Abstract
Environmental changes are expected to shift the distribution of functional trait values in plant communities through a combination of species turnover and intraspecific variation. The strength of these shifts may depend on the availability of individuals with trait values adapted to new environmental conditions, represented by the functional diversity (FD) of existing community residents or dispersal from the regional species pool. We conducted a 3-year nutrient- and seed-addition experiment in old-field plant communities to examine the contributions of species turnover and intraspecific variation to community trait shifts, focusing on four key plant functional traits: vegetative height, leaf area, specific leaf area (SLA), and leaf dry matter content (LDMC). We further examined the influence of initial FD and seed availability on the strength of these shifts. Community mean height, leaf area, and SLA increased in response to fertilization, and these shifts were driven almost entirely by intraspecific variation. The strength of intraspecific shifts in height and leaf area was positively related to initial intraspecific FD in these traits. Intraspecific trait responses to fertilization varied among species, with species of short stature displaying stronger shifts in SLA and LDMC but weaker shifts in leaf area. Trait shifts due to species turnover were generally weak and opposed intraspecific responses. Seed addition altered community taxonomic composition but had little effect on community trait shifts. These results highlight the importance of intraspecific variation for short-term community functional responses and demonstrate that the strength of these responses may be mediated by community FD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trait-based approaches are increasingly used to understand and predict changes in community structure in response to environmental change (Lavorel and Garnier 2002; McGill et al. 2006; Jung et al. 2014). Local environmental conditions deterministically select or filter individuals based on their functional trait values (Keddy 1992; Weiher and Keddy 1995; Cornwell and Ackerly 2009), such that environmental changes in space and time lead to predictable shifts in community trait composition, as demonstrated by numerous studies showing relationships between environmental conditions and community mean trait values (Fonseca et al. 2000; Ackerly et al. 2002; Garnier et al. 2004). These community-level patterns reflect processes operating at different levels of organization (Ackerly 2003):
-
1.
Phenotypic plasticity—modifications in trait values within individuals in response to the environment.
-
2.
Adaptive evolution—heritable changes in trait values within populations resulting from natural selection.
-
3.
Species sorting—changes in occurrence or relative abundance of species within communities (i.e., species turnover) resulting from differential success based on interspecific trait differences.
All three processes may combine and interact to generate community-level functional responses to the environment, but most previous studies have only considered species sorting, under the assumption that interspecific trait differences are much larger than intraspecific differences arising from phenotypic plasticity or heritable genetic variation (Garnier et al. 2001a). However, recent studies have shown that intraspecific trait variation may be substantial at the community level and contribute strongly to shifts in community mean trait values in response to environmental variation (e.g., Jung et al. 2010; Messier et al. 2010; Hulshof and Swenson 2010; Lepš et al. 2011), highlighting the need to consider intraspecific trait responses when quantifying community trait responses to the environment.
Another unresolved question is how community functional structure, including the amount of interspecific and intraspecific trait variation present in a community, controls the strength of community trait responses to environmental change. In evolutionary biology, it is well known that the amount of evolutionary change in a trait is a function of the strength of selection and the amount of heritable trait variation within the population, as expressed in the Breeder’s equation (Lush 1937; Falconer 1960). By extension, the amount of change in a trait within a community in response to selection may be influenced by the amount of trait variation, or functional diversity (FD), present within the community (Shipley et al. 2006). Communities with high interspecific FD, reflecting large trait differences among species, are more likely to include species with traits pre-adapted to new environmental conditions, facilitating rapid shifts in community trait composition through species turnover. Similarly, communities with high intraspecific FD, reflecting phenotypic plasticity of resident genotypes or large genetic variation, may have a strong capacity to respond to an environmental change through intraspecific trait shifts, including plastic responses and genetic adaptation (Grime et al. 2008).
These predictions assume that communities already include species and genotypes with traits suited to the new conditions created by an environmental change. If not, the ability of a community’s functional composition to track the environment will depend on dispersal of individuals with appropriate traits from the surrounding area (Ackerly 2003). Dispersal limitation may therefore limit community trait responses to environment change, particularly responses due to species sorting (Foster et al. 2011). Metacommunity theory predicts that the strength of species sorting and the correspondence between environment and species composition should be greatest in systems with high connectivity due to dispersal (Leibold et al. 2004), and this prediction has been supported by experiments manipulating seed dispersal in grassland communities (Questad and Foster 2008; Foster et al. 2011). On the other hand, dispersal limitation may have less of an effect on functional than taxonomic composition due to functional redundancy among species and the potential for intraspecific variation to generate functional responses in the absence of species turnover (Messier et al. 2010; Swenson et al. 2011; Siefert et al. 2013).
The strength of community trait shifts may also be influenced by interactions between species turnover and intraspecific responses. Previous studies examining trends in community mean traits along environmental gradients have found that trait shifts due to species turnover and intraspecific variation may either reinforce or oppose each other (e.g., Lepš et al. 2011; Pérez-Ramos et al. 2012; Kichenin et al. 2013). Negative interactions between species turnover and intraspecific responses may occur if changes at one level preempt changes at the other. For example, Grime et al. (2008) suggested that rapid plastic responses and expansion of genotypes of resident species prevented large shifts in species composition in response to simulated climate change in grassland communities. Conversely, rapid migration may allow species with traits pre-adapted to new environmental conditions to replace residents before they have time to adapt (Donoghue 2008). Examining the relationship between species turnover and intraspecific trait shifts in communities with varying levels of inter- and intraspecific FD and dispersal limitation will shed light on these potential scenarios.
We conducted a 3-year field experiment in old-field plant communities in central New York to investigate community functional responses to environmental change and to assess the effects of dispersal limitation and initial community FD on these responses. We manipulated nutrient availability by adding fertilizer to experimental communities and removed dispersal limitation by adding seeds of species from the local species pool. We focused on four functional traits known to be related to plant resource acquisition and use strategies: vegetative height, leaf area, specific leaf area (SLA), and leaf dry matter content (LDMC). We addressed four main questions:
-
1.
How do community mean trait values respond to increased nutrient availability, and what are the contributions of, and interactions between, species turnover and intraspecific trait responses?
-
2.
How are community trait responses influenced by removal of dispersal limitation through seed addition?
-
3.
How are community trait responses influenced by inter- and intraspecific FD?
-
4.
How are trait responses of individual species influenced by their ecological and functional characteristics?
Materials and methods
Experimental design
The study was conducted at Green Lakes State Park in central New York, USA (43°2.5′N, 76°0.0′W). The experiment was established in a 1-km2 area of old fields abandoned from agricultural use in the 1960s through 1990s and presently dominated by native Solidago spp. and non-native grasses such as Bromus inermis and Poa pratensis. In summer 2010, we established eight 5 × 5-m square plots with 1-m2 quadrats located at the four corners, making a total of 32 quadrats. Individual quadrats served as experimental units and plots as experimental blocks. The plots were not cleared or otherwise disturbed prior to initiating the experiment. Within each block, we randomly applied a set of 2 × 2 factorial treatments, including fertilization (no fertilizer; 16 g N m−2 year−1) and seed addition (no seeds; seeds of 19 species added). We applied the treatments each year from 2011 to 2013. For the fertilization treatment, we used Osmocote slow-release fertilizer (18-6-12 N-P-K) and applied the appropriate amount to add 8 g N m−2 in April and July of each year. This rate of nutrient addition is typical of high-nutrient treatments in previous grassland fertilization experiments (e.g., Tilman 1987; Dickson and Foster 2008). For the seed-addition treatment, we added seeds of 19 species (Table S1), representing a wide range of functional strategies and trait values, that occurred within the study site. Seeds of most species were added at a rate of 1 g m−2 year−1, with a lower limit of 200 and upper limit of 2000 seeds/species per year (Table S1), representing a tradeoff between equalizing seed mass and seed number across species (Jakobsson and Eriksson 2000). Though this rate of seed addition is higher than that of natural seed rain in grassland communities (Myers and Harms 2009), the purpose of the treatment was not to mimic natural seed rain but to overcome dispersal barriers that could limit species establishment in experimental plots. Seeds were hand broadcast into quadrats, with half the yearly amount added in November and half in April. We manually agitated the vegetation and litter layer in all quadrats to allow seeds to contact the soil.
Data collection
We sampled quadrats in July–August 2010 (prior to application of experimental treatments) and 2013 (after 3 years of treatment application). To determine species richness and composition, we visually estimated the percent cover of each species within each quadrat (Peet et al. 1998). To quantify community trait distributions, we measured traits of 50 randomly selected individuals per quadrat. The number of individuals sampled per species was proportional to relative species abundance, with a minimum of three per species (Siefert 2012). On each individual sampled, we measured four functional traits related to plant resource acquisition and use strategies: vegetative height, leaf area, SLA, LDMC. Vegetative height relates to light acquisition and competitive ability (Gaudet and Keddy 1988). Leaf area relates to stress tolerance, with low nutrient availability and other types of stress selecting for small leaves (Perez-Harguindeguy et al. 2013). SLA is related to plant growth and photosynthetic rates, representing a general plant strategy spectrum that runs from fast (high SLA) to slow (low SLA) return on investment in nutrients and dry mass (Wright et al. 2004; Reich 2014). LDMC relates to leaf resistance to physical stress and is also correlated with growth and photosynthetic rates (Perez-Harguindeguy et al. 2013). We expected that increased nutrient availability would favor plants with fast, competitive strategies, resulting in community-level shifts toward increased vegetative height, leaf area, and SLA and decreased LDMC. Vegetative height was measured as the distance between the ground and the top of the general canopy of the plant. One mature, healthy looking leaf from the upper third of the canopy of each selected individual was collected for leaf trait measurements. We measured the area of one side (square millimeters) and fresh mass (milligrams) of each leaf after full rehydration (Garnier et al. 2001b) and dry mass (in milligrams) after oven drying at 80 °C for 48 h. SLA was calculated as fresh leaf area divided by dry mass, and LDMC was calculated as dry mass divided by fresh mass. Leaf area and SLA were log transformed prior to analysis to meet assumptions of linear models.
Data analysis
Using data collected prior to the start of the experiment and after 3 years of fertilization and seed addition, we quantified changes in species richness, species composition, and community mean trait values within each quadrat. We measured the change in species richness as the difference between the number of species recorded within a given quadrat in 2010 and 2013. We measured changes in species composition in two ways. First, we quantified the magnitude of change as the Bray–Curtis dissimilarity between initial and final community composition. Second, we characterized the direction of change in multivariate space by conducting a non-metric multidimensional scaling (NMDS) ordination using all quadrats from 2010 and 2013 and recording the difference in quadrat scores between years for each ordination axis. We tested for effects of fertilization, seed addition, and their interactions on changes in species richness and composition (Bray–Curtis dissimilarity) using linear mixed models with “plot” as a random effect and fertilizer addition, seed addition, and their interactions as fixed effects. We used non-parametric multivariate ANOVA to analyze changes in species composition, using a matrix of Euclidean distances among quadrats based on shifts in NMDS axis scores as the response. Significance was assessed using permutation tests (n = 999) with plot as a grouping factor.
We measured community-level changes in functional traits and the contributions of species turnover and intraspecific variation using a modification of the approach of Lepš et al. (2011). We measured the total change in community-mean trait values between 2010 and 2013 (ΔComm) for each quadrat as:
where p ij13 and p ij10 are the relative cover of species i in quadrat j in 2013 and 2010, respectively, and \(\bar{x}_{ij13}\) and \(\bar{x}_{ij10}\) are the mean trait values of species i in quadrat j in 2013 and 2010, respectively, and S is the number of species recorded in the study. We measured the contribution of species turnover (i.e., changes in species relative abundance) to the total change in community mean traits between years (ΔTurn) as:
where \(\bar{x}_{ij}\) is the mean trait value of species i in quadrat j averaged across years, thus assuming no intraspecific shifts in trait values between years. Positive ΔTurn indicates an increase in trait values at the community level due to an increase in abundance of species with high trait values, and negative ΔTurn indicates a decrease in trait values at the community level due to an increase in abundance of species with low trait values. We calculated the contribution of intraspecific trait responses to the change in community mean traits between years (ΔIntra) as the difference between the total change in community mean traits and the change due to species turnover:
Positive ΔIntra indicates an overall increase in trait values within species, and negative ΔIntra indicates an overall decrease in trait values within species. We used linear mixed models with plot as a random effect to test the effects of fertilization, seed addition, and their interactions on changes in community mean traits (ΔComm) and the contributions of species turnover (ΔTurn) and intraspecific variation (ΔIntra). To test whether community trait responses to fertilization were influenced by community FD, we also included initial interspecific and intraspecific FD and their interactions with fertilization as fixed effects. For each quadrat and trait, interspecific FD was calculated as the variance of species mean trait values weighted by species relative cover. Intraspecific FD was calculated as the weighted mean of within-species trait variances (Lepš et al. 2006). We tested for relationships between ΔTurn and ΔIntra across all treatments for each trait using major axis regression.
Finally, we examined intraspecific trait responses of individual species to fertilization. For each case in which a species was found in the same fertilized quadrat in both 2010 and 2013, we calculated the difference in species mean trait values between years within the quadrat, then averaged across quadrats to obtain a mean trait response to fertilization for each species. To assess whether species’ trait responses to fertilization could be explained by their ecological and functional characteristics, we tested for correlations between mean trait response and initial mean trait value, trait variance, frequency (number of quadrats in which species occurred), and mean cover across species for each trait. We also hypothesized that fertilization would influence leaf traits indirectly by increasing shade, and that responses to shade would be strongest for species lower in the canopy. To test this hypothesis, we tested for correlations between mean trait responses and mean vegetative height across species.
All analyses were conducted in R (R Development Core Team 2012) using the vegan (Oksanen et al. 2012) and lmodel2 (Legendre 2013) packages.
Results
Species richness and composition
Species richness in 2010 at the start of the experiment ranged from six to 19 species/quadrat (mean = 6.4). Fertilization had a significant negative effect on the change in species richness between 2010 and 2013 (F 1,21 = 35.1; P < 0.001; Fig. 1a). Species richness decreased by 1.4 species on average in fertilized quadrats, compared to an average increase of 2.4 species in unfertilized quadrats. Seed addition had a significant positive effect on the change in species richness (F 1,21 = 5.6; P = 0.03; Fig. 1a), with an average increase of 1.2 species in seed-addition quadrats, compared to an average loss of 0.3 species in quadrats not receiving seed addition. There was no significant interaction between fertilization and seed addition. Seed addition had a marginally positive effect on the change in total cover of seeded species (F 1,21 = 1.7; P = 0.10), with an average increase of 52—60 % in seed-addition quadrats. The change in the cover of seeded species was not significantly affected by fertilization (P = 0.4).
Fertilization had a significant effect on changes in species composition within quadrats between 2010 and 2013, as measured by shifts of quadrats in NMDS ordination space (F 1,31 = 6.82; P = 0.007; Fig. S1). However, the magnitude of change in species composition, measured as the Bray–Curtis dissimilarity between 2010 and 2013, did not differ between fertilized and unfertilized plots (F 1,21 = 0.19; P = 0.66; Fig. 1b). In contrast, seed addition significantly increased the magnitude of species composition change (F 1,21 = 4.7; P = 0.04; Fig. 1b) but had no effect on shifts in ordination space (F 1,21 = 0.66; P = 0.55; Fig. S1).
Community-level trait shifts
Prior to the start of the experiment, community mean vegetative height of quadrats ranged from 22.8 to 99.9 cm (mean = 60.2), community mean leaf area ranged from 4.0 to 13.3 cm2 (mean = 8.0); community mean SLA ranged from 16.8 to 24.8 mm2 mg−1 (mean = 19.8), and community mean LDMC ranged from 0.23 to 0.34 g g−1 (mean = 0.29; Table S2). While there was large natural variation in community mean traits among quadrats, no trait varied significantly between fertilization or seed-addition treatments (P > 0.5).
Fertilization had a positive effect on overall changes in community mean trait values between 2010 and 2013 for vegetative height (F 1,17 = 8.0; P = 0.01; Fig. 2a) and leaf area (F 1,17 = 6.8; P = 0.02; Fig. 2b) but no effect on changes in community mean SLA or LDMC (Fig. 2c, d). Community mean vegetative height increased by 18.4 cm on average (SD = 12.8) in fertilized plots, compared to an average increase of 9.8 cm (SD = 7.8) in unfertilized plots (Fig. 2a; Table S2). Community mean leaf area increased by 2.3 cm2 on average (SD = 1.6) in fertilized plots, compared to an average increase of 0.45 cm2 (SD = 2.1) in unfertilized plots (Fig. 2b; Table S2). Changes in community mean SLA and LDMC were negligible in both fertilized and unfertilized plots (Fig. 3c, d; Table S2). Seed addition had no effect on shifts in community mean values of any trait (P > 0.2).
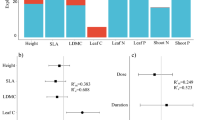
Effects of F on changes in community mean trait values in experimental quadrats. Bars are mean ± SE (n = 32 quadrats) 2010–2013. Results are shown for overall change in community mean traits (ΔComm), change due to species turnover (ΔTurn), and change due to intraspecific variation (ΔIntra). Significance of F effects are indicated; *P < 0.05, ***P < 0.001. +F Plots fertilized, −F plots not fertilized, SLA specific leaf area, LDMC leaf dry matter content
Effects of initial functional diversity (FD), measured as community-weighted trait variance, on community trait shifts in experimental quadrats: a effect of initial intraspecific FD on intraspecific shift (ΔIntra) in vegetative height; b effect of initial intraspecific FD on ΔIntra in leaf area; c effect of initial interspecific FD on trait shift due to species turnover (ΔTurn) in LDMC; d effect of initial interspecific FD on ΔIntra in LDMC. Significance of FD effects are indicated; *P < 0.05; **P < 0.01. For other abbreviations, see Fig. 2
Changes in community mean traits occurred almost entirely through intraspecific trait responses, whereas shifts due to species turnover were negligible (Fig. 2a–d). Fertilization had a significant positive effect on intraspecific traits shifts for height (F 1,17 = 23.4; P < 0.001; Fig. 2a), leaf area (F 1,17 = 18.5; P < 0.001; Fig. 2b), and SLA (F 1,17 = 4.7; P = 0.04; Fig. 2c). There was no effect of fertilization on species turnover responses for any trait except SLA, for which fertilization had a marginally negative effect (F 1,17 = 3.3; P = 0.08; Fig. 2c).
Overall shifts in community mean trait values and the contributions of species turnover and intraspecific responses were influenced by initial interspecific and intraspecific FD and seed addition and their interactions with fertilization in several cases (Fig. 3). For intraspecific responses in vegetative height, there was a significant negative interaction between initial interspecific FD and fertilization (F 1,17 = 9.1; P = 0.008) and a significant positive interaction between intraspecific FD and fertilization (F 1,17 = 6.3; P = 0.02; Fig. 3a). This indicates that intraspecific height responses to fertilization were strongest in communities that had high intraspecific variation in height at the start of the experiment and suppressed in communities with high initial interspecific variation. Intraspecific FD also had a significant positive effect on intraspecific shifts in leaf area (F 1,17 = 4.7; P = 0.04; Fig. 3b). For shifts in community mean SLA, although the main effects of fertilization and seed addition were not significant, there was a significant negative fertilization by seed addition interaction (F 1,17 = 6.2; P = 0.02), reflecting a weak positive effect of fertilization on SLA in the absence of seed addition and a negative effect with seed addition. For LDMC, initial interspecific FD had a positive effect on trait shifts due to species turnover (F 1,17 = 7.4; P = 0.01; Fig. 3c) and a negative effect on intraspecific trait shifts (F 1,17 = 11.8; P = 0.003; Fig. 3d). Communities with high initial interspecific FD in LDMC had positive shifts in LDMC due to species turnover and negative shifts due to intraspecific responses.
Across all treatments, there were significant negative relationships between species turnover and intraspecific trait shifts for SLA (R 2 = 0.21; P = 0.01; Fig. 4c) and LDMC (R 2 = 0.26; P = 0.003; Fig. 4d) but no relationships for vegetative height or leaf area (Fig. 4a, b).
Relationship between trait shifts due to species turnover and intraspecific variation within experimental quadrats for a vegetative height, b leaf area, c specific leaf area, and d LDMC. Dotted line represents the 1:1 line. Major axis regression lines and statistics are shown for statistically significant relationships (α = 0.05). For abbreviations, see Fig. 2
Single species trait responses to fertilization
We examined intraspecific trait responses to fertilization for the 13 study species that occurred in at least three fertilized plots in both 2010 and 2013 (Table S3). Of these 13 species, 12 increased in height in response to fertilization (mean increase of 18.6 cm); 11 species increased in leaf area (mean increase of 1.06 cm2); 11 species increased in SLA (mean increase of 1.8 mm2 mg−1); and nine species decreased in LDMC (mean decrease of 17.2 mg g−1). The strength of intraspecific responses of SLA and LDMC to fertilization increased with decreasing species mean vegetative height (Table 1). In contrast, leaf area responses were strongest for tall species and those with low initial mean and variance in leaf area (Table 1). The strength of intraspecific trait responses was not significantly related to species frequency or mean cover for any trait.
Discussion
Community trait shifts in response to 3 years of fertilization were mainly driven by intraspecific variation. Significant intraspecific responses to fertilization occurred in three of the four traits examined. Vegetative height, leaf area, and SLA increased within species in response to increasing nutrient availability, reflecting a shift toward a strategy of rapid resource uptake and growth (Wright et al. 2004; Reich 2014). Previous studies have also found that intraspecific variation plays a large role in community responses to resource availability. In a longer-term (10 years) fertilization experiment in grasslands in the Czech Republic, Lepš et al. (2011) found that variation in community mean height, SLA, LDMC, and other leaf traits was mostly caused by intraspecific variation. Similarly, Jung et al. (2014) found that intraspecific variation contributed more than species turnover to changes in community mean trait values in subalpine meadows in response to simulated drought. Together with the findings of the present study, these results highlight the importance of accounting for intraspecific trait variation when quantifying community functional responses to environmental change.
This study is the first to our knowledge to examine how community FD mediates responses of community mean trait values to an environmental change. By analogy with models of evolutionary trait changes within populations (Lush 1937; Falconer 1960), we predicted that the magnitude of community-level trait changes would be positively related to the amount of within-community trait variation. In general, our results offer limited support for this hypothesis. Initial FD did not influence overall changes in community mean trait values in response to fertilization for any trait. However, intraspecific changes in vegetative height and leaf area were strongest in communities with high initial intraspecific trait variance, indicating that communities with large reservoirs of intraspecific FD, arising from phenotypic plasticity and heritable genetic variation, have greater capacity to respond to environmental change through intraspecific trait shifts. Interestingly, intraspecific shifts in height were suppressed in communities with high interspecific FD. This finding suggests that when community trait space is occupied by species with different mean trait values, there may be few openings for individual species to fill new regions of trait space through intraspecific trait shifts.
A major aim of this study was to test whether community functional composition and responses to fertilization were influenced by seed availability. Previous studies in grassland communities have found that seed addition enhances species diversity and species sorting along environmental gradients (e.g., Questad and Foster 2008; Houseman and Gross 2011; Foster et al. 2011), demonstrating that dispersal plays an important role in community assembly and responses to the environment. In the present study, seed addition had a modest positive effect on species richness and increased the strength of shifts in species composition in response to fertilization but had almost no effect on shifts in community mean traits. The only exception was a negative interaction between seed addition and fertilization on community mean SLA, likely due to increasing cover of competitive, low-SLA species (e.g., B. inermis) added to fertilized plots, which overwhelmed the positive response of SLA to fertilization in plots without seed addition. These results are consistent with previous findings that stochastic processes such as dispersal have a stronger influence on species composition than functional composition (Fukami et al. 2005; Swenson et al. 2012; Siefert et al. 2013). Dispersal limitation may prevent particular species with suitable trait values from establishing in a community, but this will only have a strong influence on community functional composition if no other species with suitable trait values are available. The high initial FD and strong intraspecific responses observed in this experiment suggest that communities could track environmental changes without immigration from the local species pool. Dispersal is likely to have a more important influence on community responses to strong, long-term environmental shifts that favor trait values or trait combinations outside the range of values possessed by resident species (Ackerly 2003; Smith et al. 2009). In addition, seedling establishment may be limited in undisturbed perennial grasslands such as our study site (Hartnett and Bazzaz 1985), further limiting the impact of dispersal. Dispersal and species sorting likely play a larger role in disturbed communities that offer more opportunities for establishment of new species.
Previous studies examining intraspecific trait variation along environmental gradients have found that trait responses are highly idiosyncratic among species (e.g., Albert et al. 2010; Kichenin et al. 2013). Intraspecific trait responses also varied among species in this study, but some of this variation could be explained by species’ functional characteristics. In particular, shifts in SLA and LDMC in response to fertilization were strongest for species of short stature, consistent with patterns observed across natural soil resource availability gradients within the study site (Siefert 2012). These patterns may be explained if intraspecific shifts in SLA and LDMC are driven by decreasing light availability in the understory of fertilized plots due increased plant growth. Plants are known to respond to shading by increasing SLA and decreasing LDMC to maximize light capture per unit of leaf mass (Evans and Poorter 2001), and these responses are likely to be strongest in relatively short species, which are most subject to shading (Rozendaal et al. 2006). Leaf area showed the opposite pattern: intraspecific responses to fertilization were greater for tall species. Increases in leaf area are likely part of a general growth response to fertilization, which may be stronger for tall species that can take advantage of increased soil resource availability because they have greater access to light (Chapin et al. 1987). This finding is consistent with the dominant plasticity hypothesis, which predicts that competitive species have strong phenotypic plasticity to maximize resource capture and competitive ability (Ashton et al. 2010). Overall, the results of this experiment demonstrate that the strength of intraspecific trait responses may be partially explained by species’ functional characteristics, but these relationships appear to be trait specific.
There are several possible explanations for the weak contributions of species turnover to community traits shifts. First, the 3-year duration of the experiment may not have been long enough for large changes in species composition to occur. This explanation may be partly discounted because fertilization did lead to significant shifts in species composition, and previous fertilization experiments in old fields have also seen significant changes in composition even within a single year (Mellinger and McNaughton 1975; Bakelaar and Odum 1978). Nevertheless, while short-term community trait responses to environmental manipulations have been shown to be driven primarily by intraspecific variation in this and previous experiments, shifts in community composition are expected to play a more important role over longer time scales (Smith et al. 2009; Sandel et al. 2010). Second, although there were significant changes in the relative cover of species in response to fertilization, these changes were not explained by species’ trait values. Species that had the most positive responses to fertilization included grasses such as P. pratensis, Dactylis glomerata, and B. inermis, and species with negative responses included forbs such as Solidago juncea, Euthamia graminifolia, and Trifolium repens. Aside from the signal of growth form, there were no consistent differences in the measured traits between species that increased or decreased in response to fertilization, indicating that other traits or trait combinations mediated these responses. Finally, functional trade-offs among species generate multiple strategies with similar fitness in a given environment (Marks and Lechowicz 2006; Reich 2014), leading to high interspecific trait variance within communities and blurring interspecific trait-environment relationships (Marks 2007). In contrast, multivariate trait combinations are likely more constrained within species, possibly allowing a stronger signal of environment on single traits at the intraspecific level.
The contribution of interspecific responses to community trait shifts was further diluted by the negative relationship with intraspecific responses, particularly for SLA. A marginally significant negative interspecific response to fertilization was cancelled out by a positive response due to intraspecific variation, resulting in no overall trait response at the community level. Opposing trait shifts due to species turnover and intraspecific responses have been observed in previous studies, but the causes of these patterns appear to be context dependent (Lepš et al. 2011; Kichenin et al. 2013; Jung et al. 2014). The opposing changes in SLA observed in this study may be related to increasing aboveground competition for light in fertilized plots. The negative species turnover response was driven mainly by increasing cover of tall, competitive, relatively low-SLA grasses (e.g., B. inermis) and decreasing cover of small, subordinate, high-SLA forbs (e.g., T. repens), whereas the positive intraspecific shifts were likely driven by phenotypic plasticity of shaded individuals to increase light capture. This result is consistent with the idea that plastic responses that maximize short-term resource acquisition may oppose selection for trait values that maximize long-term competitive ability (Ryser and Eek 2000).
Implications
The results of this study demonstrate that intraspecific trait shifts may play a key role in community functional responses to environmental change. There is growing recognition that community functional composition exerts a strong influence on ecosystem processes (Diaz and Cabido 2001; Lavorel and Garnier 2002), but previous studies aimed at linking changes in community trait composition with changes in ecosystem function have focused exclusively on trait responses arising from species turnover (Garnier et al. 2004; Suding et al. 2008). In this experiment, fertilization and removal of dispersal barriers via seed addition caused changes in species composition, but these changes did not translate to directional shifts in community mean traits. These findings suggest that intraspecific trait shifts may track changing environmental conditions more reliably than trait shifts due to species turnover, highlighting the importance of accounting for intraspecific variation when quantifying and predicting community and ecosystem responses to environmental change.
The relative importance of intraspecific trait variation in community functional responses to the environment may depend on a number of factors, including the speed and magnitude of environmental change, the extent and sources of trait variation within local communities, and the FD of species and genotypes in the regional pool and their ability to disperse to and become established in a given community. In this study, intraspecific trait variation, likely reflecting rapid plastic trait responses, drove community trait shifts in response to a short-term environmental change. Moreover, we demonstrate for the first time that the capacity for such shifts may be greatest in communities with high initial intraspecific FD. Species turnover and evolutionary responses are likely to become more important in responses to long-term environmental changes that select for trait values outside the range of plasticity of resident genotypes. While dispersal limitation did not influence functional trait shifts in the perennial grassland communities examined in this study, we expect that dispersal and establishment of new species and genotypes play more important roles in communities of shorter-lived plants with more opportunities for seedling establishment. Future experiments manipulating the extent and sources of trait variation within local communities and in regional species and genotype pools will help further disentangle the effects of FD and dispersal on community functional responses to environmental change.
References
Ackerly DD (2003) Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci 164:S165–S184
Ackerly DD, Knight CA, Weiss SB et al (2002) Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia 130:449–457
Albert CH, Thuiller W, Yoccoz NG et al (2010) A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Funct Ecol 24:1192–1201
Ashton IW, Miller AE, Bowman WD, Suding KN (2010) Niche complementarity due to plasticity in resource use: plant partitioning of chemical N forms. Ecology 91:3252–3260
Bakelaar RG, Odum EP (1978) Community and population level responses to fertilization in an old-field ecosystem. Ecology 59:660–665
Chapin FI, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. Bioscience 37:49–57
Cornwell WK, Ackerly DD (2009) Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol Monogr 79:109–126
Diaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Dickson TL, Foster BL (2008) The relative importance of the species pool, productivity and disturbance in regulating grassland plant species richness: a field experiment. J Ecol 96:937–946
Donoghue M (2008) A phylogenetic perspective on the distribution of plant diversity. Proc Natl Acad Sci USA 105:11549–11555
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24:755–767
Falconer DS (1960) Introduction to quantitative genetics. Ronald Press, New York
Fonseca CCR, Overton J, Collins B, Westoby M (2000) Shifts in trait-combinations along rainfall and phosphorus gradients. J Ecol 88:964–977
Foster BL, Questad EJ, Collins CD et al (2011) Seed availability constrains plant species sorting along a soil fertility gradient. J Ecol 99:473–481
Fukami T, Bezemer TM, Mortimor SR, van der Putten WH (2005) Species divergence and trait convergence in experimental plant community assembly. Ecol Lett 8:1283–1290
Garnier E, Cortez J, Billès G et al (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85:2630–2637
Garnier E, Laurent G, Debain S et al (2001a) Consistency of species ranking based on functional leaf traits. New Phytol 152:69–83
Garnier E, Shipley B, Roumet C, Laurent G (2001b) A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct Ecol 15:688–695
Gaudet CL, Keddy PA (1988) A comparative approach to predicting competitive ability from plant traits. Nature 334:242–243
Grime JP, Fridley JD, Askew AP et al (2008) Long-term resistance to simulated climate change in an infertile grassland. Proc Natl Acad Sci USA 105:10028–10032
Hartnett DC, Bazzaz FA (1985) The genet and ramet population dynamics of Solidago canadensis in an abandoned field. J Ecol 73:407–413
Houseman GR, Gross KL (2011) Linking grassland plant diversity to species pools, sorting and plant traits. J Ecol 99:464–472
Hulshof CM, Swenson NG (2010) Variation in leaf functional trait values within and across individuals and species: an example from a Costa Rican dry forest. Funct Ecol 24:217–223
Jakobsson A, Eriksson O (2000) A comparative study of seed number, seed size, seedling size and recruitment in grassland plants. Oikos 88:494–502
Jung V, Albert CH, Violle C et al (2014) Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. J Ecol 102:45–53
Jung V, Violle C, Mondy C et al (2010) Intraspecific variability and trait-based community assembly. J Ecol 98:1134–1140
Keddy P (1992) Assembly and response rules: two goals for predictive community ecology. J Veg Sci 3:157–164
Kichenin E, Wardle DA, Peltzer DA et al (2013) Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct Ecol 27:1254–1261
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16:545–556
Legendre P (2013) lmodel2: model II regression. R package version 1.7-1
Leibold MA, Holyoak M, Mouquet N et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Lepš J, de Bello F, Šmilauer P, Doležal J (2011) Community trait response to environment: disentangling species turnover vs intraspecific trait variability effects. Ecography 34:856–863
Lepš J, de Bello F, Lavorel S, Berman S (2006) Quantifying and interpreting functional diversity of natural communities: practical considerations matter. Preslia 78:481–501
Lush J (1937) Animal breeding plans. Iowa State College Press, Ames
Marks CO, Lechowicz MJ (2006) Alternative designs and the evolution of functional diversity. Am Nat 167:55–66
Marks CO (2007) The causes of variation in tree seedling traits: the roles of environmental selection versus chance. Evolution 61:455–469
McGill BJ, Enquist BJ, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends Ecol Evol 21:178–185
Mellinger MV, McNaughton S (1975) Structure and function of successional vascular plant communities in central New York. Ecol Monogr 45:161–182
Messier J, McGill BJ, Lechowicz MJ (2010) How do traits vary across ecological scales? A case for trait-based ecology. Ecol Lett 13:838–848
Myers JA, Harms KE (2009) Seed arrival, ecological filters, and plant species richness: a meta-analysis. Ecol Lett 12:1250–1260
Oksanen J, Blanchet FG, Kindt R et al (2012) Vegan: community ecology package. R package version 2.0-4
Peet RK, Wentworth TR, White PS (1998) A flexible, multipurpose method for recording vegetation composition and structure. Castanea 63:262–274
Perez-Harguindeguy N, Diaz S, Garnier E et al (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234
Pérez-Ramos I, Roumet C, Cruz P et al (2012) Evidence for a “plant community economics spectrum” driven by nutrient and water limitations in a Mediterranean rangeland of southern France. J Ecol 100:1315–1327
Questad EJ, Foster BL (2008) Coexistence through spatio-temporal heterogeneity and species sorting in grassland plant communities. Ecol Lett 11:717–726
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Reich PB (2014) The world-wide “fast-slow” plant economics spectrum: a traits manifesto. J Ecol 102:275–301
Rozendaal DMA, Hurtado VH, Poorter L (2006) Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct Ecol 20:207–216
Ryser P, Eek L (2000) Consequences of phenotypic plasticity vs. interspecific differences in leaf and root traits for acquisition of aboveground and belowground resources. Am J Bot 87:402–411
Sandel B, Goldstein LJ, Kraft NJB et al (2010) Contrasting trait responses in plant communities to experimental and geographic variation in precipitation. New Phytol 188:565–575
Shipley B, Vile D, Garnier E (2006) From plant traits to plant communities: a statistical mechanistic approach to biodiversity. Science 314:812–814
Siefert A (2012) Spatial patterns of functional divergence in old-field plant communities. Oikos 121:907–914
Siefert A, Ravenscroft C, Weiser MD, Swenson NG (2013) Functional beta-diversity patterns reveal deterministic community assembly processes in eastern North American trees. Glob Ecol Biogeogr 22:682–691
Smith M, Knapp A, Collins S (2009) A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90:3279–3289
Suding KN, Lavorel S, Chapin FI et al (2008) Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob Chang Biol 14:1125–1140
Swenson NG, Anglada-Cordero P, Barone JA (2011) Deterministic tropical tree community turnover: evidence from patterns of functional beta diversity along an elevational gradient. Proc R Soc B 278:877–884
Swenson N, Erickson D, Mi X (2012) Phylogenetic and functional alpha and beta diversity in temperate and tropical tree communities. Ecology 93:112–125
Tilman D (1987) Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecol Monogr 57:189–214
Weiher E, Keddy PA (1995) Assembly rules, null models, and trait dispersion: new questions from old patterns. Oikos 74:159–164
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Acknowledgments
We thank Maureen Carey for assisting with functional trait measurements and Tom Hughes for providing access to field sites. Jason Fridley provided valuable comments on an earlier version of the manuscript. This research was supported by the National Science Foundation Graduate Research Fellowship (DGE-1247399) and NSF grant DEB-03089. The experiments comply with the current laws of the country (USA) in which the experiments were performed.
Author contribution statement
A. S. and M. E. R. conceived and designed the experiment. A. S. performed the experiment, analyzed the data, and wrote the manuscript. M. E. R. provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Katherine L. Gross.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Siefert, A., Ritchie, M.E. Intraspecific trait variation drives functional responses of old-field plant communities to nutrient enrichment. Oecologia 181, 245–255 (2016). https://doi.org/10.1007/s00442-016-3563-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3563-z