Abstract
Aims
Belowground legume nitrogen (N) composed of roots and rhizodeposition is an important N input to soils, but published data of belowground N vary broadly, probably due to extrapolation from short-term experiments and dissimilar growing conditions. We quantified belowground N inputs of red clover (Trifolium pratense L.) during two consecutive years in a clover-grass sward along a soil nutrient availability gradient.
Methods
We established a red clover-perennial ryegrass (Lolium perenne L.) model sward in microplots located in field plots of the DOK experiment, which has a 33-year history of organic and conventional cropping, resulting in a soil nutrient availability gradient. Four treatments were examined: the zero fertilisation control, bio-organic with half and full dose manure application, and the conventional system with mineral fertilisation at full dose. We studied the development of clover aboveground and belowground N using multiple pulse 15N urea leaf labelling.
Results
Belowground clover N increased over time and with rising nutrient availability and was proportional to aboveground clover N at all times. Belowground clover N amounted to 40% of aboveground clover N during two consecutive years, irrespective of the nutrient availability status. Belowground clover N development was initially dominated by fast root growth, followed by enhanced root turnover during the second year. Potassium availability limited clover growth and total N accumulation in treatments with low nutrient availability.
Conclusions
Belowground red clover N inputs could be estimated from aboveground N by a constant factor of 0.4, regardless of the nutrient availability and cultivation time. Root turnover led to a distinct absolute increase of N rhizodeposition over time. Hence, N rhizodeposition, with an 80% share of belowground N, was the predominant N pool at the end of the second year.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Legume-rhizobia symbioses provide annually around 40 Tg reactive nitrogen (N) to agro ecosystems worldwide (Herridge et al. 2008), which compares to one third of the amount of technically bound N from the Haber-Bosch process (121 Tg, Galloway et al. 2008). About 40% of legume-rhizobia fixed N is contributed by fodder legumes, which acquire on average 70% of their N by symbiotic N2 fixation (Herridge et al. 2008). The proportion of N derived from symbiotic N2 fixation of red clover (Trifolium pratense L.) generally exceeds 80% if grown in mixture with grass and fertilised moderately with mineral N (Boller and Nösberger 1987; Nesheim and Øyen 1994; Huss-Danell et al. 2007; Oberson et al. 2013).
Perennial ryegrass (Lolium perenne L.), a common mixture partner in clover-grass swards, is highly competitive for soil mineral N (Nyfeler et al. 2011), due to the greater root length and root surface area compared with red clover (Mengel and Steffens 1985). Several studies have shown that grasses cultivated in clover-grass swards can obtain 30% to 60% of their N from clover (Boller and Nösberger 1987; Dahlin and Stenberg 2010a; Oberson et al. 2013; Schipanski and Drinkwater 2012). This significant N transfer is probably due to the regular cutting of the sward, which might induce root turnover of forage plants (Hamilton et al. 2008), followed by microbial incorporation and mineralisation (Haystead and Marriott 1979). Indeed, Sierra et al. (2007) and Trannin et al. (2000) observed cutting-induced N transfer rates from legume trees to grasses, which strongly increased after pruning. The cutting-induced N transfer found by Sierra et al. (2007) was 12 times higher than the N transfer via exudation. This observation suggests that cutting triggers root decay followed by a fast turnover of the root debris, as assumed by several authors (Fustec et al. 2010; Hamilton et al. 2008; Haystead and Marriott 1979; Ta and Faris 1987; Thilakarathna et al. 2016).
Root debris N is part of N derived from rhizodeposition (NdfR), comprising all kinds of compounds lost from living plant roots, including exuded organic compounds, ions, and volatile compounds (Uren 2007). For operational reasons, Mayer et al. (2003) defined NdfR as root derived N remaining in the soil after sorting out visible roots. Hence, belowground N (BGN) comprises root N and NdfR. As much as 70% of total N in fodder legumes was reported to be BGN (Herridge et al. 2008), which thus plays an important role in the N cycle of clover-grass mixtures. However, the published data basis is small and varies strongly, from 20% to 30% (Dahlin and Mårtensson 2008; Gylfadóttir et al. 2007), to 50% (Dahlin and Stenberg 2010b), and up to 70% (McNeill et al. 1997). While Herridge et al. (2008) attributed the variation to effects of species, soil, and climate on the partitioning of N within the plant, management (cutting vs. grazing, intensity and frequency; Dahlin and Mårtensson 2008; Dahlin and Stenberg 2010a, b) and biotic as well as abiotic stress (e.g. pests, diseases, temperature, water; Haase et al. 2007) also might affect the partitioning. Beside these effects, a strong variation might come from extrapolation to full years of short-lived examination periods and dissimilar growing conditions.
Nitrogen derived from rhizodeposition is usually determined using 15N stable isotope labelling of the root N, with the isotope being applied via stems or leaves (Fustec et al. 2010; Wichern et al. 2008). The percentage of NdfR can then be calculated from the 15N enrichment of the root-free soil assuming that NdfR and root N have the same 15N isotopic composition (Janzen and Bruinsma 1989).
In the DOK long-term experiment, Oberson et al. (2013) observed that low nutrient-supplied clover-grass swards were limited in potassium (K) and possibly co-limited in phosphorus (P). Nutrient limitations generally reduce aboveground growth, but may affect the shoot to root ratio in different manners. Potassium limitation was found to extend the shoot to root ratio of grain legumes, due to an inhibition of photosynthate-translocation to the root (Cakmak et al. 1994). Low N and P supplies, however, generally result in lower shoot to root ratios in grain legumes (Cakmak et al. 1994), leguminous (Almeida et al. 1999; Hill et al. 2006), and gramineous pasture plants (Hill et al. 2006) by increasing the absorptive root surface at the expense of aboveground biomass (Hill et al. 2006). The active release of exudates to mobilise nutrients from the soil represents a further plant strategy. Especially carboxylates and phosphatases are exuded to mobilise P (Neumann and Römheld 2012).
While red clover aboveground N (AGN) in response to nutrient availability gradients has been studied extensively (e.g. Boller and Nösberger 1987; Davis 1991; Nyfeler et al. 2011; Oberson et al. 2013; Tucker and Smith 1952), little is known about the effect of the nutrient availability status on the relative size of red clover AGN, BGN, NdfR, and root N. To our knowledge, the effect of N, P, and K availability on the red clover BGN to AGN ratio has not yet been examined under a well-established nutrient availability gradient with identical climatic and pedologic conditions. Furthermore, red clover BGN was usually determined at one point of time (Dahlin and Stenberg 2010b; Høgh-Jensen and Schjoerring 2001; Huss-Danell et al. 2007) and was only once determined throughout two consecutive years (Høgh-Jensen and Schjoerring 2001). Therefore, little is known about the development of red clover root N and NdfR during a two-year cultivation period.
The objectives of our study were i) to investigate the development of red clover BGN and its components root N and NdfR in relation to red clover AGN in a red clover-perennial ryegrass model sward during two consecutive years and ii) to study the effects of different nutrient availabilities in organic and conventional cropping systems on the relative sizes of red clover AGN, BGN, root N, and NdfR. Root N and AGN development of the grass-partner in the sward were also investigated.
We expected a first phase with fast red clover root growth during the first months of establishment and a second phase with a steady root turnover. In this second phase, red clover BGN was expected to further increase through a continued release of rhizodeposits, leading to an increasing proportion of NdfR to BGN over time. With decreasing nutrient availability, we expected enhanced red clover root growth and rhizodeposition relative to red clover aboveground biomass and AGN development.
Material and methods
The DOK long-term field experiment (Maeder et al. 2002), which compares bio-Dynamic, bio-Organic, and conventional (Konventionell) cropping systems (DOK), was used as an experimental platform. A microplot study with a model red clover-perennial ryegrass sward was carried out in the regular clover-grass sward of the DOK crop rotation in 2011 and 2012.
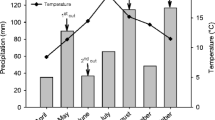
The DOK experiment was established in 1978 in Therwil (near Basle, Switzerland; 307 m above sea level; 7°33′ E, 47°30´ N) at a site with a haplic Luvisol developed on loess (Maeder et al. 2002). During the two consecutive years of study, the mean annual temperature was 11.6 °C in 2011 and 10.9 °C in 2012 and the mean annual precipitation was 688 mm in 2011 and 1048 mm in 2012 (Fig. S1 and Fig. S2). The management and the experimental design were described thoroughly by Mayer et al. (2015). From 2006 until 2012, the seven year crop rotation comprised silage maize (Zea mays L.), winter wheat I (Triticum aestivum L.), soybean (Glycine max (L.) Merr.), potato (Solanum tuberosum L.), winter wheat II, and two full years of clover-grass sward (2011 and 2012). Treatments differ in amount and form of applied fertilisers and in crop protection. The following four DOK treatments, characterised by increasing nutrient availability (Table 1), were examined in the present study: the unfertilised control (NOFERT), two bio-organic treatments receiving either manure at full dose at fertilisation level 2 (BIOORG2) or at half dose at fertilisation level 1 (BIOORG1), and a treatment representing a stockless conventional cropping system receiving only mineral fertiliser at full dose (CONMIN2) according to Swiss fertilisation guidelines (Flisch et al. 2009).
Manure application in the two organic treatments corresponds to a phosphorous (P) amount of 1.4 livestock units or 22 kg P ha−1 year−1 at level 2 and 50% of that at level 1. Soil properties, available soil nutrient contents (N, P, K), and nutrient inputs to the investigated swards are given in Table 1. The clover-grass swards received no pest or disease control agents. Details of the sward management are presented in Oberson et al. (2013). Mean fertiliser nutrient applications in 2011 and 2012 differed from the long-term applications (Table 1), due to a variation in nutrient contents of manure and an adaptation of the fertilisation to recent fertilisation guidelines. Overall, short- and long-term management with different amounts of fertiliser application led to a differentiation in nutrient availability of DOK treatments in soils of the swards in the order NOFERT < BIOORG1 < BIOORG2 < CONMIN2 (Table 1).
Microplot study
Per field plot of the examined treatments (4 treatments × 4 replications = 16 field plots), five microplots were installed by inserting a PVC tube (0.375 m inner-diameter corresponding to an area of 0.11 m2; 0.3 m height) to a depth of 0.25 m into the undisturbed soil before sowing the clover-grass sward (Swiss standard mixture SM 330, Suter et al. 2012) in August 2010. Microplots were arranged in a line between the border and central area of the field plot. In contrast to the field plots, microplots remained uncultivated until spring 2011. On February 8, 2011, red clover (Trifolium pratense L., cv. Dafila) and perennial ryegrass (Lolium perenne L., cv. Lacerta) were seeded into pots containing soil of the future field plots, were pre-cropped in the greenhouse, and afterwards cold hardened in a cold frame outside the greenhouse. On March 29, 2011, microplots were each planted with 11 clover and 20 grass seedlings corresponding to an overall density of 300 plants m−2 (Fig. 1).
Setup of the microplot-study with timeline of red clover plant labelling in labelled microplots (LMP), harvesting and regrowth (green / grey line) in LMP and in non 15N labelled reference microplots (RMP), and excavation of LMP and RMP for belowground N determination. LMP4: 1st to 2nd harvest; 1st labelling before 2nd harvest; excavation at 4 months, after the 2nd harvest. LMP8: 1st to 4th harvest; labelling before 2nd, 3rd, and 4th harvest; excavation at 8 months, after the 4th harvest. LMP19: 1st to 9th harvest; labelling before 5th, 6th, 7th, and 8th harvest; excavation at 19 months, after the 9th harvest. RMP8: 1st to 4th harvest; excavation at 8 months. RMP19: 1st to 9th harvest; excavation at 19 months
To determine red clover NdfR, clover plants were 15N multiple-pulse labelled in three out of five microplots (LMP, Fig. 1). The remaining two microplots were kept unlabelled and served as reference microplots (RMP, Fig. 1) to determine the 15N natural abundance background of the plot. One of each LMP was excavated after 4, 8, and 19 months of clover-grass sward cultivation, and one of each RMP was excavated after 8 and 19 months of cultivation. In total, the design comprised 48 LMP (4 treatments × 4 replicated field plots × 3 LMP per field plot) and 32 RMP (4 treatments × 4 replicated field plots × 2 RMP per plot). To avoid contamination of RMP microplots, LMP and RMP microplots were separated in two sub-units per field plot and were randomised within the respective sub-block.
Multiple 15N pulse labelling
While all eleven clover plants per LMP were labelled by 15N enriched urea solution, with the urea having an atom fraction 15N of 990,000 ppm (ReseaChem, Switzerland), perennial ryegrass was not fed with 15N (Fig. 1). To achieve a uniform 15N root enrichment targeted at 5000 ppm excess atom fraction (EAF) 15N, volume and urea concentration of the solution applied per plant ranged from 0.5 to 1.5 ml and from 0.3% to 0.5% (w/v), respectively, depending on the predicted N uptake as related to the treatment. The prediction was derived from data of Oberson et al. (2013). The label was generally applied about two weeks after the preceding sward harvest at one single leaf per clover plant and labelling event (Fig. 1) using the method according to Ledgard et al. (1985). The method was modified by squashing the trifoliate clover-leaf manually to damage the cuticle, thus facilitating uptake of the labelling solution. The squashed leaf was then inserted into a 2 ml vial. After pipetting the labelling solution, the vial was sealed airtight with Terostat X (Henkel, Germany) to prevent losses of labelling solution. Generally, the labelling solution was completely absorbed by the plant within 24 h. Vials together with the inserted leaves were removed not later than 72 h after starting the labelling. However, if the solution uptake was incomplete, which was the case at less than 10%, the remaining volume was recorded to enable 15N recovery calculations (cf. Eq. 13). Leaf litter was collected from the soil surface once per week to prevent decomposition of labelled aboveground litter and, hence, tracer translocation to the soil.
The first LMP (LMP4) was labelled once in 2011, three weeks before the 2nd harvest, and was excavated after the 2nd harvest in July 2011, 4 months after planting. The second LMP (LMP8) was labelled three times in 2011, before the 2nd, 3rd, and 4th harvest (Fig. 1), and was excavated after the 4th harvest in October 2011, 8 months after planting. The third LMP (LMP19) was kept unlabelled in 2011, was labelled four times in 2012, before the 5th, 6th, 7th, and 8th harvest, and was excavated after the 9th harvest in October 2012, 19 months after planting (Fig. 1). The two RMP were kept unlabelled and were excavated after 8 and 19 months, respectively. The entire amount of red clover NdfR accumulated in the soil at 19 months was determined by summing NdfR determined from LMP8 in 2011 and NdfR determined from LMP19 in 2012 (cf. Fig. 1, Eq. 8).
Sampling and processing of plant and soil samples
We quantified clover and grass total N, AGN, root N, and, additionally for clover, NdfR. To investigate the fate of NdfR in specific soil N pools (Hammelehle et al. unpublished), the soil was split in specific soil N pools using a sequential extraction. Nitrogen derived from rhizodeposition was subsequently determined for each single soil N pool (cf. Eq. 6) and summed up.
The sward’s aboveground biomass growing in the microplots was harvested four times in 2011 (1st to 4th harvest) and five times in 2012 (5th to 9th harvest, cf. Fig. 1). Clover and grass plants were harvested separately by cutting them 0.05 m above the ground using manual garden shears. Before excavating the microplots, clover and grass stubble were separately cut off at the soil surface. Aboveground clover and grass samples were stored in cooling boxes for a maximum of 12 h until drying at 60 °C for 72 h. Dried samples were ground with a centrifuge mill (Retsch GmbH, Germany) to a size < 0.08 mm.
The microplot soil was split into six equal wedges each excavated to a depth of 0.25 m and weighed separately. Three out of six wedges were combined to a composite sample and used for further processing. Additionally, composite subsoil samples consisting of five cores each from 0.25 to 0.6 m taken with a gouge auger (diameter 0.05 m, Eijkelkamp, Netherlands) were excavated at 8 and 19 months from LMP8 and LMP19, respectively (Fig. 1). Soils were stored for between one and four weeks at 4 °C until further processing.
Field moist soil samples were crumbled and separated manually from visible macro roots. The water content was determined from subsamples of crumbled soil dried at 105 °C until constant weight. After macro root separation, the field moist crumbled soil sample was sieved at 3 mm. Roots (> 3 mm) remaining on the sieve were collected. Macro roots and remaining roots from sieving were divided visually into nodulated clover roots, grass roots, and non-classifiable roots (mixture of clover and grass roots). Subsequently, roots were thoroughly cleaned using deionised water.
According to the procedure used by Mayer et al. (2003), non-classifiable rootlets < 3 mm were quantified using three aliquots of 150 g field moist soil (sieved at 3 mm) by shaking each aliquot overhead together with 500 ml 0.05 M K2SO4 at 39 r min−1 for 0.5 h. Subsequently, the soil-extract suspension was poured through a 0.5 mm sieve. Remaining non-classifiable rootlets were thoroughly cleaned with deionised water.
In the pre-extraction, the collected soil-K2SO4 suspension from the rootlet separation was then vacuum filtrated through a membrane filter (porafil® CA, Machery-Nagel, Germany) resulting in the root-free soil residue and the pre-extract. The root-free soil residue was further processed by chloroform fumigation at 20 °C for 24 h (Brookes et al. 1985) using 50 ml ethanol-free chloroform (Lichrosolv®, Merck Nr. 1.02444.1000, Germany). Subsequently, the fumigated soil was extracted with 300 ml 0.05 M K2SO4 (chloroform fumigation extraction, Wichern et al. 2007) and finally filtrated with a paper filter (MN 640d, Marcherey-Nagel, Germany) to separate the root-free soil from the extract containing microbial biomass N (Hammelehle et al. unpublished). Pre-extracts, comprising dissolved mineral N and organic N, and extracts from the chloroform fumigation extraction (CFE), containing microbial N, were processed by oxidation according to Cabrera and Beare (1993) and subsequently by NH3 diffusion to quartz filter disks according to Mayer et al. (2003).
The total N concentration and the isotopic ratio 15N to 14N of plant samples, soil samples, and diffusion quartz-filter discs were determined using a FlashEA 1112 NC analyser coupled with a ConFlo IV universal continuous flow interface to a DELTA V isotope ratio mass spectrometer (Thermo Fisher Scientific Inc., USA). Dissolved N in extracts was analysed with a TOC/TNb analyser (DIMA-TOC 100, Dimatec, Germany). Phosphorus and K concentrations of harvested clover and grass biomass were analysed per single harvest. For this purpose, plant samples were incinerated at 450 °C and ashes were solubilized with 6 M HCl. Afterwards, diluted filtrates were analysed by inductively coupled plasma atomic-emission spectroscopy using a Vista Pro ICP-OES (Varian, Agilent, USA) for P and K (Agroscope 1996).
To elucidate potential nutrient limitations of clover and grass during different development phases (0–4 months, 4–8 months, and 8–19 months) and taking into account the amounts of harvested biomass, weighted means of N, P, and K concentrations were calculated for the 1st to 2nd harvest (0 to 4 months), the 3rd to 4th harvest (5 to 8 months), and the 5th to 9th harvest (9 to 19 months).
Calculations
Cumulative AGN of red clover and perennial ryegrass [g m−2] at the time t in months was calculated according to
where stubble(t) N corresponds to the standing stubble biomass N [g m−2] at the time t when LMPt was excavated, and n corresponds to the number of harvests taken until the time t (cf. Fig. 1). Individual 15N fed leaves together with their associated stalks were removed and not included in the analyses and calculations.
Soil total N [g m−2] at the time t, when LMPt was excavated, was obtained by summing the N amounts of the soil N pools obtained from the pre-extraction and CFE extraction of the respective time:
The 15N isotopic abundance of plant and soil samples is expressed as atom fraction 15N [ppm (or μmol mol−1)] according to Coplen (2011):
where 15N or 14N is the N amount of the respective isotope.
Excess atom fraction 15N [ppm] of soil and plant samples was determined by differences in the atom fraction between LMPt samples and RMPt samples of the respective sample type and time (Jensen 1996):
Excess atom fraction 15N of total soil N [ppm] was calculated as weighted mean of EAF 15N of pre-extracts, CFE extracts, and fumigated soil residues according to
The proportion of red clover NdfR accumulated in the soil of the respective microplot LMPt until the time t of excavation was calculated according to Janzen and Bruinsma (1989):
The proportion of red clover NdfR was determined separately for each soil N pool (cf. Eq. 2) of the topsoil (0–0.25 m) and the subsoil (0.25–0.60 m).
The amount of red clover NdfR [g m−2] accumulated in the respective microplot LMPt and layer was quantified according to
Since LMP19 was only labelled during the second year, the entire amount of red clover NdfR accumulated during the two consecutive years (19 months) was calculated according to
Red clover BGN [g m−2] at the time t [months] of LMPt excavation was calculated according to
where root N (t) represents physically recoverable root N at the time t and NdfR represents the accumulated amount of NdfR until the time t.
The proportion of red clover root N of non-classifiable roots at time t was calculated using the 15N isotopic composition of the roots following the principles underlying the equation of Janzen and Bruinsma (1989):
Red clover total N [g m−2] accumulated until the time t was calculated by summing up AGN(t) and BGN(t):
Total recovered perennial ryegrass N [g m−2] accumulated until the time t comprising AGN and root N at the time t (NdfR was not determined) was calculated according to
The recovery of labelled (excess) 15N of the assimilated 15N urea solution of LMPt at the time t was calculated according to
Experimental design and statistical analyses
The experimental design was a split-split-plot situated in the Latin square of the DOK experiment, with four field replicates per treatment (Fließbach et al. 2007).
The data were fitted to a two factorial mixed effect model (treatment x time + error [paired plots, plot]) with an α level set at 0.05, below which the null hypothesis was rejected. Since treatments NOFERT and CONMIN2 on the one hand and BIOORG1 and BIOORG2 on the other hand were always situated adjacently in the Latin square design of the DOK experiment (Fließbach et al. 2007), paired plots were defined as a random factor. If the data per plot were obtained from more than one point of time or more than one microplot, plot was additionally used as second random intercept.
Prior to the statistical analysis, normal distribution of model residuals was tested using the Shapiro-Wilk test and, if violated, data were transformed, generally by log transformation. Compositional data were always centred log-ratio transformed according to Aitchison (1982) and Van den Boogaart and Tolosana-Delgado (2013). To detect differences between least significant means of the factors, a Student’s t-test was applied as a posthoc test. Since clover had been almost eliminated by deer after the 2nd harvest (4 months), microplots located in the fourth replicate of treatment CONMIN2 had to be excluded.
Back-transformation of the centred log-ratio transformed data was calculated by CoDaPack version 2.01.15 (Thió-Henestrosa et al. 2009). Statistical analyses were carried out using the software package JMP® Pro10 (SAS Institute Inc., USA).
Results
Dry matter yields
At 19 months of cultivation, the dry matter yields of clover cumulative harvests ranged from 1570 g m−2 in NOFERT to 3820 g m−2 in BIOORG2. Clover yield differed significantly in the order NOFERT < BIOORG1 ≤ CONMIN2 ≤ BIOORG2. Cumulative grass yields ranged from 224 g m−2 in NOFERT to 890 g m−2 in CONMIN2. The contribution of stubbles to the overall dry matter production was small and constituted about 50 g m−2 for clover and 80 g m−2 for grass (Table 2). The clover proportion of the sward was high in all treatments, between 78% (CONMIN2) and 87% (NOFERT) of total dry matter yields.
Clover root dry matter increased with nutrient availability, from 186 g m−2 in NOFERT to 493 g m−2 in BIOORG2, similarly to the aboveground biomass whereas grass root dry matter, with an average of 145 g m−2, did not show a clear response to the nutrient availability (Table 2).
Mineral nutrition of red clover and perennial ryegrass
Potassium concentration of clover increased from NOFERT to BIOORG1 to CONMIN2 and BIOORG2 (Table 3). Concentrations were highest in the weighted mean of the 1st to 2nd harvest (0 to 4 months) and decreased afterwards until the end of the experiment (3rd to 4th and 5th to 9th harvest), except for NOFERT. The K concentration of NOFERT remained constantly below the critical K concentration of 10 mg g−1 (Tucker and Smith 1952) throughout the two years, between 9.1 and 9.6 mg g−1.
The N concentration of clover was not affected by treatments (Table 3) and was about 29 mg g−1. Clover P concentrations were higher in CONMIN2 than in the two organic treatments BIOORG1 and BIOORG2 (Table 3). Generally, clover P concentrations were lower in the second year (5th to 9th harvest) compared to the first year (1st to 2nd and 3rd to 4th harvest). The limit of 2.2 mg g−1 (Davis 1991) was generally achieved, except for the cumulative 5th to 9th harvest in BIOORG2 (2.0 mg g−1) and BIOORG1 (2.1 mg g−1) (Table 3).
Nitrogen concentrations of grass were usually higher in CONMIN2 compared with the other treatments throughout the two years (Table 3). The critical N concentration of 25 mg g−1 for perennial ryegrass (Bolton et al. 1976) was only achieved with CONMIN2 at the weighted mean of the 5th to 9th harvest (Table 3).
The P concentration of grass was significantly different for the weighted mean of the 3rd to 4th harvest between NOFERT (3.7 mg g−1) and BIOORG2 (4.9 mg g−1) and the 5th to 9th harvest between CONMIN2 (4.5 mg g−1) and the other treatments (2.7–3.2 mg g−1) (Table 3). However, all treatments achieved the limit of the ryegrass critical P concentration of 2.5 mg g−1 (Bailey et al. 1997).
Potassium concentrations of grass were generally lower in NOFERT compared with the other treatments and were also significantly lower in BIOORG1 than in BIOORG2 and CONMIN2 for the weighted mean of the 5th to 9th harvest (Table 3). Generally, the critical K concentration of 28 mg g−1 (Smith et al. 1985) was achieved in perennial ryegrass. Only treatment NOFERT for the weighted mean of the 5th to 9th harvest did not achieve the above-mentioned threshold (second cultivation period, 16.3 mg g−1).
Enrichment and recovery of 15N
The 15N enrichment of clover parts ranged from about 2200 ppm to more than 9200 ppm EAF 15N (Table 4, details Table S1). The 15N enrichment of grass parts ranged from about 380 ppm to more than 4400 ppm EAF 15N and that of soil from 12 ppm to 240 ppm EAF 15N in the topsoil (0–0.25 m) and from 4 ppm to 26 ppm EAF 15N in the subsoil (0.25–0.6 m). Generally, the 15N enrichment was highest in clover shoots followed by clover roots, grass roots, grass shoots, the topsoil, and the subsoil. The recovery of applied 15N ranged from about 60% to 80% at 4 and 8 months. At 19 months, when the plant-soil system was only labelled during the second year (Fig. 1, LMP19), the recovery of applied 15N was lower, between 48% and 52%.
Above- and belowground nitrogen accumulation during sward cultivation
Clover AGN and BGN were clearly affected by the nutrient availability gradient increasing in the order NOFERT < BIOORG1 ≤ BIOORG2, except for CONMIN2 (Table S2, Fig. 2). Despite the highest N and P input to CONMIN2 (Table 1), amounts of AGN as well as BGN were similar to amounts of BIOORG1 (Table S2, Fig. 2). In contrast, grass AGN and root N were highest with sole mineral fertilisation in CONMIN2 but were comparable between half and full fertilised organic treatments, resulting in a treatment order NOFERT < BIOORG1 = BIOORG2 < CONMIN2 (Fig. 2, Table S2).
Allocation of red clover belowground N (BGN) and aboveground N (AGN) (a), red clover BGN to AGN ratio (b), and allocation of perennial ryegrass root N and AGN (c) at 4 months (1st to 2nd harvest, stubble1), root1), and N derived from rhizodeposition2) [NdfR] in 0–0.25 m), 8 months (1st to 4th harvest, stubble1), root1), and NdfR2) in 0–0.6 m [end of the first year]), and 19 months of sward cultivation (1st to 9th harvest, stubble1), root1), and NdfR3) in 0–0.6 m [end of the second year]) as mean (n = 4 [CONMIN2: n = 3]) +/− SEM. Columns above and below the x-axis represent AGN and BGN / root N, resp. (2a and 2c); clover NdfR was not determined in 0.25-0.6 m at 4 months of cultivation; no roots were detected in the subsoil (0.25–0.6 m); grass NdfR was not determined. 1) Standing biomass N at the time of excavation 2) At the time of microplot excavation 3) Sum of NdfR at the times 8 and 19 months (cf. Eq. 8)
While the amounts of clover AGN increased over time (Fig. 2, Table S2), clover root N was highest at 8 months (Fig. 2, Table S2). At 19 months, only 70% of clover root N found at 8 months was recovered. In contrast, clover NdfR increased three- to more than six-fold between 8 and 19 months. In consequence, clover BGN increased on average by a factor of 2 (Fig. 2, Table S2).
Clover accumulated BGN between 4 and 10 g m−2 at 4 months, 13 and 21 g m−2 at 8 months, and 19 g m−2 (NOFERT), 32 g m−2 (BIOORG1), 35 g m−2 (CONMIN2), and 58 g m−2 (BIOORG2) at 19 months (Fig. 2, Table S2). The amount of clover NdfR increased from 1.5 to 3.1 g m−2 at 4 months to 5 to 7 g m−2 at 8 months to 16 g m−2 (NOFERT), 25 to 26 m−2 (BIOORG1, CONMIN2), and 47 g m−2 (BIOORG2) at 19 months (Fig. 2, Table S2).
While amounts of grass AGN increased over time (Fig. 2, Table S2) as clover AGN did, grass root N peaked at 8 months, as observed for clover root N (Fig. 2, Table S2). At 19 months, only about 60% of grass root N found at 8 months was recovered.
The percentage of clover N of total sward N was high (Fig. 2, Table S2), between 69% (CONMIN2 at 4 months) and 93% (BIOORG2 at 8 months). However, the clover N percentage in CONMIN2 was 5% to 19% lower compared to the other treatments (69% at 4 months, 81% at 8 months, and 83% at 19 months).
Allocation of red clover nitrogen and nutrient availability
The distribution between clover AGN and BGN was never significantly affected by the treatments during the entire study (Table S2). Clover BGN constituted about 40% of AGN at all points of time (Fig. 2, Table S2).
The size of clover BGN fractions strongly changed over time both in terms of absolute amounts and of relative proportions (Table 5). While clover macro root N (> 3 mm) decreased from 8 to 19 months, clover rootlets N (< 3 mm) and NdfR increased in the meantime (Table 5). At 4 and 8 months, the contribution of clover NdfR to BGN was relatively low, between 30% and 40%. However, at 19 months, clover NdfR was the major part of clover BGN, ranging from 72% to 82% of clover BGN (Fig. 2, Table S2). In parallel, the proportion of clover rootlets N (< 3 mm) of clover total root N was about two times greater at 19 months compared with 8 months (Table 5).
No roots could be found in the subsoil (0.25–0.6 m). However, clover NdfR was found in the subsoil, ranging from 1.0 to 1.4 g m−2 at 8 months and 1.4 to 2.2 g m−2 at 19 months. At 8 months, a comparatively large proportion of about 30% of total clover NdfR was recovered in the subsoil, whereas at 19 months the proportion constituted only about 10% (Table 5).
Discussion
Evolution of red clover N partitioning between AGN and BGN over time
Clover BGN was highest at the end of the second year, at 19 months (Fig. 2). While NdfR increased from one excavation time to the following one, root N was greatest at the end of the first year (8 months). The significantly lower amount of root N at the end of the second compared with the end of the first year may be due to enhanced root decay and reduced root N regrowth (Chen et al. 2016). Root decay is indicated by a lower ratio of root N > 3 mm to rootlets N < 3 mm at the end of the second compared to the end of the first year (Table 5). Bowley et al. (1984) stated that red clover taproots normally disintegrate during the second year, which is supported by our results. In parallel, the amount of NdfR increased by a factor of 3 (NOFERT) to more than 6 (BIOORG2) between the end of the first and the end of the second year (Fig. 2). Decreasing root N together with increasing NdfR suggests that root N turnover was higher than regrowth of new roots during the second year. As a result, BGN development was reflected neither by the development of root N nor by the development of NdfR alone. Hence, both root N and NdfR are essential to obtain realistic BGN estimates.
Only a few studies have focused on red clover BGN in clover-ryegrass swards. In a red clover-perennial ryegrass sward cultivated during one growing season, Dahlin and Stenberg (2010b) found barely 60% of the amount of clover AGN and about 20% of the amount of clover root N, but three times the amount of clover NdfR compared to results of treatment CONMIN2 in the present study at 8 months of cultivation. However, the amount of clover BGN determined in our study in the respective treatment (Table S2) is comparable to the amount determined by Dahlin and Stenberg (2010b). The difference in amounts of clover NdfR and root-N may partly be related to different methods used for root separation, which may have affected the relation between clover root N and NdfR. While Dahlin and Stenberg (2010b) separated roots and soil by using a 2 mm sieve, we implemented a 0.5 mm sieving subsequent to the 3 mm sieving and recovered an average amount of clover rootlets < 3 mm of 2.4 g m−2. Fine roots recovered with this procedure additionally contributed 18% of clover root N and reduced clover NdfR by 26% (Table 5) compared to amounts recovered by 3 mm sieving alone. However, the sieving protocol did neither change the BGN to AGN ratio nor total N, but the proportion of root N and NdfR. Furthermore, differences in site factors, varieties, and fertilisation (e.g. 160% of N but 60% of P and 20% of K applied with fertilisation by Dahlin and Stenberg 2010b) might have led to differences in sward performance and belowground inputs.
Nutrient availability did not affect the BGN to AGN ratio of red clover
To evaluate nutrients that limit plant growth primarily, Römheld (2012) suggested critical nutrient concentrations in plants and McNaught and During (1970) nutrient norm ratios. We focused on N, P, and K concentrations, since screenings in a previous study (Oberson et al. 2013) revealed no limitations of other nutrients (Oberson, unpublished results).
While the P and K concentrations of cumulative clover harvests were signficantly affected by treatments, only the K concentration clearly reflected the treatment related K availability of the soil (Table 1 and Table 3). However, low K availability only seems to have limited clover growth in NOFERT, as suggested by low K concentrations (Tucker and Smith 1952) throughout the two consecutive cultivation years. Phosphors, by contrast, only limited clover growth in the both organic treatments BIOORG1 and BIOORG2 during the second cultivation year as indicated by the P concentrations (Davis 1991) together with the N to P ratios (Fystro et al. 2008) of the cumulative 5th to 9th harvests.
The N and K concentrations of cumulative grass harvests were signficantly affected by treatments. However, only K limited grass growth in NOFERT during the second cultivation year (2012) as suggested by the low K concentration, the low K to P ratio (Liebisch et al. 2013), and the high N to K ratio (Dampney 1992) of the cumulative 5th to 9th harvest. We assume that grass was generally sufficiently supplied by P and N except for N in BIOORG2 in 2011 as suggested by low N concentrations (Bolton et al. 1976) together with low N to P ratios (Liebisch et al. 2013) and low N to K ratios (Dampney 1992) of cumulative harvests in 2011.
In a previous study, Oberson et al. (2013) found similar nutrient limitations in clover-grass swards growing in DOK field plots. Both data sets clearly revealed that red clover and to a lesser degree grass were primarily K limited in NOFERT.
With rising nutrient availability, we expected a relative increase of clover AGN compared to clover BGN, corresponding to a decrease in the BGN to AGN ratio. However, the ratio was similar for all treatments at 4, 8, and 19 months of cultivation (Fig. 2, Table S2), with an average around 0.4 irrespective of the treatment, i.e. the nutrient availability. Hence, red clover BGN could roughly be estimated from AGN under field conditions independently of the management intensity and the cultivation time, using a factor of 0.4. The factor of 0.4 is below the range of factors reviewed by Herridge et al. (2008) for fodder legumes, ranging from 0.5 to 2.1. The authors proposed an average factor of 1.0 for fodder legumes to calculate BGN from AGN, but the data set did not include red clover. However, Dahlin and Stenberg (2010b) found a similar factor of 1.1 for red clover in a red clover-perennial ryegrass mixture.
We have not included the N-transfer from clover to grass, which removes clover NdfR from the soil. Thus, N-transfer to associated grass will contribute to gross clover BGN and will potentially increase the factor of 0.4. For instance, in a two-year-old clover-grass mixture growing in the DOK experiment, about 50% of grass N derived from clover (Oberson et al. 2013).
Dry matter yields in microplots versus DOK field plots
Dry matter yields of our simple two-component red clover-perennial ryegrass model sward were higher compared with the clover-grass mixture of the field plots (Table 2, Table S3). Field plots yielded between 1270 and 2400 g m−2 and the clover proportion on a dry matter basis was between 30% and 52%. These yields and clover proportions were comparable to those of previous years (Oberson et al. 2013). Dry matter yields in microplots were about 1.5 to 2.1 fold higher than in field plots, however, while clover yielded up to 4.5 times as much, grass yielded only about half. We attributed the higher dry matter yields of clover to the substitution of the red clover varieties ‘Merian’ and ‘Global’ with the more productive, competitive, and persistent variety ‘Dafila’ (Suter et al. 2014) and to the simplification of the sward because white clover has lower yields than red clover (Oberson et al. 2013). Lower grass dry matter yields in the microplots were thus the result of the very strong clover competition. In consequence, dry matter yields of the sward in the microplots were highest in BIOORG2, whereas in the field plot the higher yields were found in CONMIN2. Finally, higher yields in microplots than surrounding field plots have been reported previously, e.g. by Oberson et al. (2007) for soybean, suggesting that additional factors such as protection by the microplot frame, border effects, careful management of small experimental units, and scale may affect the yields. Therefore, upscaling of our data should take into account the clover proportion and N yield measured at more realistic field scales.
Methodological considerations
To detect red clover NdfR in the bulk soil, a sufficient 15N enrichment of the soil via rhizodeposition from 15N labelled roots is required (Wichern et al. 2008). With a target enrichment of 5000 ppm EAF 15N of clover roots, we achieved a sufficient bulk soil enrichment, between 12 (LMP4) and 240 ppm (LMP19) EAF 15N in the topsoil (0–0.25 m) and between 4 ppm and 26 ppm EAF 15N in the subsoil (0.25–0.6 m) (Table 4). Grass roots and shoots showed high 15N enrichments, ranging from 377 ppm (shoot NOFERT, LMP4) to 4469 ppm EAF 15N (root NOFERT, LMP8), thus indicating a strong BGN transfer from clover to grass (Oberson et al. 2013) due to a fast turnover of red clover NdfR (Hamilton et al. 2008; Haystead and Marriott 1979).
The recovery of labelled 15N was generally in the medium range compared to similar studies with red clover-perennial ryegrass swards under field conditions. While Rasmussen et al. (2007) recovered only 25% of applied 15N after 65 days of labelling, Høgh-Jensen and Schjoerring (2001) could recover 85% of applied 15N after two consecutive growing seasons. However, we removed the highly 15N labelled leaves with their stalks after completion of tracer uptake, but did not account for the removed 15N amount contained therein when calculating the recovery (Eq. 13). In addition, gaseous ammonia losses from the leaves into the atmosphere (Gooding and Davies 1992) and losses from red clover NdfR by denitrification or nitrate leaching into deeper soil layers below 0.6 m (Robertson and Vitousek 2009) may explain 15N label losses. Higher precipitation during the second compared with the first year (Fig. S2) might have resulted in greater leaching of red clover NdfR into deeper soil layers and higher denitrification rates, due to oxygen limiting conditions as a consequence of increased soil moisture (Butterbach-Bahl et al. 2013).
The principle underlying Eq. 6 for calculating the proportion of clover NdfR assumes that i) clover NdfR has the same 15N enrichment as clover root N (Janzen and Bruinsma 1989). This implies that the 15N enrichment of the root is constant over ii) space (Jensen 1996) and iii) time (Sawatsky and Soper 1991). Leakage of highly 15N enriched tracer or tracer metabolites shortly after labelling is one of the main factors violating assumption i), leading to an overestimation of NdfR (Gardner et al. 2014; Gasser et al. 2015). Gasser et al. (2015) found 0.5% of applied 15N enriched tracer or metabolites leaked after one day from mono-cropped red clover in a model greenhouse study, leading to a vast overestimation of NdfR. However, the bias would decrease if clover or the competitive grass partner (Snaydon and Howe 1986) reabsorbed soluble tracer 15N, which is suggested in our case by the fast N transfer from clover to grass after the first labelling in samples taken at 4 months of cultivation (Table 4, Table S1). Thus, we assume that biases from possible tracer leakage in soil were small. Gasser et al. (2015) found spatial root enrichments varying by a factor of 3, hence violating assumption ii). The observed non-uniform 15N labelling of the root (e.g. Gasser et al. 2015; Khan et al. 2002; Russell and Fillery 1996) might be partially the result of symbiotic N2 fixation diluting the 15N enrichment of nodulated compared to unnodulated root segments (Russell and Fillery 1996). However, since defoliation of red clover also results in nodule turnover (Bowley et al. 1984), the average 15N enrichment of the nodulated root should be comparable with the average 15N enrichment of NdfR. This assumption is supported by Gasser et al. (2015), who detected only small biases of NdfR due to the spatial heterogeneity of the root 15N enrichment. Therefore, we sampled the entire root and homogenised the ground samples before the analyses to minimise the spatial bias in 15N enrichment of the clover root. Assumption iii), equal root enrichment over time, was not met in the first year, as root 15N enrichment recorded at 4 and at 8 months differed by a factor of 2, although we adjusted the multiple pulse labelling to the predicted N uptake. Gasser et al. (2015) and Sawatsky and Soper (1991) concluded that biases of NdfR estimations should be small if the 15N enrichment of the root at the time of sampling is comparable with the mean root enrichment over time. However, the nature of the clover grass sward is very complex, due to species mixtures, frequent harvests inducing root turnover, and tracer removal by harvests. Induced root turnover releases soluble 15N enriched compounds to the rhizosphere, which can be taken up by either grass or, less likely, due to the strong competition of grass (Nyfeler et al. 2011), by clover immediately or subsequently to the mineralisation (Gasser et al. 2015; Hart et al. 1986; Mayer et al. 2003).
Because we did not know how these processes affect clover NdfR estimations in a two year clover grass sward, we split the determination of NdfR on two microplots, one being labelled in the first and one being labelled in the second year (Fig. 1). Both results were then summed to determine clover NdfR over 19 months of cultivation (Eq. 8). As a control, we used a second microplot (LMP19B, Table S4), which was 15N labelled during the first year according to LMP8, but was not further labelled during the second year. The LMP19B was excavated at the same time as LMP19. In LMP19B, the 15N enrichment of the clover root probably decreased continuously during the second year, as suggested by the decreasing aboveground 15N enrichment of the grass partner (data not shown). Clover root N decrease was the result of i) dilution due to regrowth of new root biomass with unlabelled N from symbiotic N2 fixation or from uptake of soil N and ii) of tracer 15N removal from the system by harvesting aboveground biomass. Therefore, clover NdfR of LMP19B was calculated using the geometric mean of clover root N EAF 15N at 8 (LMP8) and at 19 (LMP19B) months. Clover NdfR differed only in treatment BIOORG2 between the labelling strategies, with 48 g m−2 in LMP19 and 21 g m−2 in LMP19B (Table S4). This might partly be the result of about 40% higher AGN accumulations in LMP19 (112 g m−2) than in LMP19 B (82 g m−2) in BIOORG2. However, the ratios BGN to AGN were not significantly different between treatments and labelling strategies.
Conclusions
During two consecutive years of red clover-perennial ryegrass sward cultivation, dry matter yields and total N uptake responded to varying nutrient availabilities established by organic and conventional cropping systems. Potassium availability was the main growth-limiting factor for red clover in treatments with low nutrient availability. In contrast, organic treatments were limited in P during the second cultivation year. However, independently of the nutrient availability, red clover BGN increased proportionally to cumulative AGN. Hence, red clover BGN could be estimated from red clover AGN by multiplying AGN with a factor of 0.4. The increase of red clover BGN with time was the result of a strong increase of red clover NdfR between 8 and 19 months, the latter thus compensating the decrease of root N between these two time points. Consequently, red clover BGN was reflected neither by the development of root N nor by the development of NdfR. The inverse relation between red clover root N and NdfR development between 8 and 19 months was probably triggered by enhanced root turnover, resulting in a distinct absolute and relative increase of NdfR until the end of the second year, independently of the nutrient availability.
Abbreviations
- AGN:
-
Aboveground N
- BGN:
-
Belowground N, comprising physically recoverable root N at the time of excavation plus NdfR
- BIOORG1:
-
Bio-organic treatment of the DOK experiment with half dose fertilisation
- BIOORG2:
-
Bio-organic treatment of the DOK experiment with full dose fertilisation
- CONMIN2:
-
Conventional treatment of the DOK experiment with full dose sole mineral fertilisation
- CFE:
-
Chloroform fumigation extraction
- DOK:
-
Long-term experiment comparing Bio-Dynamic, Bio-Organic, and conventional (K) cropping systems
- EAF:
-
Excess atom fraction
- LMP(t):
-
Labelled microplot, delimiting the 15N labelled plant-soil system (excavated after t months of sward cultivation)
- NdfR:
-
Nitrogen derived from rhizodeposition
- NOFERT:
-
Unfertilised control treatment of the DOK experiment
- RMP(t):
-
Reference microplot, delimiting the unlabelled plant-soil system (excavated after t months of sward cultivation)
- t:
-
Time from planting of red clover and perennial ryegrass until microplot excavation in months
References
Agroscope (1996) Schweizerische Referenzmethoden der Forschungsanstalt Agroscope. Band 1: Bodenuntersuchung und Substratuntersuchung zur Düngeberatung. Zürich-Reckenholz
Aitchison J (1982) The statistical analysis of compositional data. J R Stat Soc Series B Stat Methodol 44:139–177. https://doi.org/10.2307/2345821
Almeida JPF, Lüscher A, Frehner M, Oberson A, Nösberger J (1999) Partitioning of P and the activity of root acid phosphatase in white clover (Trifolium Repens L.) are modified by increased atmospheric CO2 and P fertilisation. Plant Soil 210:159–166. https://doi.org/10.1023/a:1004625801141
Bailey JS, Cushnahan A, Beattie JAM (1997) The diagnosis and recommendation integrated system (DRIS) for diagnosing the nutrient status of grassland swards: II. Model calibration and validation. Plant Soil 197:137–147. https://doi.org/10.1023/a:1004288505814
Boller BC, Nösberger J (1987) Symbiotically fixed nitrogen from field- grown white and red clover mixed with ryegrasses at low levels of 15N-fertilization. Plant Soil 104:219–226. https://doi.org/10.1007/bf02372535
Bolton J, Nowakowski TZ, Lazarus W (1976) Sulphur–nitrogen interaction effects on the yield and composition of the protein-N, non-protein-N and soluble carbohydrates in perennial ryegrass. J Sci Food Agric 27:553–560. https://doi.org/10.1002/jsfa.2740270611
Bowley SR, Taylor NL, Dougherty CT (1984) Physiology and morphology of red clover. Adv Agron 37:317–347. https://doi.org/10.1016/S0065-2113(08)60457-5
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842. https://doi.org/10.1016/0038-0717(85)90144-0
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Phil Trans R Soc B Biol Sci 368:20130122. https://doi.org/10.1098/rstb.2013.0122
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007–1012. https://doi.org/10.2136/sssaj1993.03615995005700040021x
Cakmak I, Hengeler C, Marschner H (1994) Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J Exp Bot 45:1245–1250. https://doi.org/10.1093/jxb/45.9.1245
Chen SM, Lin S, Loges R, Reinsch T, Hasler M, Taube F (2016) Independence of seasonal patterns of root functional traits and rooting strategy of a grass-clover sward from sward age and slurry application. Grass Forage Sci 71:607–621. https://doi.org/10.1111/gfs.12222
Coplen TB (2011) Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Spectrom 25:2538–2560. https://doi.org/10.1002/rcm.5129
Dahlin AS, Mårtensson AM (2008) Cutting regime determines allocation of fixed nitrogen in white clover. Biol Fertil Soils 45:199–204. https://doi.org/10.1007/s00374-008-0328-9
Dahlin AS, Stenberg M (2010a) Transfer of N from red clover to perennial ryegrass in mixed stands under different cutting strategies. Eur J Agron 33:149–156. https://doi.org/10.1016/j.eja.2010.04.006
Dahlin AS, Stenberg M (2010b) Cutting regime affects the amount and allocation of symbiotically fixed N in green manure leys. Plant Soil 331:401–412. https://doi.org/10.1007/s11104-009-0261-1
Dampney PMR (1992) The effect of timing and rate of potash application on the yield and herbage composition of grass grown for silage. Grass Forage Sci 47:280–289. https://doi.org/10.1111/j.1365-2494.1992.tb02272.x
Davis MR (1991) The comparative phosphorus requirements of some temperate perennial legumes. Plant Soil 133:17–30. https://doi.org/10.1007/bf00011895
Fließbach A, Oberholzer HR, Gunst L, Mäder P (2007) Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric Ecosyst Environ 118:273–284. https://doi.org/10.1016/j.agee.2006.05.022
Flisch R, Sinaj S, Charles R, Richner W (2009) Grundlagen für die Düngung im Acker- und Futterbau (GRUDAF). Agrarforsch Schweiz 16:1–97
Fustec J, Lesuffleur F, Mahieu S, Cliquet JB (2010) Nitrogen rhizodeposition of legumes. A review. Agron Sustain Dev 30:57–66. https://doi.org/10.1051/agro/2009003
Fystro G, Nesheim L, Bakken AK (2008) The N:P ratio in plant tissues as a diagnostic tool for P supply. NJF report. http://www.bioforsk.no/ikbViewer/Content/38595/NJF401_gf.pdf. Accessed 24 Apr 2017
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892. https://doi.org/10.1126/science.1136674
Gardner M, Peoples M, Condon J, Li G, Conyers M, Dear B (2014) Evaluating the importance of a potential source of error when applying shoot 15N labelling techniques to legumes to quantify the belowground transfer of nitrogen to other species. Proceedings of the 16th Australian agronomy conference, Armidale, NSW, Australia
Gasser M, Hammelehle A, Oberson A, Frossard E, Mayer J (2015) Quantitative evidence of overestimated rhizodeposition using 15N leaf-labelling. Soil Biol Biochem 85:10–20. https://doi.org/10.1016/j.soilbio.2015.02.002
Gooding MJ, Davies WP (1992) Foliar urea fertilization of cereals: a review. Fertil Res 32:209–222. https://doi.org/10.1007/bf01048783
Gylfadóttir T, Helgadóttir Á, Høgh-Jensen H (2007) Consequences of including adapted white clover in northern European grassland: transfer and deposition of nitrogen. Plant Soil 297:93–104. https://doi.org/10.1007/s11104-007-9323-4
Haase S, Ruess L, Neumann G, Marhan S, Kandeler E (2007) Low-level herbivory by root-knot nematodes (Meloidogyne Incognita) modifies root hair morphology and rhizodeposition in host plants (Hordeum Vulgare). Plant Soil 301:151–164. https://doi.org/10.1007/s11104-007-9431-1
Hamilton EW, Frank DA, Hinchey PM, Murray TR (2008) Defoliation induces root exudation and triggers positive rhizospheric feedbacks in a temperate grassland. Soil Biol Biochem 40:2865–2873. https://doi.org/10.1016/j.agee.2017.04.003
Hart PBS, Rayner JH, Jenkinson DS (1986) Influence of pool substitution on the interpretation of fertilizer experiments with 15N. Eur J Soil Sci 37:389–403. https://doi.org/10.1111/j.1365-2389.1986.tb00372.x
Haystead A, Marriott C (1979) Transfer of legume nitrogen to associated grass. Soil Biol Biochem 11:99–104. https://doi.org/10.1016/0038-0717(79)90083-X
Herridge D, Peoples M, Boddey R (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311:1–18. https://doi.org/10.1007/s11104-008-9668-3
Hill JO, Simpson RJ, Moore AD, Chapman DF (2006) Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 286:7–19. https://doi.org/10.1007/s11104-006-0014-3
Høgh-Jensen H, Schjoerring JK (2001) Rhizodeposition of nitrogen by red clover, white clover and ryegrass leys. Soil Biol Biochem 33:439–448. https://doi.org/10.1016/S0038-0717(00)00183-8
Huss-Danell K, Chaia E, Carlsson G (2007) N2 fixation and nitrogen allocation to above and belowground plant parts in red clover-grasslands. Plant Soil 299:215–226. https://doi.org/10.1007/s11104-007-9376-4
Janzen HH, Bruinsma Y (1989) Methodology for the quantification of root and rhizosphere nitrogen dynamics by exposure of shoots to 15N-labelled ammonia. Soil Biol Biochem 21:189–196. https://doi.org/10.1016/0038-0717(89)90094-1
Jensen ES (1996) Rhizodeposition of N by pea and barley and its effect on soil N dynamics. Soil Biol Biochem 28:65–71. https://doi.org/10.1016/0038-0717(95)00116-6
Khan WDF, Peoples MB, Herridge DF (2002) Quantifying below-ground nitrogen of legumes. Plant Soil 245:327–334. https://doi.org/10.1023/A:1020407006212
Ledgard SF, Freney JR, Simpson JR (1985) Assessing nitrogen transfer from legumes to associated grasses. Soil Biol Biochem 17:575–577. https://doi.org/10.1016/0038-0717(85)90028-8
Liebisch F, Bünemann EK, Huguenin-Elie O, Jeangros B, Frossard E, Oberson A (2013) Plant phosphorus nutrition indicators evaluated in agricultural grasslands managed at different intensities. Eur J Agron 44:67–77. https://doi.org/10.1016/j.eja.2012.08.004
Maeder P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697. https://doi.org/10.1126/science.1071148
Mayer J, Buegger F, Jensen ES, Schloter M, Heß J (2003) Estimating N rhizodeposition of grain legumes using a 15N in situ stem labelling method. Soil Biol Biochem 35:21–28. https://doi.org/10.1016/S0038-0717(02)00212-2
Mayer J, Gunst L, Mäder P, Samson MF, Carcea M, Narducci V, Thomsen IK, Dubois D (2015) Productivity, quality and sustainability of winter wheat under long-term conventional and organic management in Switzerland. Eur J Agron 65:27–39. https://doi.org/10.1016/j.eja.2015.01.002
McNaught KJ, During C (1970) Relations between nutrient concentrations in plant tissues and responses of white clover to fertilisers on a gley podzol near westport. New Zeal J Agr Res 13:567–590. https://doi.org/10.1080/00288233.1970.10421604
McNeill AM, Zhu C, Fillery IRP (1997) Use of in situ 15N-labelling to estimate the total below-ground nitrogen of pasture legumes in intact soil–plant systems. Aust J Agric Res 48:295–304. https://doi.org/10.1071/A96097
Mengel K, Steffens D (1985) Potassium uptake of rye-grass (Lolium Perenne) and red clover (Trifolium Pratense) as related to root parameters. Biol Fertil Soils 1:53–58. https://doi.org/10.1007/bf00710971
Nesheim L, Øyen J (1994) Nitrogen fixation by red clover (Trifolium Pratense L.) grown in mixtures with timothy (Phleum Pratense L.) at different levels of nitrogen fertilization. Acta Agr Scand B- S P 44:28–34. https://doi.org/10.1080/09064719409411254
Neumann G, Römheld V (2012) Rhizosphere chemistry in relation to plant nutrition. In: Marschner P (ed) Marschner's mineral nutrition of higher plants, 3rd edn. Academic, San Diego, pp 347–368
Nyfeler D, Huguenin-Elie O, Suter M, Frossard E, Lüscher A (2011) Grass-legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric Ecosyst Environ 140:155–163. https://doi.org/10.1016/j.agee.2010.11.022
Oberson A, Nanzer S, Bosshard C, Dubois D, Mäder P, Frossard E (2007) Symbiotic N2 fixation by soybean in organic and conventional cropping systems estimated by 15N dilution and 15N natural abundance. Plant Soil 290:69–83. https://doi.org/10.1007/s11104-006-9122-3
Oberson A, Frossard E, Bühlmann C, Mayer J, Mäder P, Lüscher A (2013) Nitrogen fixation and transfer in grass-clover leys under organic and conventional cropping systems. Plant Soil 371:237–255. https://doi.org/10.1007/s11104-013-1666-4
Rasmussen J, Eriksen J, Jensen ES, Esbensen KH, Høgh-Jensen H (2007) In situ carbon and nitrogen dynamics in ryegrass–clover mixtures: transfers, deposition and leaching. Soil Biol Biochem 39:804–815. https://doi.org/10.1016/j.soilbio.2006.10.004
Robertson GP, Vitousek PM (2009) Nitrogen in agriculture: balancing the cost of an essential resource. Annu Rev Environ Resour 34:97–125. https://doi.org/10.1146/annurev.environ.032108.105046
Römheld V (2012) Diagnosis of deficiency and toxicity of nutrients. In: Marschner P (ed) Marschner's mineral nutrition of higher plants, 3rd ed. academic, San Diego, pp 299–312
Russell CA, Fillery IRP (1996) In situ 15N labelling of lupin below-ground biomass. Aust J Agric Res 47:1035–1046. https://doi.org/10.1071/AR9961035
Sawatsky N, Soper RJ (1991) A quantitative measurement of the nitrogen loss from the root system of field peas (Pisum avense L.) grown in the soil. Soil Biol Biochem 23:255–259. https://doi.org/10.1016/0038-0717(91)90061-N
Schipanski ME, Drinkwater LE (2012) Nitrogen fixation in annual and perennial legume-grass mixtures across a fertility gradient. Plant Soil 357:147–159. https://doi.org/10.1007/s11104-012-1137-3
Sierra J, Daudin D, Domenach A-M, Nygren P, Desfontaines L (2007) Nitrogen transfer from a legume tree to the associated grass estimated by the isotopic signature of tree root exudates: a comparison of the 15N leaf feeding and natural 15N abundance methods. Eur J Agron 27:178–186. https://doi.org/10.1016/j.eja.2007.03.003
Smith GS, Cornforth IS, Henderson HV (1985) Critical leaf concentrations for deficiencies of nitrogen, potassium, phosphorous, sulphur, and magnesium in perennial ryegrass. New Phytol 101:393–409. https://doi.org/10.1111/j.1469-8137.1985.tb02846.x
Snaydon R, Howe C (1986) Root and shoot competition between established ryegrass and invading grass seedlings. J Appl Ecol 23:667–674. https://doi.org/10.2307/2404044
Suter D, Rosenberg E, Frick R, Mosimann E (2012) Swiss standard mixtures for ley farming, revision 2013-2016. Agrarforsch Schweiz 3:1–12
Suter D, Frick R, Hirschi H, Aebi P (2014) Substantial progress in variety testing with red clover. Agrarforsch Schweiz 5:272–279
Ta TC, Faris MA (1987) Effects of alfalfa proportions and clipping frequencies on timothy-alfalfa mixtures. II: nitrogen fixation and transfer. Agron J 79:820–824. https://doi.org/10.2134/agronj1987.00021962007900050013x
Thilakarathna MS, Papadopoulos YA, Rodd AV, Grimmett M, Fillmore SAE, Crouse M, Prithiviraj B (2016) Nitrogen fixation and transfer of red clover genotypes under legume–grass forage based production systems. Nutr Cycl Agroecosyst 106:233–247. https://doi.org/10.1007/s10705-016-9802-1
Thió-Henestrosa S, Barceló-Vidal C, Martín-Fernandez A, Pawlowsky-Glahn V (2009) CoDaPack. An excel and visual basic based software of compositional data analysis: current version and discussion for upcoming versions. In: proceedings of the 6th international workshop on compositional data analysis: Girona, June 1-5, 2015
Trannin WS, Urquiaga S, Guerra G, Ibijbijen J, Cadisch G (2000) Interspecies competition and N transfer in a tropical grass-legume mixture. Biol Fertil Soils 32:441–448. https://doi.org/10.1007/s003740000271
Tucker TC, Smith FW (1952) The influence of applied boron, magnesium, and potassium on the growth and chemical composition of red clover grown under greenhouse conditions1. Soil Sci Soc Am J 16:252–255. https://doi.org/10.2136/sssaj1952.03615995001600030006x
Uren NC (2007) Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In: Pinton R, Varanini Z, Nannipieri P (eds) The Rhizosphere, 2nd edn. CRC Press, Boca Raton, pp 1–21
Van Den Boogaart KG, Tolosana-Delgado R (2013) Analyzing compositional data with R. Springer, Dordrecht
Wichern F, Mayer J, Joergensen RG, Muller T (2007) Release of C and N from roots of peas and oats and their availability to soil microorganisms. Soil Biol Biochem 39:2829–2839. https://doi.org/10.1016/j.soilbio.2007.06.006
Wichern F, Eberhardt E, Mayer J, Joergensen RG, Müller T (2008) Nitrogen rhizodeposition in agricultural crops: methods, estimates and future prospects. Soil Biol Biochem 40:30–48. https://doi.org/10.1016/j.soilbio.2007.08.010
Acknowledgments
We warmly thank the Agroscope field team, especially their head Ernst Brack, Lucie Gunst and Monika Schnider from Agroscope for their versatile help in the DOK experiment and in the lab, the FiBL field team for their help in the DOK experiment, Stephano Bernasconi from the Geological Institute at ETH Zurich for isotopic analysis, Claude Renaux from the statistical consulting service of the seminar of statistics at the ETH Zürich, and Juliane Hirte from Agroscope for the final internal review of the manuscript. The work was funded by the Swiss National Science Foundation Grant 205321_132770 / 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Richard J. Simpson.
Andreas Hammelehle is currently affiliated at Landesbetrieb Landwirtschaft Hessen, Fachinformation Pflanzenbau, 61169 Friedberg, Germany.
Electronic supplementary material
ESM 1
(DOCX 98 kb)
Rights and permissions
About this article
Cite this article
Hammelehle, A., Oberson, A., Lüscher, A. et al. Above- and belowground nitrogen distribution of a red clover-perennial ryegrass sward along a soil nutrient availability gradient established by organic and conventional cropping systems. Plant Soil 425, 507–525 (2018). https://doi.org/10.1007/s11104-018-3559-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3559-z