybean (Glycine max (L.) Merr. cv. Bunya) were germinated in rolled paper towel suspended vertically in tap water. After 3 d, seedlings were placed in Perspex strips on glass beakers filled with 650 mL of 1 mM CaCl2 and 5 μM H3BO3 at pH 5.6 (unadjusted) for a further 24 h. For seedlings that were later to be exposed to Ag, this basal solution was prepared using 1 mM Ca(NO3)2 instead of 1 mM CaCl2. Similarly, for seedlings that were later to be exposed to Al, this basal solution was prepared at pH 4.5 using 0.1 M HCl. All solutions were continuously aerated.
Dose-response curves
Experiment 1 aimed to provide dose-response curves for the metals of interest. A total of 52 treatments were investigated, consisting of nine different metals (each with five to seven concentrations) plus three controls (Supplementary Table S1). With two replicates per treatment (each replicate consisting of seven seedlings), this experiment contained a total of 104 experimental units. The nine metals selected were: Ag, Al, Ca, Cu, Hg, Na, Sr, ‘mixed salts’, and mannitol. These metals were selected because they vary widely in the strengths with which they bind to hard and soft ligands (Fig. 1) (Kinraide 2009). The mixed salts treatment consisted of a mixture of Ca, Mg, K, and Na added at concentrations which contributed equally to osmolarity (Kopittke et al. 2011). The ‘mixed salts’ and mannitol treatments were included to compare to the Na and Ca treatments in which RER would likely be reduced due to osmotic effects rather than due to a direct ionic toxicity (Kopittke et al. 2011; Munns and Termaat 1986). Although mannitol is not a metal, it is listed as such for brevity.
Strength with which metals bind to hard and soft ligands, with data taken from Kinraide (2009). The presence or absence of ruptures were assessed using light microscopy in Experiment 1
Following growth in the basal solution for 24 h, the Perspex strips were transferred to the appropriate treatment (also containing at least 1 mM Ca and 5 μM B) in separate beakers. Light microscopy (Olympus SZX16, Tokyo) was used to examine the nature and timing of any symptoms of rhizotoxicity. To calculate root length using ImageJ (http://imagej.nih.gov/ij/), seedlings were photographed at the time of transfer and again after 48 h exposure (Kopittke et al. 2008).
Investigating the timing of growth reductions
Experiment 2 investigated the speed with which these nine metals reduced RER. Following growth in basal solution for 24 h, seedlings were transferred to treatment-containing solutions in separate beakers. Images were captured using the digital camera at the time of transfer and again after exposure for 4, 8, 12, 24, 36, and 48 h exposure. A total of 21 treatments were investigated, each with two replicates (with seven seedlings per replicate). For each metal, two concentrations were selected as corresponding to a 50 or 90% reduction in RER over 48 h. These concentrations were: 0.28 and 0.75 μM Ag, 14 and 75 μM Al, 69 and 120 mM Ca, 0.83 and 2 μM Cu, 1 and 4 μM Hg, 75 and 140 mM Na, 6.5 and 13 mM Sr, 310 and 380 mOsmol ‘mixed salts’, and 320 and 440 mOsmol for mannitol. Nutrient solutions were sampled at the beginning (0 h) and completion (48 h), filtered to 0.22 μm, acidified using HCl, and analyzed using inductively coupled plasma optical emission spectroscopy or flow injection mercury atomic absorption spectroscopy. Solution analyses were not conducted for the mannitol or ‘mixed salts’ treatments.
Kinematic analyses
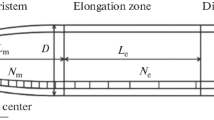
Experiment 3 aimed to (i) refine the assessment of the effects of metals on RER through measurement at 5 to 10 min intervals, and (ii) determine the extent to which changes in RER result from changes in either the MEER or the LEZ. For this experiment, only 1 μM Hg was chosen for investigation, which was chosen because it binds strongly to both hard and soft ligands (Fig. 1). The method used for the kinematic analyses is similar to that described previously by Kopittke et al. (2015). Briefly, a stereo microscope (Olympus SZX16) with a 10 megapixel camera (Olympus SC100) was placed horizontally in order to allow images to be obtained of roots growing vertically. Seedlings were grown in a basal solution for 24 h before being dipped in a 1 mM CaCl2 solution with suspended activated carbon particles (242,276, Sigma Aldrich). Some of the carbon particles adhered to the root surface, thereby later allowing for changes in root length to be assessed. The strip was then returned to the basal solution, with 60 images captured at 0.5 min intervals in order to allow assessment of RER, MEER, and LEZ under non-limiting conditions. Thereafter, the beaker was replaced with one containing 1 mM CaCl2 and 5 μM H3BO3 plus 1 μM Hg. Following placement of the strip in this treatment-containing beaker, images were captured for the next 12 h at 0.5 min intervals. Four replicate roots were examined, corresponding to a total of ca. 6000 images. These images were later analyzed using KineRoot (Basu et al. 2007) with data then averaged within 5 min intervals. Growth velocity profiles, MEER, and LEZ were calculated as outlined by Kopittke et al. (2015). Briefly, the LEZ was determined using the relative velocity profile defined as the region in which 80% of root elongation occurs (i.e. between 10% and 90% of the relative velocity profile) and the MEER was calculated as the derivative of the growth velocity profile.
Results
Concentrations decreasing root elongation and changes in root morphology
For all metals examined, the highest concentrations added were sufficient to almost completely inhibit RER (Fig. 2). The concentrations required to reduce RER by ca. 50% over 48 h were (μM): 0.28 Ag < 0.83 Cu < 1 Hg < 14 Al < 6500 Sr < 69,000 Ca < and 75,000 Na, with corresponding values being 290 mOsmol for ‘mixed salts’ and 300 mOsmol for mannitol (Fig. 2). Similarly, concentrations reducing RER by ca. 90% over 48 h were (μM) 0.75 Ag < 2 Cu < 4 Hg < 75 Al < 13,000 Sr < 120,000 Ca < and 140,000 Na, with corresponding values being 380 mOsmol for ‘mixed salts’ and 417 mOsmol for mannitol.
Using light microscopy, the symptoms associated with these decreases in RER were examined. Broadly, two types of symptoms were observed. Firstly, for all nine metals, roots were observed to swell radially (Fig. 3). After exposure for 24 h, the swelling was generally observed in the region from ca. 1 mm behind the apex to 4–8 mm behind the apex (Fig. 3 and Supplementary Video S1), although this varied depending upon the treatment. In addition, it was noted that the root apical tissues (i.e. < 1 mm from the apex) did not swell and were of similar diameter to the control. The extent of swelling varied, with root diameter increasing from ca. 0.7 mm in the control to 1.0–1.2 mm in the metal-treated roots (Fig. 3).
The second symptom observed was a rupturing and tearing of the roots, with this being observed in roots exposed to Ag, Al, Cu, and Hg but not Ca, Na, Sr, mixed salts, or mannitol (Fig. 3). This rupturing was caused by the tearing and separation of the outer tissues of the root cylinder coupled with the continued elongation of the inner tissues (Fig. 4). Often, but not always, at the higher concentrations Ag, Al, Cu, and Hg, roots were observed to both be ruptured and swollen.
Timing of the decrease in root elongation
Experiment 2 extended the first experiment by examining changes in RER over time using concentrations sufficient to reduce RER by 50 or 90% over 48 h. In all instances, the decrease in RER was comparatively rapid. For the treatments sufficient to reduce RER by 50% over 48 h, RER decreased by an average of 37 to 100% within the first measurement period (0–4 h after exposure) (Fig. 5). Similarly, for the treatments sufficient to reduce RER by 90% over 48 h, RER decreased by an average of 57 to 142% within the first measurement period. These initial decreases in RER were greatest for treatments where osmotic stress would have been the most pronounced, including for Ca, Na, mixed salts, and mannitol (Fig. 5). Following this initial rapid decrease, RER generally increased again somewhat, with RER in the initial measurement period (0–4 h) often being the lowest of any of the six measurement periods (Fig. 5).
Changes in root elongation rate (RER) of soybean seedlings over time. Two concentrations were chosen for each as corresponding to an average reduction in RER over 48 h of ca. 50 or 90% (Fig. 1). Data are the arithmetic mean of two replicates (each replicate with seven seedlings), ± standard deviation
Measured solution concentrations were generally similar to nominal values (Supplementary Table S2). However, particularly for Ag and Hg, concentrations decreased during the 48 h experimental period. For example, for the 1 μM Hg treatment, average concentrations measured in the nutrient solution decreased from 0.82 μM after 0 h to 0.64 μM after 48 h (Supplementary Table S2).
Kinematic analyses
Experiment 3 used kinematic analyses to examine changes in RER in 5 min intervals. In addition, kinematic analyses can show whether the decrease in RER is due to a decrease in either MEER or LEZ. It was found that 1 μM Hg reduced RER rapidly, decreasing by 50% (from ca. 2 to 1 mm/h) within 40 min of exposure (Fig. 7). Following this initial rapid decrease, RER was consistently observed to increase slightly after ca. 1.75 h before decreasing again to a rate of 0.02–0.05 mm/h after 2.5 h. Thereafter, RER gradually increased again across the remaining experimental period, reaching a maximum of ca. 0.7 mm/h after 12 h (Fig. 7).
This initial rapid decrease in RER tended to be associated primarily with a decrease in MEER, with LEZ also decreasing somewhat, although the difference between MEER and LEZ was not significant (Figs. 6 and 7). For example, after 1 h exposure, relative MEER had decreased to 51% of the original value in non-limiting conditions, while LEZ decreased to 74% of the original value (Figs. 6 and 7). Thereafter, the LEZ continued to decrease steadily before reaching ca. 30–40% of the non-limited value. Rather, changes in RER were generally more closely associated with changes in MEER. For example, the increase in RER after 1.75 h was associated with an increase in MEER, as was the gradual increase in RER from 3 to 12 h (Figs. 6 and 7). Indeed, after 12 h exposure, MEER had increased to values corresponding to those achieved in non-limiting conditions (Figs. 6 and 7).
Changes in velocity, relative velocity, and strain rate of soybean seedlings exposed to 1 μM Hg for up to 12 h. These values were used for the calculation of MEER and LEZ as shown in Fig. 7
Changes in root elongation rate (RER) in soybean seedlings exposed to 1 μM Hg. For clarity, the standard deviations (four replicates) are not shown for RER. The dotted vertical line (0 h) corresponds to when the seedlings were exposed to Hg. The relative maximum elemental elongation rate (MEER) and the relative length of the elongation zone (LEZ) explain the overall changes in RER. The figure on the right is the same as the one on the left (other than the scaling of the X axis) but better shows changes during the initial stages of the experiment
Discussion
Changes in root morphology
The present study investigated a wide range of metals, from Hg which is toxic in contaminated soils, to Al which is elevated in acid soils, and Na which reduces growth in saline soils. However, despite this wide range in metals, it was observed that all caused the radial swelling of the root cylinder, often increasing root diameter from ca. 0.7 mm in the control to 1.0–1.2 mm (Fig. 3). This observation is interesting given that these metals would differ markedly in the mechanisms by which they would reduce RER. For example, in the Na, Ca, mixed salts, and mannitol treatments, RER would be decreased by osmotic effects (Munns and Termaat 1986), whilst the other metals would reduce RER by binding strongly to the cell wall (Jones et al. 2006; Kopittke et al. 2015) or through other mechanisms. These swellings generally formed ca. 2–8 mm behind the apex, corresponding with the zone of elongation (Fig. 6). Indeed, using images captured from the kinematic analyses for Hg, it was clear that the swelling forms in the proximal elongation zone (Supplementary Video S1). Radial swellings have been reported in roots in response to a number of stresses, including Al toxicity, Cd toxicity, and salt stress (Burssens et al. 2000; Jones et al. 2006; Kopittke et al. 2015; Sasaki et al. 1997; Valentovičová et al. 2012; Zelinova et al. 2011). We hypothesize that this non-specific radial swelling of the root cylinder forms in the elongation zone due to the effects of ethylene or auxin, with the symptoms similar in appearance to “thick root syndrome” which can be observed in greenhouses and is due to ethylene (Alarcon et al. 2013; Pierik et al. 1999). Indeed, it has been noted that the radial swellings are similar to the symptoms caused by the addition of ACC (1-aminocyclopropane-1-carboxylic acid, an ethylene precursor), IAA (indole-3-acetic acid), and NPA (1-N-naphthylphthalamic acid, an auxin transport inhibitor), with these swellings also forming in the proximal elongation zone (Supplementary Video S2). Furthermore, it has also been reported that both Al- and Cd-induced radial swellings can be reduced markedly by the addition of ethylene synthesis inhibitors (Kopittke et al. 2015; Valentovičová et al. 2012). Regardless, it is not clear if the radial swelling in Al- and Cd-toxic roots (Kopittke et al. 2015; Sasaki et al. 1997; Valentovičová et al. 2012; Zelinova et al. 2011) is part of a specific response to that toxic metal (such as Al and Cd) as hypothesised previously (Kopittke et al. 2015). Rather, that all nine treatments investigated in the present study caused this same symptom is intriguing, suggesting that these swellings are likely a secondary effect associated with an inhibition of root elongation. Further work is required in order to understand the cause of this radial swelling, as well as its implications.
A second symptom was observed to form in the current short-term study. Specifically, for roots exposed to Ag, Al, Cu, and Hg (but not Ca, Na, Sr, mixed salts, or mannitol), the outer tissues of the root cylinder were observed to rupture (Figs. 3 and 4). These ruptures have been reported previously for a range of plant species, including for toxicities of Ag, Al, Cu, La, Ga, Gd, Hg, In, Ru, and Sc (Blamey et al. 2010; Kopittke et al. 2009; Matsumoto and Motoda 2012; Osawa et al. 2011; Sheldon and Menzies 2005). For Al, this rupturing is associated with an inhibition of wall loosening for the outer cells of the root cylinder in which the Al binds strongly to the cell wall (Kopittke et al. 2015). Indeed, it is known that the rupturing of roots is related to the strength with which the metal binds to hard ligands (such as the carboxylate ligands of the cell wall pectic matrix) (Kopittke et al. 2014). Other physiological damage caused by increased lipid peroxidation and lignin deposition is also potentially involved in the rupturing of roots (Sasaki et al. 1996; Yamamoto et al. 2001).
Speed and nature of the decrease in root elongation
The decrease in RER upon exposure to the various metals was rapid. For example, for the treatments sufficient to reduce RER by 50% over 48 h, RER decreased by an average of 37 to 100% within the first measurement period alone (i.e. 0–4 h after exposure) (Fig. 5). That RER decreases so rapidly across all treatments was perhaps surprising, especially given that following this initial rapid decrease, RER generally increased again somewhat, with RER in the initial measurement period (0–4 h) often the lowest of any of the six measurement periods (Fig. 5). The reason for this subsequent increase in RER is unknown, but has been observed previously for Al (Barceló and Poschenrieder 2002; Kopittke et al. 2015; Parker 1995). It was suggested by Kopittke et al. (2015) that this observation is related to changes in the biosynthesis and distribution of ethylene and auxin in the root apical tissues (see Fig. 3 of Kopittke et al. 2015). Regardless, given the comparatively rapid decrease in RER observed for all treatments demonstrates the need for future studies to focus on changes that occur after these short periods of exposure – by focusing on longer time periods (for example, 48 h) some important responses may be missed. Longer-term experiments would need to investigate changes after the 48 h experimental period of the present study. Nevertheless, for the future, where studies aim to identify the underlying mechanisms by which these metals are toxic to plant roots, it is necessary to design experiments to investigate the changes that are occurring rapidly upon exposure.
Certainly, the speed (and magnitude) with which RER is reduced suggest that the initial reduction in RER is associated with a reduction in cell elongation rate rather than cell division. Indeed, cell division itself does not result in elongation of the root, but rather it is its ongoing gradual supply of new cells into the zone of elongation that results in overall root elongation. Thus, even if the presence of toxic concentrations of metals immediately reduced cell division, RER would not decrease markedly until these reduced numbers of cells had ‘transited’ from the meristem to the zone of elongation. Clearly, further work is required to test this hypothesis (also see Matsumoto 2000). Furthermore, it should be noted that a reduction in elongation rate could occur either due to an inhibition of wall loosening or due to an inability to maintain turgor (Winship et al. 2010). Certainly, that RER decreases rapidly upon exposure to metals is consistent with observations in a range of plant species for Al which often decreases RER markedly within 5–120 min (Blamey et al. 2004; Blancaflor et al. 1998; Jones and Kochian 1995; Kopittke et al. 2015; Liu et al. 2008; Llugany et al. 1995; Massot et al. 2002). Similarly, elevated levels of Na, such as in saline soils, are known the decrease RER rapidly (for example, see Cramer et al. 1988).
Given the speed with which the various metals decreased RER, kinematic analyses were used to examine Hg further – this being a metal that binds strongly to both hard and soft ligands (Fig. 1). It was found that RER decreased by 50% within ca. 40 min. Importantly, for this initial rapid decrease in RER, the decrease in MEER tended to be greater than the decrease in the LEZ (Figs. 6 and 7). Interestingly, LEZ tended to decrease steadily to a value of ca. 30–40% of the original value (i.e. LEZ decreased from 6.9 mm to ca. 2.0–2.5 mm, Fig. 6), but in contrast, MEER tended to vary somewhat. For example, although MEER decreased rapidly upon initial exposure, the consistent (slight) increase in RER after 1.75 h was due to an increase in MEER, and the gradual increase in RER from 3 to 12 h was also due to an increase in MEER (Fig. 6 and 7). Changes in the LEZ involve the addition or subtraction of cells undergoing elongation (Baskin 2013) and is thus related to cell division. In contrast, responses in which MEER changes while the LEZ stays constant are known to reflect a direct influence at the level of the expansion mechanism (Baskin 2013). Thus, it is clear that the rate at which cells are elongating plays a crucial role in the expression of the toxic effects of at least some metals, as shown here for Hg.
Finally, although not considered in detail here, careful consideration needs to be given to the impact of solution composition on RER. For example, the gradual increase in RER for roots exposed to Ag and Hg (Figs. 5 and 7) can presumably be attributed, at least in part, to decreases in their solution concentrations over the 48 h experimental period (Supplementary Table S2). Changes in solution composition will impact upon root elongation. It is also possible, however, that the gradual increase in RER for roots exposed to Ag and Hg (Figs. 5 and 7) can be attributed to physiological resistance mechanisms. Further study is required in this regard.
Conclusions
Despite their wide range of properties, all nine treatments examined caused similar changes in root morphology, with the root cylinder swelling in the zone of elongation. This non-specific symptom that forms upon a decrease in RER is possibly associated with the effects of ethylene or auxin, although more studies are required in this regard. A second symptom was observed in roots exposed to Ag, Al, Cu, and Hg, being a tearing and rupturing of the outer tissues of the root cylinder associated with an inhibition of wall loosening in these outer cells. The decrease in RER upon exposure to the various metals was rapid – for the treatments sufficient to reduce RER by 50% over 48 h, RER decreased by an average of 37 to 100% within only 0–4 h after exposure. The speed of the decrease in RER suggests that this initial reduction in RER is associated with a reduction in cell elongation rate rather than cell division. Finally, kinematic analyses were used to more closely examine the effects of Hg toxicity in 5 min intervals. It was found that RER decreased by 50% after only 40 min, with this decrease in RER initially due primarily to a decrease in the rate at which cells were elongating in the elongation zone (i.e. MEER) rather than a decrease in the length of the elongation zone. The information provided here will assist in understanding the mechanisms by which toxic levels of metals reduce root elongation.
Abbreviations
- LEZ:
-
Length of the elongation zone
- MEER:
-
Maximum elemental elongation rate
- RER:
-
Root elongation rate
References
Alarcon MV, Lloret PG, Salguero J (2013) Auxin-ethylene interaction in transversal and longitudinal growth in maize primary root. Botany 91:680–685
Barceló J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48:75–92
Baskin TI (2013) Patterns of root growth acclimation: constant processes, changing boundaries. Wiley Interdiscip Rev Dev Biol 2:65–73
Basu P, Pal A, Lynch JP, Brown KM (2007) A novel image-analysis technique for kinematic study of growth and curvature. Plant Physiol 145:305–316
Blamey FPC, Nishizawa NK, Yoshimura E (2004) Timing, magnitude, and location of initial soluble aluminium injuries to mungbean roots. Soil Sci Plant Nutr 50:67–76
Blamey FPC, Kopittke PM, Wehr JB, Kinraide TB, Menzies NW (2010) Rhizotoxic effects of silver in cowpea seedlings. Environ Toxicol Chem 29:2072–2078
Blancaflor EB, Jones DL, Gilroy S (1998) Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiol 118:159–172
Burssens S, Himanen K, van de Cotte B, Beeckman T, Van Montagu M, Inzé D, Verbruggen N (2000) Expression of cell cycle regulatory genes and morphological alterations in response to salt stress in Arabidopsis thaliana. Planta 211:632–640
Cramer GR, Epstein E, Läuchli A (1988) Kinetics of root elongation of maize in response to short-term exposure to NaCl and elevated calcium concentration. J Exp Bot 39:1513–1522
Eswaran H, Reich P, Beinroth F (1997) Global distribution of soils with acidity. In: Plant-soil interactions at low pH. Ed. A C Moniz. Brazilian Soil Science Society, Sao Paulo, pp 159–164
Ghassemi F, Jakeman A J, Nix H A (1995) Salinisation of land and water resources: human causes, extent, management and case studies. Canberra, Australia: the Australian National University, Wallingford, Oxon, UK: CAB international. CAB International, Wallingford pp. 544
Jones DL, Kochian LV (1995) Aluminum inhibition of the inositol 1,4,5-trisphosphate signal transduction pathway in wheat roots: a role in aluminum toxicity? Plant Cell 7:1913–1922
Jones DL, Blancaflor EB, Kochian LV, Gilroy S (2006) Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ 29:1309–1318
Kinraide TB (1988) Proton extrusion by wheat roots exhibiting severe aluminum toxicity symptoms. Plant Physiol 88:418–423
Kinraide TB (2009) Improved scales for metal ion softness and toxicity. Environ Toxicol Chem 28:525–533
Kinraide TB, Yermiyahu U (2007) A scale of metal ion binding strengths correlating with ionic charge, Pauling electronegativity, toxicity, and other physiological effects. J Inorg Biochem 101:1201–1213
Kopittke PM, Blamey FPC, Menzies NW (2008) Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant Soil 303:217–227
Kopittke PM, McKenna BA, Blamey FPC, Wehr JB, Menzies NW (2009) Metal-induced cell rupture in elongating roots is associated with metal ion binding strengths. Plant Soil 322:303–315
Kopittke PM, Blamey FPC, Kinraide TB, Wang P, Reichman SM, Menzies NW (2011) Separating multiple, short-term deleterious effects of saline solutions to the growth of cowpea seedlings. New Phytol 189:1110–1121
Kopittke PM, Menzies NW, Wang P, McKenna BA, Wehr JB, Lombi E, Kinraide TB, Blamey FPC (2014) The rhizotoxicity of metal cations is related to their strength of binding to hard ligands. Environ Toxicol Chem 33:268–277
Kopittke PM, Moore KL, Lombi E, Gianoncelli A, Ferguson BJ, Blamey FPC, Menzies NW, Nicholson TM, McKenna BA, Wang P, Gresshoff PM, Kourousias G, Webb RI, Green K, Tollenaere A (2015) Identification of the primary lesion of toxic aluminum (Al) in plant roots. Plant Physiol 167:1402–1411
Liu Q, Yang JL, He LS, Li YY, Zheng SJ (2008) Effect of aluminum on cell wall, plasma membrane, antioxidants and root elongation in triticale. Biol Plantarum 52:87–92
Llugany M, Poschenrieder C, Barceló J (1995) Monitoring of aluminium-induced inhibition of root elongation in four maize cultivars differing in tolerance to aluminium and proton toxicity. Physiol Plant 93:265–271
Massot N, Nicander B, Barcelo J, Poschenrieder C, Tillberg E (2002) A rapid increase in cytokinin levels and enhanced ethylene evolution precede Al3+-induced inhibition of root growth in bean seedlings (Phaseolus vulgaris L.) Plant Growth Regul 37:105–112
Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200:1–46
Matsumoto H, Motoda H (2012) Aluminum toxicity recovery processes in root apices. Possible association with oxidative stress. Plant Sci 185–186:1–8
Munns R, Termaat A (1986) Whole-plant responses to salinity. Aust J Plant Physiol 13:143–160
NLWRA (2002) Australians and natural resource management, http://www.nlwra.gov.au/. National Land and Water Resources Audit, Canberra
Osawa H, Endo I, Hara Y, Matsushima Y, Tange T (2011) Transient proliferation of proanthocyanidin-accumulating cells on the epidermal apex contributes to highly aluminum-resistant root elongation in camphor tree. Plant Physiol 155:433–446
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Parker DR (1995) Root growth analysis: an underutilised approach to understanding aluminium rhizotoxicity. Plant Soil 171:151–157
Pierik R, Verkerke W, Voesenek RACJ, Blom KWPM, Visser EJW (1999) Thick root syndrome in cucumber (Cucumis sativus L.): a description of the phenomenon and an investigation of the role of ethylene. Ann Bot 84:755–762
Sasaki M, Yamamoto Y, Matsumoto H (1996) Lignin deposition induced by aluminum in wheat (Triticum aestivum) roots. Physiolgia Plantar 96:193–198
Sasaki M, Yamamoto Y, Ma JF, Matsumoto H (1997) Early events induced by aluminum stress in elongating cells of wheat root. Soil Sci Plant Nutr 43:1009–1014
Sheldon AR, Menzies NW (2005) The effect of copper toxicity on the growth and root morphology of Rhodes grass (Chloris gayana Knuth.) in resin buffered solution culture. Plant Soil 278:341–349
Valentovičová K, Mistrík I, Zelinová V, Tamás L (2012) How cobalt facilitates cadmium- and ethylene precursor-induced growth inhibition and radial cell expansion in barley root tips. Cent Eur J Biol 7:551–558
Veitch FP (1904) Comparison of methods for the estimation of soil acidity. J Am Chem Soc 26:637–662
Winship LJ, Obermeyer G, Geitmann A, Hepler PK (2010) Under pressure, cell walls set the pace. Trends Plant Sci 15:363–369
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Zelinova V, Haluskova L, Huttova J, Illes P, Mistrik I, Valentovicova K, Tamas L (2011) Short-term aluminium-induced changes in barley root tips. Protoplasma 248:523–530
Acknowledgements
Dr. Kopittke is the recipient of an Australian Research Council (ARC) Future Fellowship (FT120100277).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Electronic supplementary material
ESM 1
(PDF 179 kb)
Supplementary Video S1
A time-lapse video compiled from the kinematic analysis (Experiment 3) showing the growth of soybean roots in a solution containing 1 μM Hg, with a radial swelling forming. The video represents the period from 447 to 745 min after initially exposing the root to Hg. (AVI 10987 kb)
Supplementary Video S2
A time-lapse video compiled showing the growth of soybean roots in a solution containing 1 μM NPA, with a radial swelling forming. The video represents the period from 564 to 764 min after initially exposing the root to NPA. (AVI 8393 kb)
Rights and permissions
About this article
Cite this article
Kopittke, P.M., Wang, P. Kinetics of metal toxicity in plant roots and its effects on root morphology. Plant Soil 419, 269–279 (2017). https://doi.org/10.1007/s11104-017-3342-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3342-6