Abstract
The protective effect of polyamines against Cd toxicity of rice (Oryza sativa) leaves was investigated. Cd toxicity to rice leaves was determined by the decrease in protein content. CdCl2 treatment results in (1) increased Cd content, (2) induction of Cd toxicity, (3) increase in H2O2 and malondialdehyde (MDA) contents, (4) decrease in ascorbic acid (ASC) and reduced glutathione (GSH) contents, and (5) increase in the activities of antioxidative enzymes (superoxide dismutase, glutathione reductase, ascorbate peroxidase, catalase, and peroxidase). Spermidine (Spd) and spermine (Spm), but not putrescine (Put), were effective in reducing CdCl2-induced toxicity. Spd and Spm prevented CdCl2-induced increase in the contents of H2O2 and MDA, decrease in the contents of ASC and GSH, and increase in the activities of antioxidative enzymes. Spd and Spm pretreatments resulted in a decrease in Cd content when compared with H2O pretreatment, indicating that Spd and Spm may reduce the uptake of Cd. Results of the present study suggest that Spd and Spm are able to protect Cd-induced oxidative damage and this protection is most likely related to the avoidance of H2O2 generation and the reduction of Cd uptake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd), a heavy metal toxic to humans, animals, and plants, is a widespread pollutant with a long biological half-life (Wagner 1993). Cd is readily taken up by plants, leading to toxic symptoms such as growth reduction (Chen and Kao 1995). Cd damages the photosynthetic apparatus (Krupa 1988; Siedlecka and Baszynski 1993), lowers chlorophyll (Stobart et al. 1985; Larsson et al. 1998), and alters proline and polyamine contents (Sharma and Dietz 2006).
Oxygen is essential for the existence of aerobic life, but toxic reactive oxygen species (ROS), which include the superoxide anion O2 −, hydroxyl radical (OH•) and hydrogen peroxide (H2O2), are generated in all aerobic cells during metabolic processes (Asada 1999; Foyer et al. 1994, 1997). Initially, ROS were only regarded as damaging to cells (Apel and Hirt 2004). More recently, ROS emerged as ubiquitous signaling molecules participating in the recognition of and the response to stress factors (Foyer and Noctor 2005).
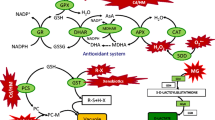
Injury caused by these ROS, known as oxidative stress, is one of the major damaging factors in plants exposed to environmental stress. Plants cope with oxidative stress by using antioxidative enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), peroxidase (POX), catalase (CAT), and the low molecular weight antioxidants, ascorbic acid (ASC) and glutathione (GSH) (Asada 1999; Noctor and Foyer 1998). Three lines of evidence indicate that one mechanism of Cd toxicity is related to oxidative stress in plant cells. First, Cd can promote the generation of ROS (Kuo and Kao 2004; Olmos et al. 2003; Piqueras et al. 1999; Romero-Puertas et al. 2003, 2004; Sandalio et al. 2001; Schützendübel et al. 2001; Shah et al. 2001). Second, Cd can inhibit or stimulate the activities of antioxidant enzymes (Chaoui et al. 1997; Dixit et al. 2001; Gallego et al. 1996; Innelli et al. 2002; Kuo and Kao 2004; León et al. 2002; Shah et al. 2001; Shaw 1995). Third, treatment with Cd results in cellular oxidative damage or lipid peroxidation (Chaoui et al. 1997; Chien et al. 2002; Dixit et al. 2001; Gallego et al. 1996; Kuo and Kao 2004; Lozano-Rodríguez et al. 1997; Shah et al. 2001; Shaw 1995).
The polyamines putrescine (Put), spermidine (Spd), and spermine (Spm) are polycationic cellular molecules and are present in all living organisms. Experimental evidence now indicates that polyamines are involved in a number of cellular and molecular processes in plants (Bouchereau et al. 1999; Wallace et al. 2003). The levels of polyamines in plants are altered in response to heavy metals (Sharma and Dietz 2006). Weinstein et al. (1986) showed an up to 10-fold increase in Put content with a marginal rise in Spd and Spm contents in Cd-treated oat seedlings and detached oat leaves. Similar results were obtained in Cd-treated detached rice leaves (Hou and Kao 1993). It has been shown that polyamines are able to protect against oxidative damage caused by paraquat (Benavides et al. 2000; Chang and Kao 1997; Kurepa et al. 1998; Minton et al. 1990), acid rain (Velikova et al. 2000) and heavy metals such as Cd and Cu (Groppa et al. 2001). Borrell et al. (1997) demonstrated that polyamines inhibited lipid peroxidation in senescing oat leaves. Evidence has been provided to show that polyamines are effective radical scavengers in a number of chemical and in vitro enzyme systems (Drolet et al. 1986) and that the reduction in polyamine content in leaves of Glycyrrhiza inflata under osmotic stress promoted the increase in the production of ROS (Li and Wang 2004). A close interrelationship between polyamines and oxidative stress was documented by the finding that leaf necrosis caused by ozone in tomato plants could be suppressed by an exogenous supply of polyamines (Ormrod and Beckerson 1986). However, Bors et al. (1989) claimed that the scavenging of radicals by polyamines cannot explain the protection against ozone damage observed after exogenous application. Recently, Tang et al. (2004) demonstrated that exogenously added polyamines recover browning tissues into normal callus cultures of Virginia pine by decreasing oxidative damage. In the present study, we investigated the effect of polyamines on Cd toxicity of rice leaves, and we observed that oxidative damage caused by CdCl2 is reduced by Spd and Spm.
Materials and methods
Plant material
Rice (Oryza sativa L., cv. Taichung Native 1) seeds were sterilized with 2.5% sodium hypochlorite for 15 min and washed extensively seeds with distilled water. These seeds were then germinated in Petri dishes with wetted filter paper at 37°C under dark conditions. After 48 h incubation, uniformly germinated seeds were selected and cultivated in a 500 ml beaker containing half-strength Kimura B solution as described previously (Hsu and Kao 2005). The hydroponically cultivated seedlings were grown for 12 days in a Phytotron (Agricultural Experimental station, National Taiwan University, Taipei, Taiwan) with natural sunlight at 30°C day/25°C night and 90% relative humidity. The apical 3 cm of the third leaf was used in all experiments. Detached rice leaves were pretreated with distilled water or polyamines for 6 h at 27°C in darkness and then transferred to distilled water or 5 mM CdCl2 for 4, 8, 12, and 18 h at 27°C in the light (40 μmol m−2 s−1).
Determination of protein, H2O2, lipid peroxidation, GSH, oxidized glutathione (GSSG), ASC, dehydroascorbate (DHA), and Cd
For protein determination, leaf segments were homogenized in a 50 mM sodium phosphate buffer (pH 6.8). The extracts were centrifuged at 17,600 × g for 20 min, and the supernatants were used for determination of protein by the method of Bradford (1976) and antioxidative enzyme activities. The H2O2 content was measured colorimetrically as described by Jana and Choudhuri (1982). H2O2 was extracted by homogenizing leaf tissue with phosphate buffer (50 mM, pH 6.5) containing 1 mM hydroxylamine. The homogenate was centrifuged at 6,000 × g for 25 min. To determine H2O2 content, the extracted solution was mixed with 0.1% titanium chloride in 20% (v/v) H2SO4. The mixture was then centrifuged at 6,000 × g for 15 min. The absorbance was measured at 410 nm. Using this method, we obtained that absorbance increased linearly with the amount of H2O2 and addition of H2O2 to leaf extracts resulted in the predicted increase of absorbance, i.e. added H2O2 was fully recovered (data not shown). The H2O2 content in leaf extracts was calculated using the extinction coefficient of 0.28 μmol−1 cm−1. In some experiments, H2O2 was also visually detected in the leaves by using 3,3-diaminobenzidine (DAB) as substrate (Orozco-Cárdenas and Ryan 1999). Detached rice leaves were supplied through the cut ends with DAB (1 mg ml−1) solution for 24 h under light at 27°C. Leaves were them decolorized in boiling ethanol (95%) for 0.5 h. This treatment decolorized the leaves except for the brown polymerization product produced by DAB with H2O2. After cooling, the leaves were extracted at room temperature with fresh ethanol. The H2O2 staining was repeated four times with similar results.
MDA, routinely used as an indicator of lipid peroxidation, was extracted with 5% (w/v) trichloroacetic acid and determined by the thioburbituric acid reaction as described by Heath and Packer (1968). GSH and GSSG in 3% sulfosalicylic acid extract and ASC and DHA in 5% (w/v) trichloracetic acid extract were determined as described previously (Hsu and Kao 2005). For determination of Cd, leaves were dried at 65°C for 48 h and the dried material ashed at 550°C for 4 days. The ash residue was incubated with 31% HNO3 and 17.5% H2O2 at 72°C for 2 h, and dissolved in distilled water. Cd was then quantified using an atomic absorption spectrophotometer (Model AA-6800, Shimadzu, Kyoto, Japan).
Polyamine determination
Leaf tissues were homogenized with 5 ml of 5% (w/v) perchloric acid. Polyamine contents were determined using high performance liquid chromatography (Waters 484, Milford, USA) after benzoylation as described previously (Chen and Kao 1991).
Enzyme extraction and assays
For extraction of enzymes, leaf tissues were homogenized with 0.1 M sodium phosphate buffer (pH 6.8) in a chilled pestle and mortar. For analysis of APX activity, 2 mM ASC was added to the extraction buffer. The homogenate was centrifuged at 12,000 × g for 20 min and the resulting supernatant was used for determination of enzyme activity. The whole extraction procedure was carried out at 4°C. SOD was determined according to Paoletti et al. (1986). One unit of SOD was defined as the amount of enzyme that inhibits by 50% the rate of NADH oxidation observed in blank sample. POX activity was measured using a modification of the procedure of MacAdam et al. (1992). The activity was calculated using the extinction coefficient (26.2 mM−1 cm−1 at 470 nm) for tetraguaiacol. One unit of POX was defined as the amount of enzyme that caused the formation of 1 μmol tetraguaiacol per min. CAT activity was assayed according to Kato and Shimizu (1987). One unit of CAT was defined as the amount of enzyme which degraded 1 μmol H2O2 per min. APX activity was determined according to Nakano and Asda (1981). One unit of activity for APX was defined as the amount of enzyme that degraded 1 μmol of ASC per min. GR was determined by the method of Foster and Hess (1980). One unit of GR was defined as the amount of enzyme that decreased 1 A340 per min.
Statistical analysis
Statistical differences between measurements (n = 4) on different treatments or on different times were analyzed following the Duncan’s multiple range test or Student’s t-test.
Results
Cd promotes protein loss
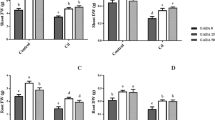
In plants, the most general symptom of Cd toxicity is chlorosis (Das et al. 1997). In rice, we have shown that detached leaves and seedlings treated with CdCl2 show chlorosis and protein loss (Chien and Kao 2000; Hsu and Kao 2003, 2005). In the present study, Cd toxicity in detached rice leaves caused by excess Cd was assessed by a decrease in protein content. Increasing concentration of CdCl2 from 0.1 to 5 mM progressively decreased protein content in detached rice leaves in the light and no further decrease was observed at 10 mM CdCl2 (data not shown). Thus, 5 mM CdCl2 was used in the present investigation. The promotion of the loss of protein by CdCl2 was evident 8 h after treatment (Fig. 1A). Cd concentration in the control leaves remained unchanged during 18 h of incubation (Fig. 1C). However, Cd concentration in CdCl2-treated leaves increased with increasing duration of incubation (Fig. 1C). The increase in Cd concentration in CdCl2-treated leaves was evident 4 h after treatment (Fig. 1C).
Changes in the contents of protein (A), MDA (B), and Cd (C) in rice leaves treated with CdCl2. Detached rice leaves were pretreated with H2O for 6 h in the dark and then treated with H2O or 5 mM CdCl2 for 4, 8, 12, and 18 h in the light. * and ** represent values that are significantly different between H2O and CdCl2 treatment at P < 0.05 and P < 0.01, respectively
Cd induces oxidative stress
MDA content in CdCl2-treated detached rice leaves was observed to be greater than that in water-treated controls at 8 h after treatment (Fig. 1B). This showed that Cd toxicity in detached rice leaves was linked to lipid peroxidation. Lipid peroxidation is caused by ROS (Thompson et al. 1987). CdCl2 treatment also caused an increase in H2O2 content (Fig. 2A). To verify in situ the increase in H2O2 in leaves treated with CdCl2, a histochemical method with DAB that is based on the formation by H2O2 of brown polymerization product was used. The development of DAB-H2O2 reaction product in H2O- and CdCl2-treated leaves is shown in Fig. 3. It is clear that the DAB-H2O2 reaction product was observed after H2O2 and CdCl2 treatments. All these results support the involvement of ROS as the chemical species inducing Cd toxicity in rice leaves.
Changes in the contents of H2O2 (A) and the activities of SOD (B), GR (C), APX (D), CAT (E), and POX (F) in rice leaves treated with CdCl2. Detached rice leaves were pretreated with H2O for 6 h in the dark and then treated with H2O or 5 mM CdCl2 for 4, 8, 12, and 18 h in the light. * and ** represent values that are significantly different between H2O and CdCl2 treatment at P < 0.05 and P < 0.01, respectively
Histochemical detection of H2O2 with DAB staining in rice leaves. Detached leaves were pretreated with H2O, Spd, and Spm, respectively, for 6 h in the dark, and then treated with either H2O, H2O2, or CdCl2 for 18 h in the light. The concentrations of Spd, Spm, H2O2, and CdCl2 were 5, 5, 1, and 5 mM, respectively
CdCl2-treated rice leaves had higher activities of SOD, GR, APX, and CAT than the controls at 4 h after treatment (Figs. 2B–E). Higher activities of POX were observed at 8 h after treatment (Fig. 2F). GSH, GSSG, and ASC contents were observed to be lower than the controls at 4 h after treatment (Figs. 4A–C). However, DHA content in Cd-treated leaves was observed to be higher than the contents at 18 h after treatment (Fig. 4D). The increased activities of antioxidative enzymes and the decreased contents of ASC and GSH in response to CdCl2 are further suggestive of strong induction of oxidative stress.
Changes in contents of GSH (A) and ASC (B) in rice leaves treated with CdCl2. Detached rice leaves were pretreated with H2O for 6 h in the dark and then treated with H2O or 5 mM CdCl2 for 4, 8, 12, and 18 h in the light. * and ** represent values that are significantly different between H2O and CdCl2 treatment at P < 0.05 and P < 0.01, respectively
Spd and Spm reduce Cd-induced oxidative damage
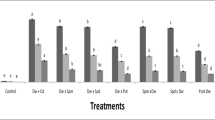
To test if polyamines could reduce the toxicity caused by CdCl2, as judged by the changes in protein levels, detached rice leaves were pretreated with either water or polyamines for 6 h in the dark and then transferred to either water or CdCl2 for 18 h in the light. Spd and Spm, but not Put, pretreatments reduced Cd toxicity (Fig. 5). We also observed that Spd and Spm were effective in reducing Cd-induced lipid peroxidation (Fig. 6A) and H2O2 production (Figs. 3, 6B), Cd-increased antioxidative enzyme activities (Fig. 7), and Cd-decreased ASC and GSH contents (Fig. 8). Furthermore, detached rice leaves pretreated with Spd or Spm for 6 h in the dark had higher endogenous levels of Spd and Spm, and Spm, respectively, than those pretreated with water (Table 1). However, Put pretreatment had no effect on endogenous levels of Spd and Spm (Table 1).
Effect of pretreatments with polyamines on the content of protein in detached rice leaves in the presence or absence of CdCl2. Detached rice leaves were pretreated with H2O, 5 mM Put, 5 mM Spd, and 5 mM Spm, respectively, for 6 h in the dark and then treated with H2O or 5 mM CdCl2 for 18 h in the light. Values with the same letter are not significantly different at P < 0.05
Effect of pretreatments with Spd and Spm on the contents of MDA (A) and H2O2 (B) in detached rice leaves in the presence or absence of CdCl2. Detached rice leaves were pretreated with H2O, 5 mM Spd, and 5 mM Spm, respectively, for 6 h in the dark and then treated with H2O or 5 mM CdCl2 for 18 h in the light. Values with the same letter are not significantly different at P < 0.05
Effect of pretreatments with Spd and Spm on the activities of antioxidative enzymes [SOD (A), GR (B), APX (C), CAT (D), and POX (E) in detached rice leaves in the presence or absence of CdCl2. Detached rice leaves were pretreated with H2O, 5 mM Spd, and 5 mM Spm, respectively, for 6 h in the dark and then treated with H2O or 5 mM CdCl2 for 18 h in the light. Values with the same letter are not significantly different at P < 0.05
Effect of pretreatments with Spd and Spm on the contents of ASC (A) and GSH (B) in detached rice leaves in the presence or absence of CdCl2. Detached rice leaves were pretreated with H2O, 5 mM Spd, and 5 mM Spm, respectively, for 6 h in the dark and then treated with H2O or 5 mM CdCl2 for 18 h in the light. Values with the same letter are not significantly different at P < 0.05
Spd and Spm inhibit the uptake of Cd
To test if endogenous Spd and Spm affect Cd uptake, Cd content in detached rice leaves pretreated with Spd or Spm followed by treatment of CdCl2 was determined. It was observed that Spd and Spm pretreatments resulted in a decrease (about 27%) in Cd content when compared with H2O pretreatment (Fig. 9).
Effect of pretreatments with Spd and Spm on the content of Cd in detached rice leaves in the presence or absence of CdCl2. Detached rice leaves were pretreated with H2O, 5 mM Spd, and 5 mM Spm, respectively, for 6 h in the dark and then treated with H2O or 5 mM CdCl2 for 18 h in the light. Values with the same letter are not significantly different at P < 0.05
Discussion
It has been shown that Cd increased ethylene production in detached rice leaves (Hou and Kao 1993). Here, we show that Cd induced H2O2 production in rice leaves (Fig. 2A, 3). Wounding is known to induce ethylene production (Yu and Yang 1980) and H2O2 generation (Orozco-Cárdenas et al. 2001). Ethylene biosynthesis shares a common precursor with Spd and Spm, thus wounding may induce a direct modification of the synthesis of Spd and Spm. When detached rice leaves are used to study H2O2, ethylene, polyamines, and senescence, wounding is always a problem. However, in the present study, each long and narrow rice leaf was cut transversely; thus, the area of wounding was very small. Therefore, H2O2 and ethylene production of detached leaves induced by Cd is unlikely to be complicated by the wounding effect.
Cd is known to increase the production of H2O2 (Kuo and Kao 2004; Schützendübel et al. 2001; Olmos et al. 2003) and induce lipid peroxidation (Chien et al. 2002; Gallego et al. 1996; Kuo and Kao 2004). These results suggest that Cd treatment causes an oxidative stress in plants. Our results not only have shown that CdCl2 increased the content of H2O2 (Figs. 2A, 3) and the activities of SOD, APX, GR, CAT, and POX (Figs. 2B–F), but also demonstrated that caused a decrease in GSH and ASC contents (Fig. 4). Meanwhile, protein loss (Fig. 1A) and lipid peroxidation (Fig. 1B) were observed in CdCl2-treated rice leaves. All these results suggest that CdCl2 causes an oxidative stress and that CdCl2-induced toxicity in rice leaves is mediated through oxidative stress.
GSH functions as a direct antioxidant of ROS and is involved in the generation of ASC, which is utilized as a substrate for APX (Noctor and Foyer 1998). In the present study, we observed that the decrease in GSH content is one of the earliest steps in oxidative stress induced by CdCl2 in rice leaves, which occurred at 4 h after treatment (Fig. 4B). It may be suspected that the decrease in GSH may favor the accumulation of ROS in Cd-treated rice leaves. In a review, Schützendübel and Polle (2002) also suggest that the depletion of GSH is apparently a critical step in Cd toxicity.
Cd induced a significant accumulation of H2O2 in rice leaves (Figs. 2A, 3). Accumulation of H2O2 has also been observed in Cd-treated pine and pea roots, pea leaves, and tobacco cells (Olmos et al. 2003; Romero-Puertas et al. 2003; 2004; Schützendübel et al. 2001). There are reports showing that NADPH oxidase was possibly involved in Cd-induced H2O2 production in pea leaves and tobacco cells (Olmos et al. 2003; Romero-Puertas et al. 2004). Our unpublished observations indicate that diphenyleneiodonium chloride and imidazole, inhibitors of NADPH oxidase, prevented Cd-induced H2O2 production in rice leaves.
Data from the present study indicate that Cd-induced oxidative damage in rice leaves is reduced by Spd and Spm. This conclusion is based on the observations that pretreatment with Spd and Spm prevented Cd-induced loss of protein (Fig. 5), increase in the contents of MDA (Fig. 6) and H2O2 (Figs. 3, 6B), decrease in the content of ASC and GSH (Fig. 8), and increase in the activities of antioxidative enzymes (Fig. 7). Groppa et al. (2001) also demonstrated that Spm and Spd were effective in reducing Cd-caused lipid peroxidation in sunflower leaf discs. It is generally accepted that polyamines are highly protonated at physiological pH, which favors electrostatic binding of polyamines to negatively charged components of membranes, leading to membrane stabilization through ionic interactions (Slocum et al. 1984). The more pronounced protective effect of Spd and Spm could be accounted for by its longer chain and greater number of positive charges, which allows membrane stabilizing ability.
The decrease in ASC and GSH contents in rice leaves treated with CdCl2 suggests that ASC and GSH contents may be regulated by the synthesis and oxidation. GSH is the precursor of phytochelatins, cysteine-rich peptides, synthesized via phytochelatin synthase (Cobbett and Goldsbrough 2002). A severe depletion of GSH is a common response to Cd caused by an increased consumption of GSH for phytochelatin production (Schützendübel and Polle 2002). Thus, the sharp decline in GSH content in CdCl2-treated rice leaves may also be due to phytochelatin biosynthesis.
The present results indicated that Spd and Spm reduced Cd-decreased ASC and GSH contents (Fig. 8). Theses observations suggest that the capacity of Spd and Spm to scavenge H2O2 might increase in rice leaves that were pretreated with Spd or Spm followed by treatment of CdCl2 (Fig. 6B).
In considering a possible mechanism for the reduction of Cd-induced oxidative damage by polyamines, we speculated that Spd and Spm might inhibit Cd uptake from the medium. Here, we show that Cd content in detached rice leaves pretreated with Spd and Spm followed by treatment of CdCl2 was lower that those pretreated with H2O. Our findings suggest that increase in endogenous Spd or Spm may block, though slightly (27%), the uptake of Cd (Fig. 9). In the present study, pretreatment of detached rice leaves with exogenous Spd or Spm was found to reverse almost completely the Cd-induced H2O2 generation and lipid peroxidation (Fig. 6). It appears that Spd and Spm were able to protect Cd-induced oxidative damage and this protection was related to the avoidance of H2O2 generation and the reduction of Cd uptake.
Abbreviations
- APX:
-
Ascorbate peroxidase
- ASC:
-
Ascorbic aicd
- CAT:
-
Catalase
- DAB:
-
3,3′-Diaminobenzidine
- DHA:
-
Dehydroascorbate
- DW:
-
Dry weight
- FW:
-
Fresh weight
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- POX:
-
Peroxidase
- Put:
-
Putrescine
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- Spd:
-
Spermidine
- Spm:
-
Spermine
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 85:235–241
Asada K (1999) The water–water cycle in chloroplasts: scavenging of reactive oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:501–639
Benavides MP, Gallego SM, Comga MZ, Tomaro ML (2000) Relationship between polyamines and paraquat toxicity in sunflower leaf discs. Plant Growth Regul 31:215–224
Borrell A, Carbonell L, Farrás R, Puig-Paellada P, Tiburcio AF (1997) Polyamines inhibit lipid peroxidation in senescing oat leaves. Physiol Plant 99:385–390
Bors W, Langebartels C, Michel C, Sandermann H (1989) Polyamines as radical scavengers and protectants against ozone damage. Phytochemistry 28:1589–1595
Bouchereau A, Aziz A, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chang CJ, Kao CH (1997) Paraquat toxicity is reduced by polyamines in rice leaves. Plant Growth Regul 22:163–168
Chaoui A, Mazhoudi S, Ghorbal MH, Ferjani EE (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Chen CT, Kao CH (1991) Senescence of rice leaves. XXX. Levels of endogenous polyamines and dark-induced senescence of rice leaves. Plant Cell Physiol 32:935–941
Chen SL, Kao CH (1995) Cd induced changes in proline level and peroxidase activity in roots of rice seedlings. Plant Growth Regul 17:67–71
Chien HF, Kao CH (2000) Accumulation of ammonium in rice leaves in response to excess cadmium. Plant Sci 156:111–115
Chien HF, Lin CC, Wang JW, Chen CT, Kao CH (2002) Changes in ammonium ion content and glutamine synthetase activity in rice leaves caused by excess cadmium are a consequence of oxidative damage. Plant Growth Regul 36:41–47
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Das P, Sammantaray S, Rout GR (1997) Studies on cadmium toxicity: a review. Environ Pollut 98:29–36
Dixit V, Pandey V, Shyam R (2001) Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J Exp Bot 52:1101–1109
Drolet G, Dumbroff EB, Legge RL, Thompson JE (1986) Radical scavenging properties of polyamines. Phytochemistry 25:367–371
Foster JG, Hess JL (1980) Response of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66:482–487
Foyer CH, Descourvies P, Kunert KJ (1994) Protection against oxygen radicals: an important defense mechanism studies in transgenic plants. Plant Cell Environ 17:507–523
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant 100:241–254
Foyer CH, Noctor G (2005) Oxidant and antioxidant signaling in plants: re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071
Gallego SM, Benavides MP, Tomaro ML (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159
Groppa MD, Tomaro ML, Benavides MP (2001) Polyamines as protectors against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci 161:481–488
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hou SM, Kao CH (1993) Characteristics of the induction of the ethylene by cadmium in detached rice leaves. Bot Bull Acad Sin 34:163–168
Hsu YT, Kao CH (2003) Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ 26:867–874
Hsu YT, Kao CH (2005) Abscisic acid accumulation and cadmium tolerance in rice seedlings. Physiol Plant 124:71–80
Innelli MA, Pietrni R, Fiore L, Petrilli L, Massacci A (2002) Antioxidant response to cadmium in Phragmites anstrals plants. Plant Physiol Biochem 40:977–982
Jana S, Choudhuri MA (1982) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. Can J Bot 65:729–735
Krupa Z (1988) Cadmium-induced changes in the composition and structure of the light-harvesting chlorophyll a/b protein complex II in radish cotyledons. Physiol Plant 73:518–524
Kuo MC, Kao CH (2004) Antioxidant enzyme activities are upregulated in response to cadmium in sensitive, but not in tolerant rice (Oryza sativa L.) seedlings. Bot Bull Acad Sin 45:291–299
Kurepa J, Smalle J, Van Montagu M, Inzé D (1998) Polyamines and paraquat toxicity in Arabidopsis thaliana. Plant Cell Physiol 39:987–992
Larsson EH, Bordman JF, Asp H (1998) Influence of UV-B radiation and Cd2+ on chlorophyll fluorescence, growth and nutrient content in Brassica napus. J Exp Bot 49:1031–1039
León AM, Palma JM, Corpas FJ, Gomez M, Romeropuertas MC, Chatterjee D, Mateos RM, del Rio LA, Sandalio LM (2002) Antioxidative enzymes in cultivars of pepper plants with different sensitivity to cadmium. Plant Phsyiol Biochem 40:813–820
Li C-Z, Wang G-X (2004) Interaction between reactive oxygen species, ethylene and polyamines in leaves of Glycyrrhiza inflata seedlings under root osmotic stress. Plant Growth Regul 42:55–60
Lozano-Rodriguez E, Hernández LE, Bonay P, Carpena-Ruiz R (1997) Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. J Exp Bot 48:123–128
MacAdam JW, Nelson CJ, Sharpe RE (1992) Peroxidase activity in the leaf elongation zone of tall fescue. Plant Physiol 99:872–878
Minton KW, Tabor H, Tabor CW (1990) Paraquat toxicity is increased in Escherichia coli defective in the synthesis of polyamines. Proc Natl Acad Sci USA 87:2851–2855
Nakano Y, Asda K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:807–880
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Olmos EO, Martínez-Solano JR, Piqueras A, Hellín E (2003) Early steps in the oxidative burst induced by cadmium in cultured tobacco cells (BY-2 line). J Exp Bot 54:291–301
Ormrod DP, Beckerson DW (1986) Polyamines as antiozonants for tomato. HortScience 21:1070–1071
Orozco-Cárdenas ML, Ryan CA (1999) Hydrogen peroxide is generated systematically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96:6553–6557
Orozco-Cárdenas M, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13:179–191
Paoletti F, Aldinucci D, Mocali A, Capparini A (1986) A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem 154:536–541
Piqueras A, Olmos E, Martínez-Solano JR, Hellín E (1999) Cd induced oxidative burst in tobacco BY-2 cell: time-course, subcellular location and antioxidant response. Free Radical Res 31:S25–S31
Romero-Puertas MC, Rodriguez-Serrano M, Corpas FJ, Gómez M, del Rio LA, Sandalio LM (2004) Cadmium-induced subcellular accumulation of O2- and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Romero-Puertas MC, Zablza A, Rodriguez-Serrano M, Gómez M, del Rio LA, Sandalio LM (2003) Antioxidative response to cadmium in pea roots. Free Radical Res 37:44
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Rio LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plant. J Exp Bot 52:2115–2126
Schützendübel A, Polle A (2002) Plant responses to biotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1366
Schützendübel A, Schwang P, Teichmann T, Gross K, Langenfeld-Heyer R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in scots pine roots. Plant Physiol 127:887–898
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Sharma SS, Dietz K-J (2006) The significance of amino acids and amino acid-derived molecules in plant response and adaptation to heavy metal stress. J Exp Bot 57:711–726
Shaw BP (1995) Effect of mercury and cadmium on the activities of antioxidative enzymes in the seedlings of Phaseolus aureus. Biol Plant 37:587–596
Siedlecka A, Baszynski T (1993) Inhibition of electron flow around photosystem I in chloroplasts of cadmium-treated maize plants is due to cadmium-induced iron deficiency. Physiol Plant 87:199–202
Slocum RD, Kaur-Sawhney R, Galston AW (1984) The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys 235:283–303
Stobart AK, Griffiths WT, Ameen-Bukhari I, Sherwood RP (1985) The effect of Cd2+ on the biosynthesis of chlorophyll of barley. Physiol Plant 63:293–298
Tang W, Newton RJ, Outhavong V (2004) Exogenously added polyamines recover browning tissues into normal callus cultures and improve plant regeneration in pine. Physiol Plant 122:386–395
Thompson JE, Legge RL, Barber RF (1987) The role of free radicals in senescence and wounding. New Phytol 105:317–344
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci 151:59–66
Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron 5:173–212
Wallace HM, Fraser AV, Hughes A (2003) A perspective of polyamines metabolism. Biochem J 376:1–14
Weinstein LH, Kaur-Sawhney R, Rajam MV, Wettlaufer SH, Galston AW (1986) Cadmium-induced accumulation of putrescine in oat and bean leaves. Plant Physiol 82:641–645
Yu YB, Yang SF (1980) Biosynthesis of wound ethylene. Plant Physiol 66:281–285
Acknowledgements
This work was supported by a research grant from the National Science Council of the Republic of China (NSC 95-2313-B002-007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsu, Y.T., Kao, C.H. Cadmium-induced oxidative damage in rice leaves is reduced by polyamines. Plant Soil 291, 27–37 (2007). https://doi.org/10.1007/s11104-006-9171-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9171-7