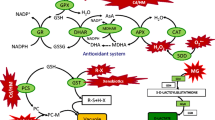
Abstract
A pot experiment was performed to assess the useful effects of seed soaking or seedling foliar spray using 0.25 mM spermine (Spm), 0.50 mM spermidine (Spd), or 1 mM putrescine (Put) on heavy metal tolerance in wheat plants irrigated with water contaminated by cadmium (2 mM Cd2+ in CdCl2) or lead (2 mM Pb2+ in PbCl2). Cd2+ or Pb2+ presence in the growth medium resulted in significant reductions in growth and yield characteristics and activities of leaf peroxidase (POD), glutathione reductase (GR), ascorbic acid oxidase (AAO), and polyphenol oxidase (PPO) of wheat plants. In contrast, significant increases were observed for Cd2+ content in roots, leaves and grains, superoxide dismutase (SOD) and catalase (CAT) activities, radical scavenging activity (DPPH), reducing power capacity, and fragmentation in DNA in comparison to controls (without Cd2+ or Pb2+ addition). However, treating the Cd2+- or Pb2+-stressed wheat plants with Spm, Spd, or Put, either by seed soaking or foliar spray, significantly improved growth and yield characteristics and activities of POD, GR, AAO, PPO, SOD, and CAT, DPPH, and reducing power capacity in wheat plants. In contrast, Cd2+ levels in roots, leaves, and yielded grains, and fragmentation in DNA were significantly reduced compared with the stressed (with Cd2+ or Pb2+) controls. Generally, seed soaking treatments were more effective than foliar spray treatments. More specifically, seed priming in Put was the best treatment under heavy metal stress. Results of this study recommend using polyamines, especially Put, as seed soaking to relieve the adverse effects of heavy metals in wheat plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide, wheat is one of the major cereals due to the richness of wheat in protein and calories. About 82% and 85% of the world’s population depend on wheat for basic protein and calories, respectively (Chaves et al. 2013).
Recently, environmental pollution from heavy metals increased due to multiple industrial activities, solid waste management, and improved agricultural techniques. Increased dependence of agriculture on chemical fertilizers, irrigation with sewage wastewater, and rapid manufacturing are shown to add some heavy metals to agricultural soils. This causes deleterious effects on the environmental system of the surrounded soil. Among the top toxicants, cadmium (Cd) has been placed at a seventh rank (Kumar 2013; Rady et al. 2019a). Cd is found in normal soils to be less than 0.5 mg per kilogram of soil but can reach up to 3.0 mg per kilogram depending on the materials of soil parent (Nazar et al. 2012). The Cd accumulation in plants causes several physio-biochemical and structural changes (Howladar 2014; Rady and Hemida 2015) due to the toxicity of Cd to plant cells causing oxidative stress with the overproduction of reactive oxygen species (ROS) (Yadav 2010). Lead (Pb), a potentially poisonous heavy metal with no recognized bio-function, has attracted more and more attention due to its widespread distribution and potential environmental hazards. As a result, soil contamination with Pb not only arouses changes of its microorganisms, their activities and relations by the deterioration of soil fertility, but also directly affects several physiological signals and decline crop growth and productivity (Majer et al. 2002).
Polyamines (PAs) are low molecular weight polycations (Cohen 1998) and are known to be pivotally needful for growth and development in eukaryotes and prokaryotes (Tiburcio et al. 1990). In plant cells, putrescine (Put; diamine), spermidine (Spd; triamine), and spermine (Spm; tetramine) are reported to constitute major PAs found as free forms or as conjugates bound to phenolic acids and other low molecular weight compounds. In addition, they are found as conjugates bound to macro-molecules such as nucleic acids and proteins. Polyamines are found to stimulate the replication, transcription, and translation of DNA (Frugier et al. 1994; Chen et al. 2019). They are involved in a wide range of bio-functions in plant growth and development, including senescence, environmental stress, and fungal and viral infections. Therefore, PAs are involved in diverse environmental stresses (Liu et al. 2007; Chen et al. 2019), which includes metal toxicity (Groppa and Benavides 2008; Shevyakova et al. 2010; Rady and Hemida 2015; Rady et al. 2016a, 2016b).
The main objective of this work was to evaluate the potential capacity of PAs seed soaking or seedling foliar spraying applications to alleviate the harmful effects of the heavy metal stress such as Cd2+ or Pb2+ in wheat plants through assessing plant growth and yield and its Cd2+ and Pb2+ levels. To elucidate the alleviation effect of different PAs on Cd2+- or Pb2+-stressed plants, the activities of some antioxidant enzymes, radical scavenging activity (DPPH), reducing power capacity, and fragmentation in DNA were also assessed.
Material and methods
Plant material, growing conditions, and treatments
Plastic pots of 35 cm in diameter and 30-cm depth were used to conduct an experimental attempt in which wheat seeds were soaked or seedlings were sprayed using the major three polyamines (PAs; ChemTex Speciality LTD, Kolkata, West Bengal, India, 100% Purity). A homogeneous lot of wheat (Triticum aestivum L.) seed, cv. Sakha 93 was obtained from the Field Crop Institute at the Research Center, Giza, Egypt. This cultivar was found to be sensitive to heavy metals (Rady et al. 2016a, 2016b). Using 0.001 M HgCl2 solution, the seeds were surface sterilized two times and then washed thoroughly several times with distilled water. The seeds were soaked in 0.25 mM Spm, 0.5 mM Spd, and 1.0 mM Put, or in distilled water for 8 h. These concentrations of PAs and soaking time were selected based on a preliminary study (data not shown). After seed soaking and air-drying, the treated seeds along with another amount of untreated seeds (for foliar spray treatments) were planted (15 wheat grains were sown in each container) in sterilized ion-free sand. The particle size of the sand was 0.25 mm, obtained through a sieve that has 0.25-mm-diameter holes. The ions (all anions and cations) were effectively removed from the sand by washing with commercial HCl (30% concentration) for 24 h. Then, the sand was washed with distilled water several times to remove the acid completely.
A ½-strength nutritious solution (Hoagland and Arnon 1950) was used for this study. The components of this nutritive solution were KNO3 (1020 mg L−1), Ca(NO3)2·4H2O (492 mg L−1), NH4H2PO4 (230 mg L−1), MgSO4·7H2O (420 mg L−1), H3BO3 (2.86 mg L−1), MnCl2·4H2O (1.81 mg L−1), H2MoO4·H2O (0.09 mg L−1), FeSO4·7H2O (0.07 mg L−1), and (CHOH)2(COOH)2 (0.02 mg L−1).
The kinds of nutritive solution were adjusted by using pure CdCl2 or PbCl2 (2 mM; Shree Bajrang Sales (P) Ltd., Nagpur - 440018, Maharashtra, India) to obtain the tested levels of heavy metals in the nutrient solution (based on a preliminary study). The experiment was comprised of 15 treatments. The experimental layout was a completely randomized block design that replicated three times. Each one of the 15 treatments was replicated 3 times and each replicate consisted of 8 pots. The description of the treatments is shown in Table 1.
Irrigation with heavy metals in the nutrient solution was done 20 times (60 days) starting from 15 days after sowing. Heavy metal concentrations were maintained and controlled in the growing medium by an inductively coupled plasma atomic emission spectrometry (ICP- AES, IRIS-Advan type, Thermo, USA). Wheat seedlings were sprayed to run-off with the mentioned concentrations of PAs three times at 20, 40, and 60 days after sowing. By using a handheld manual sprayer (model 0417.02.00; Guarany Ind. & Com. Ltd), different solutions were sprayed on the upper leaf surface until run-off (approximately 120 mL per pot), and few drops of 1% Tween-20 were added to the spray solutions as a surfactant.
The experiment was continued until harvest, but irrigation with the Cd2+- or Pb2+-containing nutritive solution was terminated on the 60th day of sowing. Plants of 75 days (at the end of the tillering; vegetative growth stage with the first node) were collected from each treatment for different growth and physio-biochemical measurements. Leaves and roots were collected from plants of each treatment, frozen in liquid N and then stored immediately at − 80 °C until use for enzymes activities (leaves only) and heavy metals contents (leaves and roots). At harvest (ripening stage), the yielded grains were used for the assessments of DPPH-antioxidant activity, antioxidant reducing power, genomic DNA assays, and their Cd2+ and Pb2+ ion levels. In addition, the yield and its components assessments were conducted.
Assessments of plant growth and yield and its components, and Cd2+ levels
Wheat plants of 75 days were removed gently from their pots and then moved to remove the adhering sand particles by dipping plant roots several times in a water-filled bucket. To record fresh weights, plant shoots were weighed and then placed in an electrical oven at 70 °C until reached constant weights and dry weights (DW) were recorded. At 150 days after planting (harvest stage), spikes were collected from all pots of each treatment to assess 1000-grain weight and grain weight per plant.
The powdery dried plant samples (leaf, root, and yielded grain) were burned at 500 °C for 12 h to obtain ash to determine the Cd2+ ion levels. Ash samples were dissolved in HNO3 (3.3%, v/v). The Cd2+ levels were assessed by inductively coupled plasma optical emission spectroscopy (ICP-OES, Varian, and Australia). Measurements of Cd2+ ions in plants were tested against the certificated Cd2+ values in different reference plant materials obtained from the National Institute of Standards and Technology (Gaithersburg, USA).
Assays of antioxidant enzymes
Using a mortar and pestle, 1 g of fresh fully expanded leaf samples was homogenized in 5 mL of ice-cold phosphate buffer (50 mM, pH 7.6) containing 0.1 mM Na-EDTA. Supernatants were obtained by centrifugation (at 4600×g for15 min) of the homogenized samples, and then repeated for the supernatants to use for enzyme assays. All previous operations were performed at 4 °C. Excluding the SOD enzyme, all enzymatic activities were performed using a 1-mL volume of supernatant. According to the methods suggested by Beer and Sizer (1952), Maehly and Chance (1954), Dawson and Magee (1955), Taneja and Sachar (1974), and Schaedle and Bassham (1977), the activities of CAT, POD, AAO, PPO, and GR, respectively, were assayed.
Superoxide dismutase (SOD) activity was performed as in the photochemical method outlined by Cakmak and Marshner (1992). Based on a SOD-inhibitable reduction of NBT by O2•–, the assay was done in an illuminated growth chamber. The components—phosphate buffer (50 mM, pH 7.6), sodium ethylenediaminetetraacetic acid (Na-EDTA; 0.1 mM), enzymatic extract (50–150 μL), Na2CO3 (50 mM, pH 10.2), L-methionine (12 mM), and NBT (75 μM)—were the reaction medium that finally received 2 μM riboflavin in a glass vial. Initiation of the reaction was done by turning lights on at an intensity of 700 μE m−2 s−1 and assays lasted 15 min. Enzyme amount which caused a 50% decrease in SOD-inhibitable NBT reduction was spectrophotometrically read at 560 nm expressing the enzyme activity.
Investigation of antioxidant activity
Extract preparation
The harvested seeds were ground to a fine powder, and a weight of 2 g of the powder was separately shaken for 48 h in methanol (85%) at a room temperature. The obtained extracts were then filtered through the Buckner funnel and filter papers (Whatman No. 1). Using the rotary evaporator and reduced pressure, the filtrates were concentrated to dryness at 40 °C. The extracts were re-suspended using methanol (the respective solvent) to obtain 100 mg per milliliter of stock solution (Taylor et al. 1996).
Antioxidant activity (DPPH assay)
The method outlined in Brand-Williams et al. (1995) was used to assess the activity of free radical scavenging by using DPPH (1.1-diphenyl-2-picryl-hydrazil) reagent. Methanol (85%, v/v) was used to dissolve wheat seed extract, and 1.0 mL DPPH solution (20 μg mL−1, freshly prepared) was added to 0.5 mL extract sample, and the mixture was then stirred. At 5 min from beginning the reaction, discoloration processes were scored at 517 nm against a blank control.
Reducing power assay
Reducing the power of methanolic extract of wheat seeds was assessed as outlined by Oyaizu (1986). To phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and K3[Fe(CN)6] (2.5 mL, 1%), a volume (0.5 mL) of crude plant extract with isolated compounds were added, and at 50 °C, the mixture was incubated for 20 min, and then 2.5 mL of TCA (10%) was added. Centrifugation (at 1000 rpm for 10 min) was then practiced. Solution upper layer (2.5 mL) was collected and mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 solution (0.1%, freshly prepared). Absorbance was read at 700 nm, and an increase in absorbance indicates the increase in reducing power.
DNA extraction
Genomic DNA was extracted from ground seed samples and preserved until extraction (Porebski et al. 1997). DNA was purified using a method that prevents degradation to prepare an adequate amount of DNA to allow for multiple end uses.
Using liquid N, the initial grinding stage was employed and samples were suspended in CTAB buffer once they were appropriately ground. For purifying the DNA, soluble protein and others were separated by the centrifugation process and insoluble particles were discarded. From the aqueous phase, DNA was precipitated and thoroughly washed to discard any contaminating salts. Purified DNA was re-suspended and then stored in Tris-EDTA buffer or sterilized distilled water. This procedure conferred the intact genomic DNA from tested samples. To test extracted DNA quality, the samples were run on the agarose gel, stained with EtBr (ethidium bromide), and then visualized under ultraviolet light (Porebski et al. 1997).
The sample (200 mg) was ground in 500 μL of CTAB buffer to a fine paste and the mixture was then transferred to a microfuge tube for incubation at 55 °C for 15 min. Thereafter, the mixture was spied (at 12000×g for 5 min) to spin down cell debris. To clean microfuge tubes, the supernatant was transferred. A volume of 250 μL of chloroform:isoamyl alcohol (24:1) was added and the solution was mixed by inversion. Then, the tubes were spied again (at 13000 rpm for 1 min). The upper aqueous phase which contained DNA was taken to clean microfuge tube, and ammonium acetate (CH3COO− NH4+; 50 μL, 7.5 M) and 500 μL of ice-cold ethanol (absolute) were added. For several times, the tubes were inverted, slowly, to precipitate DNA. In general, DNA can precipitate out of solution. As an alternative, the tubes can cool for 1 h under − 20 °C after adding the ethanol for precipitating the DNA. Thereafter, DNA was pipetted off by slowly rotating a tip in the old solution. DNA was washed by transferring the precipitate to a microfuge tube that contained ice-cold ethanol (500 μL, 70%) and by inverting the tube slowly. Precipitate can also be isolated through tube spinning (at 13000 rpm for 1 min) to form the DNA pellet. Then, the supernatant was discarded and the DNA pellet was washed by the ice-cold ethanol (70%). DNA was then spied to a pellet through a centrifugation (at 13000 rpm for 1 min) process. The supernatant was discarded and the DNA pellet was allowed to dry (for about 15 min). DNA was re-suspended and then incubated for 20 min at 65 °C for destroying any found DNase and was then stored at 4 °C (Porebski et al. 1997).
DNA quality confirmation
For a couple of minutes, agarose solution (1%) was cooled and EtBr (2.5 μL) was added, and then they were mixed well. On a flat surface for at least 20 min, the gel was allowed to set at a room temperature. The following was loaded into separate wells: 10 μL 1kb ladder, 5 μL sample + 5 μL water +2 μL 6× loading buffer; the gel was run for 30 min at 100 V, and was then exposed to ultraviolet light and photographed (demonstration). The quality of DNA was confirmed by the presence of highly resolved high molecular weight bands, which indicates good DNA quality, while the presence of smeared bands indicates degraded DNA (Porebski et al. 1997).
Statistical analysis
By using SAS (Statistical Analysis System) version 9.0 (2002), data of the current study were analyzed. The means (n = 3) of different treatments were separated using Duncan’s multiple range test, and the P value ≤ 0.05 was identified as significant.
Results and discussion
Few studies including the current work have focused on the modulating effect of exogenous PA application on heavy metal–stressed plants. Plants are negatively affected by heavy metal stress (Alzahrani et al. 2018; Desoky et al. 2019; Rady et al. 2019b). However, exogenous PA applications mitigated the heavy metal stress effects by increasing the endogenous contents of PAs and decreasing the contents of heavy metals in different plant parts (Rady and Hemida 2015; Rady et al. 2016a, 2016b). The Cd2+ and Pb2+ stress adversely affected growth, physio-biochemical attributes, and antioxidant defense systems in wheat plants; however, modulating effects for these attributes were occurred with exogenous PAs treatment (Rady and Hemida 2015; Rady et al. 2016a, 2016b). In addition, enzyme activities were completely restored by PA treatments (Chen et al. 2019).
Effect of different polyamine applications on growth and yield characteristics of a heavy metal–stressed wheat plant
Data set in Table 2 show that the presence of CdCl2 (2 mM) or PbCl2 (2 mM) in the growing medium led to significant reductions in growth and yield traits (i.e., shoot fresh and dry weights, 1000-grain weight, and grain weight per plant) of wheat plants compared with the normal controls (without Cd2+ or Pb2+ addition). The reductions were 68 or 65% for shoot fresh weight, 78 or 54% for shoot dry weight, 73 or 67% for 1000-grain weight, and 85 or 73% for grain weight per plant by Cd2+ or Pb2+, respectively, showing that Cd2+ was more toxic on wheat plants. However, treating wheat plants subjected to Cd2+ or Pb2+ with Spm, Spd, or Put, either by seed soaking or by foliar spraying, significantly improved the aforementioned growth and yield characteristics. Seed soaking in Put at 1 mM was the best treatment under the stress of either 2 mM Cd2+ or 2 mM Pb2+. This best treatment increased shoot fresh weight (FW), shoot dry weight (DW), 1000-grain weight, and grain weight per plant by 94 or 97%, 109 or 84%, 180 or 127%, and 397 or 177% under Cd2+ or Pb2+, respectively.
Results of the current study reveal that Cd2+ was more toxic than Pb2+ as reported earlier by Lewis (1995). The Cd2+ stress reduced plant vigor and inhibited plant growth and productivity (Aldesuquy et al. 2014; Semida et al. 2015; Rady and Hemida 2015). Kopittke et al. (2010) reported that toxic concentrations of Cd2+ and Pb2+ are 0.3 μM and 5 μM, respectively. The Cd2+-induced growth inhibition and yield limitation have been proved to come from inhibiting the cell elongation and division rates, and consequently the decline in the production of plant biomass that adversely reflects in plant yield. This occurs mainly due to a permanent inhibition of the proton pump, which is responsible for these processes (Choudhury and Panda 2004). The reduction in wheat plant growth and yield was associated with Cd2+-induced increase in Cd2+ ions concentrations (Fig. 1). These results are in agreement with those of Rady et al. (2016a, 2016b), which indicated that the wheat (cv. Sakha 93) plant had reduced tolerance to 2 mM Cd2+ or 2 mM Pb2+ stress. This reduced heavy metal tolerance in wheat (cv. Sakha 93) may be due to the elevated uptake of Cd2+ or Pb2+ by roots and translocation rapidly to shoots in toxic amounts that affect physiological and biochemical processes in plant cells via Cd2+ or Pb2+-stimulated oxidative damages to different cellular components (Lamhamdi et al. 2013; Rady and Hemida 2015; Rady et al. 2016a, 2016b). Moreover, Kaur et al. (2012) have reported the inhibitory effect of Pb2+ on wheat plant growth due to a reduction in meristematic cells in the shoot region by the accumulation of Pb2+. The inhibition of cell division in the meristematic zone of root and shoot systems by Pb2+ is also reported earlier (Bashmakov et al. 2005; Jili et al. 2009). They have attributed the effects of heavy metals on meristematic cells to some of the enzymes found in the cotyledon and endosperms, which are affected by heavy metals, as well as to the reduced mitotic cells in meristematic zone of root and shoot.
Changes in the concentration of Cd2+ in heavy metal–stressed wheat plants as a response to polyamines (PAs—spermine, Spm; spermidine, Spd; and putrescine, Put) applied as seed soaking or foliar spraying. Values are means of triplicate measurements ± SD. Control means seeds or plants without any treatments, Dw + Cd means soaking seeds in distilled water (Dw) and foliar spray with Dw under Cd2+ contamination, Dw + Spm means soaking seeds in distilled water and foliar spray with spermine under Cd2+ contamination, Dw + Spd means soaking seeds in distilled water and foliar spray with spermidine under Cd2+ contamination, Dw + Put means soaking seeds in distilled water and foliar spray with putrescine under Cd2+ contamination, Spm + Dw means soaking seeds in spermine and foliar spray with distilled water under Cd2+ contamination, Spd + Dw means soaking seeds in spermidine and foliar spray with distilled water under Cd2+ contamination, and Put + Dw means soaking seeds in putrescine and foliar spray with distilled water under Cd2+ contamination
To alleviate and repair the damage caused by ROS overproduced by the Cd2+ or Pb2+ presence in plant growing medium, exogenous application of antioxidants such as PAs enables crop plants to stimulate their antioxidant defense systems to increase the cellular defense strategy against Cd2+- or Pb2+-induced oxidative stress (Wu et al. 2013). Exogenous Spm, Spd, or Put application minimized the poisonous influences of Cd2+ or Pb2+ and considerably improved growth and yield characteristics of the wheat plant (Table 2). These improved growth and yield traits of plant growing under Cd2+ or Pb2+ stress could be due to the minimized endogenous levels of Cd2+ ion by PA application (Fig. 1). In this connection, Alexandra and Tiburcio (1997) have stated that PAs are implicated in various physio-biochemical functions related to plant growth and development, as well as the functions related to the quality of grain yield such as development of reproductive organ and fruits, formation of tubers, initiation of flowers, and fruit ripening (Tiburcio et al. 2002; Chen et al. 2019), which could elucidate the preventive role of Spm, Spd, or Put in Cd2+- or Pb2+-stimulated inhibition of plant growth. The elevated yield induced by PA applications may be attributed to the elevation in biomass of wheat plant (Table 2) and leaf longevity that may be contributed to grain filling by improving the supplementation period of photosynthates into grains (Kaur et al. 2012). In addition, several studies reported that PAs are implicated in several defense mechanisms during environmental stresses (Groppa and Benavides 2008; Rady and Hemida 2015). The increases in the activity of the antioxidative enzymes (Table 3), radical scavenging activity, and reducing power capacity (Fig. 2) as a result of Cd2+ or Pb2+ stress are monitored in the current study, and the different PAs treatments induced further increases in these antioxidative signals in wheat plant, which offer additional mechanisms by which PAs ameliorated the toxic influences of Cd2+ and Pb2+ stresses on wheat growth and yield.
Changes in the reducing power capacity and the radical scavenging activity (DPPH) in heavy metal–stressed wheat plants as responses to polyamines (PAs—spermine, Spm; spermidine, Spd; and putrescine, Put) applied as seed soaking (SS) or foliar spraying (FS). Values are means of triplicate measurements ± SD
Among the applied PAs, Put was most effective through either seed soaking or foliar spray in this regard in the current study. This result comes from the most positive effect of Put, in our study, on inducing the activity of the antioxidative enzymes, radical scavenging activity, and reducing power capacity under Cd2+ and Pb2+ stresses. It has been reported that Put could be sufficient to minimize the oxidative damages induced, indirectly, by heavy metals (Mandal et al. 2013, 2014). Also, the composition nature of Put as a diamine (having lower molecular mass) may confer it the preference to rapidly translocate to bind with the negatively charged domain of the cell membranes compared with both Spd and Spm as triamine and tetraamine (having higher molecular masses), respectively, and consequently perhaps prevent the proteins from its oxidation by ROS (Rady and Hemida 2015). Our results confirm this suggestion that Spd conferred better results than Spm (Tables 2 and 3, Figs. 1 and 2). In addition, seed priming with PAs generally induced better results than PAs foliar spraying. This result may be due to that seeds supporting with PAs give the powerful for seeds to confer strong seedlings against the tested stresses. Seed priming with PAs might increase their endogenous levels within plant tissues (Rady and Hemida 2015), improving seed vigor and seedling growth that could be a result of the improved metabolic activities: effective mobilization and employment of seed reserves and preferable genetic rehabilitation (Farooq et al. 2008). PAs play key roles in modulating cell enlargement and division rates (Cvikrova et al. 1999) and increasing cell division within the apical meristem (Farooq et al. 2008). These results could be monitored via the improved fresh and dry weights of plant shoots that significantly reflected in higher grain yield under stress.
Effect of different polyamine applications on Cd2+ ion levels in a heavy metal–stressed wheat plant
Data set in Fig. 1 indicate that the presence of 2 mM Cd2+ in the growing medium resulted in significant increases in wheat root, leaf, and yielded grain concentrations of Cd2+ compared with the normal controls (without Cd2+ addition) in which Cd2+ was scored traces. However, treating wheat plants subjected to Cd2+ with Spm, Spd, or Put, either by seed soaking or by foliar spraying, significantly declined the concentrations of Cd2+ in all wheat plant organs. Generally, seed soaking treatments were more effective than foliar spray treatments. In addition, seed priming using Put was the best treatment under Cd2+ stress, reducing the concentrations of Cd2+ by 50% in roots, 53% in leaves, and 61% in grains, showing the prolonged improving effect of Put.
PAs stabilize and protect cell membrane systems against metal ion stress (Groppa et al. 2007) through modulating Cd2+ stress effect on tissue relative water content and membrane integrity (Rady and Hemida 2015), reducing Cd2+ ion content (Fig. 1). Under Cd2+ stress, disruptions occur in antioxidant defense systems when plant cells cannot maintain a low level of Cd2+ through effective detoxification mechanisms. Thus, oxidative damage might occur to various components of cells, resulting in reduced plant growth (Groppa et al. 2007; Rady and Hemida 2015) as reported in the current study. Pretreatment using PAs has supported plant cells to maintain a minimized level of Cd2+ ion (Fig. 1), which lead, consequently, to healthy seedling growth under Cd stress.
There is no specific transport channel for the non-essential element, including Cd2+ within the plant. The non-essential elements were transported into plants via transporters (Clemens et al. 1998). Wheat plants accumulated high amounts of Cd2+ in roots than in shoots, and in turn than in grains. Semida et al. (2018) recorded a higher Cd2+ accumulation in cucumber roots and lower in leaves in an experiment, determining Cd2+ uptake and its localization in cucumber transplants that confirmed our results (Fig. 1). Compared with shoot growth, root growth is more sensitive to heavy metals, and this evidence is correlated to data indicated in this study a significant lower root length under Cd2+ treatment than that of the normal control (Rady and Hemida 2015). The ameliorative effects of PAs on heavy metals and reducing their contents in wheat plant organs (Fig. 1) come through inducing the synthesis of specific proteins that increases the tolerance of wheat plants to heavy metals (Chen et al. 2019). This PA-stimulated repairing effect may be attributed to that PAs elevate phytochelatins (PCs) production, especially in root (Pál et al. 2017), elevate cell wall and vacuolar storage of these toxic metals, increase the detoxification of heavy metals by increasing the accumulation of these metals in trichomes of leaves and peduncles of wheat plants (Alsokari and Aldesuquy 2011), and act as an effective antioxidants and free radical scavengers under these stresses (Ferreira et al. 2002), as well as PAs stimulate the root exudates including heavy metals into the soil (biosphere) (Aldesuquy 2016). Among the tested PAs, Put was the most effective, greatly reducing the wheat plant organs contents of Cd2+ compared with Spd and Spm. The best improving effects of Put may be attributed to that Put is actively synthesized via ornithine decarboxylase (ODC) and arginine decarboxylase (ADC), sustaining enough endogenous Put content and/or poor Put conversion into Spd. In addition, the better effect of Spd than Spm may be due to that Spd is actively produced by Spd synthase (SPDS) to form enough endogenous Spd content and/or poor Spd conversion into Spm or thermospermine.
Effect of different polyamine applications on antioxidant enzymes activity of a heavy metal–stressed wheat plant
Data set in Table 3 display that the presence of 2 mM Cd2+ or Pb2+ in the growth medium resulted in significant increases in the activities of SOD and CAT, while resulted in significant reductions in the activities of POD, GR, AAO, and PPO of wheat leaves compared with the normal controls (without Cd2+ or Pb2+ addition). The increases in the SOD and CAT activities were 41 or 53%, and 36 or 43%, respectively, while the reductions in the POD, GR, AAO, and PPO activities were 41 or 28%, 26 or 29%, 36 or 45%, and 35 or 24%, respectively. However, treating wheat plants exposed to Cd2+ or Pb2+ with Spm, Spd, or Put, either by seed soaking or by foliar spraying, significantly elevated the activities of POD, GR, AAO, and PPO, and further increased the activities of SOD and CAT in wheat plant leaves. Generally, seed soaking treatments were more effective than foliar spray treatments. In addition, seed priming using Put was the best treatment under the stress of either Cd2+ or Pb2+, increasing the activities of SOD, CAT, POD, GR, AAO, and PPO by 38 or 31%, 47 or 45%, 189 or 117%, 69 or 68%, 271 or 267%, and 191 or 131%, respectively.
Enzymatic antioxidants are outstanding biochemical signals of stress. Increase in the activities of these antioxidant enzymes could be a potential to ameliorate the heavy metal stress-stimulated oxidative stress. Under Cd2+ or Pb2+ stress, PAs induced increases in the activities of all tested enzymes in different degrees, which might be attributed to the antioxidant characteristics of PAs against ROS generation; besides that, the plant tries to force this by stimulating its antioxidants defenses (Li et al. 2011). Excessive production of ROS is a common outcome of different stressors, including Cd2+ and Pb2+. Under a stress condition, a balance is required between generation and degradation of ROS to maintain healthy metabolic functions; otherwise, oxidative damages may eventuate. ROS levels are controlled in plant tissues by antioxidant defense systems, including enzymatic antioxidants (SOD, CAT, POD, PPO, AAO, and GR tested in the current study) and non-enzymatic antioxidants like proline, glutathione (GSH), tocopherols, and carotenoids (Rady and Hemida 2015; Schutzendubel and Polle 2002). Thus, it was expected that wheat plant exposure to Cd2+ or Pb2+ stress enhance the activities of enzymatic antioxidant, but present study results showed the reverse state, except for SOD and CAT activities which were increased. However, all enzyme activities tested in this study were improved or further improved in wheat leaves by PAs treatments (by seed soaking or by foliar spraying) under the stress of Cd2+ or Pb2+. The interesting thing in the current study is that seed soaking in PAs was better than a foliar spray, improving the enzymatic activities that play a pertinent role in reducing the harmful effects of heavy metal stress. Many researchers have investigated that treatment of heavy metals promotes ROS formation, and therefore, substantially elevates the activities of ascorbate peroxidase (APX), CAT, and SOD (Bashri and Prasad 2015). As the first defense line to cope with the superoxide (O2•−) radical, SOD stimulates O2•− conversion to H2O2, which in turn is converted by POD to H2O (Alscher et al. 2002), by functioning several reductants like guaiacol, ascorbate, and phenolic compounds (Apel and Hirt 2004). This explanation elucidates the increased activity of SOD under Cd2+ or Pb2+ stress in our study. Like POD, CAT scavenges H2O2 by converting it to H2O and O2. The increased expression of GR maintains the GSH pool in the reduced state that, in turn, reduces dehydroascorbate to ascorbate and enhances tolerance to oxidative stress (Noctor and Foyer 1998). Many researchers suggested different mechanisms to elucidate the increase of the antioxidant status occurred due to PAs, which can act as direct effective scavengers of ROS (Drolet et al. 1986). They can be bound to enzymatic antioxidants or can be linked to antioxidant molecules and allow them to permeate oxidative stress sites (Poduslo and Curran 1996), or may be interacted with molecular complexes to stabilize the thylakoid membranes (Besford et al. 1993). Functions of plasma membrane negatively affected rapidly by toxic levels of heavy metals (Quartacci et al. 2001); however, PAs contribute to stabilize the plasma membrane and maintain its functions. PA applications showed a greater increase in the activities of enzymatic antioxidants as a proved synergy between PAs and enzymatic antioxidants as an effective protective mechanism (Gill and Tuteja 2010). Antioxidative protection conferred by PAs for plant tissues is attributed to maintain the integrity of membranes via stabilization of membrane lipids and to avoid inorganic leakage under Cd2+- or Pb2+-induced oxidative stress. Otherwise, Kurepa et al. (1998) have stated that the resistance of paraquat is not correlated with increased contents of PAs. It has been proposed also that the antioxidative impacts of PAs are attributed to combined properties linked to anions and cations. PA binding to anions (nucleic acids, phospholipid membranes) is contributed to high local levels at specific sites prone to the oxidations, while binding to cations is effectively prevented at specific sites for the generation of hydroxyl radical, OH–, and singlet oxygen, 1O2 (Lovaas 1997).
Effect of different polyamine applications on DPPH radical scavenging activity of a heavy metal–stressed wheat plant
Antioxidants can prevent, inhibit, or even delay the oxidation of material that is able to be oxidized by ROS scavenging and, therefore, minimize the oxidative stress effects. DPPH has been extensively employed as an empirical free radical to assess reducing materials. Figure 2 displays the inhibition level of DPPH radical generation. The stress of Cd2+ or Pb2+ increased the DPPH radical scavenging activity by 36 ± 0.4% or 37 ± 0.2%, respectively, compared with the untreated control plants (19 ± 0.5%).
The elevated activity of DPPH radical scavenging may be due to the increased content of some secondary metabolites such as polyphenolic compounds (phenols and flavonoids) (Ali et al. 2018). The increase in these metabolites under stress contributes to the increase of the antioxidative defense because they are considered as the main responsible compounds in the antioxidant activity. In Cd2+- or Pb2+-stressed plants, treatment of PAs (Sp, Spd, or Put) reduced the DPPH radical scavenging activity, but it is still higher than the control (without any treatments). Our results are in the similar trend of those obtained by Groppa et al. (2001) and Zhao and Yang (2008). In the first report, exogenously added PAs mitigated the negative effects of Cd2+- or Cu2+-induced oxidative damage in sunflower leaf discs, and they alleviated the lipid peroxidation induced by Cd2+ stress in Malus hupehensis in the second report. Polyphenols (and 2,2-diphenyl-1-picrylhydrazyl; DPPH) are implicated in plant defense mechanisms and their concentrations are improved as a response to environmental stressors (Dudjak et al. 2004). There is evidence that phenolic metabolism induction occurs as a response to metal stress (Michalak 2006; Singh and Malik 2011). Phenolic compounds have powerful antioxidative activities in the heavy metal–stressed plant. In their chemical structure, the antioxidant action is established mainly. By POD, phenols are oxidized and contributed to H2O2 scavenging (Singh and Malik 2011).
Effect of different polyamine applications on reducing power ability of a heavy metal–stressed wheat plant
A compound reducing capacity may be functioned as a pivotal indicator of its potential antioxidative activity (Duh et al. 1999). Increased absorbance of their reaction mixture indicates increased reducing power. It has been suggested that reducing power and antioxidative activity are related to each other (Duh 1998). Results of reducing power capacity are presented in Fig. 2 which illustrates that either Cd2+ or Pb2+ stress increased the reducing power capacity of plants. It was increased by about 193 or 260% under Cd2+ or Pb2+ stress, respectively, compared with the control (without any treatments). This means that the increase in some secondary metabolites increases the reducing power capacity in wheat plants. Treatment with PAs as seed soaking or foliar spray reduced the reducing power capacity, but it is still higher than the control.
Many studies have confirmed the intricacy of the reaction between ROS and PAs, particularly when the plant is grown under stress (Velarde-Buendía et al. 2012; Pottosin et al. 2014). Also, when the cellular PA contents are increased, their catabolism is also increased; this is in conjunction with increasing the level of H2O2, various ROS, and different antioxidant defense systems in the plant. Their function in alleviating damages from stresses such as heavy metal is valuable. The benefit of PAs in enhancing antioxidant defense systems to increase the adaptation of the plant against several stresses was considered the main reason in the production of superoxide molecule in cells (Chmielowska-Bak et al. 2013; Mandal et al. 2013; Scoccianti et al. 2013). Other studies have investigated that PA effects may be increased in cellular PA titers participated in both ROS-antioxidant equation sides under stress conditions. Otherwise, ROS are participated in environmental, abiotic and biotic, stressors as pathway parts of signal transduction to increase the defense system (Moschou et al. 2012).
Effect of different polyamine applications on genomic DNA of a heavy metal–stressed wheat plant
Figure 3 shows agarose gel electrophoresis of genomic DNA samples extracted from seeds of wheat treated with Spm, Spd, or Put and cultivated under Cd2+ or Pb2+ stress, as well as the control using 1% agarose gel in 1X TBE buffer.
Response of genomic DNA in heavy metal–stressed wheat plants to polyamines (PAs—spermine, Spm; spermidine, Spd; and putrescine, Put). Control without treatments (1), Cd2+ only (2), Pb2+ only (3), Spm − seed soaking + Cd2+ (4), Spm − foliar spray + Cd2+ (5), Spm − seed soaking + Pb2+ (6), Spm − foliar spray + Pb2+ (7), Spd − seed soaking + Cd2+ (8), Spd − foliar spray + Cd2+ (9), Spd − seed soaking + Pb2+ (10), Spd − foliar spray + Pb2+ (11), Put − seed soaking + Cd2+ (12), Put − foliar spray + Cd2+ (13), Put − seed soaking + Pb2+ (14), Put − foliar spray + Pb2+ (15)
Lane (1) represents the DNA of untreated control that shows no fragmentation. Lanes (2) to (7) represent DNA samples of Cd2+ and Pb2+ stresses, as well as seed soaking and plant spraying using Spm under Cd2+ and Pb2+ stresses, which show moderate degree of fragmentation that represented by moderate smearing on the gel, while lanes (8) to (11) represent samples of seed soaking and plant spraying using Spd under Cd2+ and Pb2+ stresses that exhibit intact DNA with a minor degree of fragmentation. In addition, lanes (12) to (15) represent DNA of samples treated with Put (seed soaking and foliar spraying) in Cd2+- and Pb2+-stressed plants that reveal small degree of fragmentation, which indicates that Spd treatment is the most effective PAs in alleviating the harmful effect of heavy metal stress on the DNA of plants, followed by Put treatment. As small aliphatic amines found in all plant kinds, PAs are considered as a new category of growth substances. It has been reported that PAs are implicated in various plant processes such as cell division, fruit ripening, organ development, gene transcription, and regulation of DNA replication, as well as they are considered as anti-senescence (Yin 1994).
It has been elucidated that the exogenous PA application scavenged the destructive influences of salinity stress in the plant. Further, PAs have the merit of catalyzing the biosynthesis of DNA and RNA, and protecting the replication of DNA from the oxidative damage (Khan et al. 1992; D’Agostino et al. 2005). The plant should also develop a group of bio-defenses to ameliorate the destructive sources through the expression of a wide range of functions of proteins and genes in response to different environmental stressors (Voronova et al. 2014; Finatto et al. 2015).
Conclusions
It can be concluded, from our results, that exogenous application of 0.25 mM Spm, 0.50 mM Spd, or 1.0 mM Put, particularly when used as seed soaking, could alleviate the harmful effects of 2.0 mM Cd2+ or 2 mM Pb2+ stress in wheat plants. Exogenously applied PAs, particularly Put, significantly improved antioxidant enzymes activities, DPPH radical scavenging activity, reducing power capacity, and genomic DNA, and significantly declined Cd2+ and Pb2+ levels in roots, leaves, and yielded grains in Cd2+- or Pb2+-stressed wheat plants. These results were positively reflected in plant growth and yield. Generally, the best PA treatment was seed soaking in Put concerning all of the above attributes, except for genomic DNA (Spd was the best). In the same time in which numerous works have confirmed the positive roles of PAs in alleviating the adverse effects of heavy metal stress, more studies are requested to specify exactly the mechanisms by which Put maintain the DNA free from fragmentation and improve the DPPH radical scavenging activity and the reducing power capacity under heavy metal stress.
References
Aldesuquy HS (2016) Polyamines in relation to metal concentration, distribution, relative water content and abscisic acid in wheat plants irrigated with waste water heavily polluted with heavy metals. Intl J Bioassays 5(5):4534–4546
Aldesuquy HS, Samia H, Samy A, Abdel-Whab E (2014) Involvement of spermine and spermidine in the control of productivity and biochemical aspects of yielded grains of wheat plants irrigated with waste water. Egypt J Basic Appl Sci 1:16–28
Ali AMA, El-Nour MEM, Yagi SM (2018) Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J Genet Engin Biotechnol 16:677–682 In Press
Alscher PG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plant. J Exp Bot 53:1331–1341
Alsokari SS, Aldesuquy HS (2011) Synergistic effect of polyamines and waste water on leaf turgidity, heavy metals accumulation in relation to grain yield. J Appl Sci Res 7(3):376–384
Alzahrani Y, Kuşvuran A, Alharby HF, Kuşvuran S, Rady MM (2018) The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol Environ Saf 154:187–196
Apel K, Hirt H (2004) Reactive oxygen species: metabolism oxidatives and signal transduction. Annu Rev Plant Physiol Plant Mol Biol 55:373–399
Bashmakov DI, Lukatkin AS, Revin VV, Duchovskis P, Brazaityte A, Baranauskis K (2005) Growth of maize seedlings affected by different concentrations of heavy metals. Ekologija 3:22–27
Bashri G, Prasad SM (2015) Indole acetic acid modulates changes in growth, chlorophyll a fluorescence and antioxidant potential of Trigonella foenum-graecum L. grown under cadmium stress. Acta Physiol Plant 37:1745
Beer RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133
Besford RT, Richardson CM, Campos JL, Tiburcio AF (1993) Effect of polyamines on stabilization of molecular complexes of thylakoid membranes of osmotically stressed oat leaves. Planta 189:201–206
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Leben smittel Wissenschaften und Technologi 28:25–30
Cakmak I, Marshner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Chaves MS, Martinelli JA, Wesp-Guterres C, Graichen FAS, Brammer S, Scagliusi SM, Da Silva PR, Wiethölter P, Torres GAM, Lau EY, Consoli L, Chaves ALS (2013) The importance for food security of maintaining rust resistance in wheat. Food Sec 5:157–176
Chen D, Shao Q, Yin L, Younis A, Zheng B (2019) Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. A Review Front Plant Sci 9:1945. https://doi.org/10.3389/fpls.2018.01945
Chmielowska-Bak J, Lefèvre I, Lutts S, Deckert J (2013) Short term signaling responses in roots of young soybean seedlings exposed to cadmium stress. J Plant Physiol 170:1585–1594
Choudhury S, Panda SK (2004) Role of salicylic in regulating cadmium induced oxidative stress in Oryza sativa roots. Bulg J Plant Physiol 30:95–110
Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI (1998) The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc Natl Acad Sci U S A 95:12043–12048
Cohen SS (1998) A guide to the polyamines. Oxford University Press, New York, NY
Cvikrova M, Binarova P, Cenklova V, Edera J, Machackova I (1999) Reinitiation of cell division and polyamine and aromatic monoamine levels in alfalfa explants during the induction of somatic embryogenesis. Physiol Plant 105:330–337
D’Agostino L, Di-Pietro M, Di-Luccia A (2005) Nuclear aggregates of polyamines are supra molecular structures that play a crucial role in genomic DNA protection and conformation. FEBS J 272:3777–3787
Dawson CR, Magee RJ (1955) Ascorbic acid oxidase. In: Colowich SP (ed) Methods in enzymology. Vol. Academic, New York, pp 831–835
Desoky EM, Elrys AS, Rady MM (2019) Integrative Moringa and licorice extracts application improves Capsicum annuum fruit yield and declines its contaminant contents on a heavy metals contaminated saline soil. Ecotoxicol Environ Saf 169:50–60
Drolet G, Dumbroff EB, Legg R, Thompson JE (1986) Radical scavenging properties of polyamines. Phytochem 25:367–371
Dudjak J, Lachman J, Miholová D, Kolihová D, Pivec V (2004) Effect of cadmium on polyphenol content in young barley plants (Hordeum vulgare L.). Plant Soil Environ 50:471–477
Duh PD (1998) Antioxidant activity of burdock (Arctium lappa Linne): its scavenging effect on free radical and active oxygen. J Amer Oil Chem Soc 75:455–461
Duh P, Du P, Yen G (1999) Action of methanolic extract of mung bean hull as inhibitors of lipid peroxidation and non-lipid oxidative damage. Food Chem Toxicol 37:1055–1061
Farooq M, Basra SMA, Rehman H, Hussain M (2008) Seed priming with polyamines improves the germination and early seedling growth in fine rice. J New Seeds 9:145–155
Ferreira RR, Fornazier RF, Vitoria AP, Lea PJ, Azevedo RA (2002) Changes in antioxidant enzyme activities in soybean under cadmium stress. J Plant Nutr 25:327–342
Finatto T, Costa de Oliveira A, Chaparro C, da Maia LC, Farias DR, Woyann LG, Mistura CC, Soares-Bresolin AP, Liauro C, Panaud O, Picault N (2015) A biotic stress and genome dynamics: specific genes and transposable elements response to iron excess in rice. Rice 8:13
Frugier M, Florentz C, Hosseini MW, Lehn JM, Giegé R (1994) Synthetic polyamines stimulate in vitro transcription by T7 RNA polymerase. Nucleic Acids Res 22(14):2784–2790
Gill SS, Tuteja N (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 5:26–33
Groppa MD, Benavides MP (2008) Polyamines and a biotic stress: recent advances. Amino Acids 1:35–45
Groppa MD, Tomaro ML, Benavides MP (2001) Polyamines as protectors against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci 161:481–488
Groppa MD, Ianuzzo MP, Tomaro ML, Benavides MP (2007) Polyamine metabolism in sunflower plants under long-term cadmium or copper stress. Amino Acids 32:265–275
Hoagland DR, Arnon DI (1950) The water-culture for growing plants without soil. Cal Agric Exp Sta Cir 347:1–32
Howladar SM (2014) A novel Moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol Environ Saf 100:69–75
Jili K, ZhiJun Z, Liu Y (2009) Effects of lead (Pb) stress on seed germination and seedling growth of wheat. Guangxi Acad Agric Sci 40(2):144–146
Kaur G, Singh HP, Batish DR, Kohli RK (2012) Growth, photosynthetic activity and oxidative stress in wheat (Triticum aestivum) after exposure of lead Pb to soil. J Environ Biol 33:265–269
Khan AU, Di-Mascio P, Medeiros MHG, Wilson T (1992) Spermine and spermidine protection of plasmid DNA against single-strand breaks induced by singlet oxygen. Proc Natl Acad Sci U S A 89:11428–11430
Kopittke PM, Pax F, Blamey C, Colin J, Neal A, Menzies W (2010) Trace metal phytotoxicity in solution culture: a review. J Exp Bot 61:945–954
Kumar M (2013) Crop plants and abiotic stresses. Biomol Res Ther 3:e125
Kurepa J, Smalle J, Vanmontagu M, Inze D (1998) Polyamines and paraquat toxicity in Arabidopsis thaliana. Plant Cell Physiol 39:987–992
Lamhamdi M, El Galiou O, Bakrim A, Novoa-Munoz JC, Arias-Estevez M, Aarab A, Lafont R (2013) Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi J Biol Sci 20(1):29–36
Lewis MA (1995) Use of freshwater plants for phytotoxicity testing: a review. Environ Pollut 87(3):319–336
Li CL, Xu HM, Xu J, Chun XY, Ni DJ (2011) Effects of aluminum on ultrastructure and antioxidant activity in leaves of tea plant. Acta Physiol Plant 33:973–978
Liu JH, Kitashiba H, Wang J, Ban Y, Moriguchi T (2007) Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol 24:117–126
Lovaas E (1997) Antioxidant and metal-chelating effects of polyamines. In: Sies, H. (Ed.). Advances in pharmacology. Antioxidants in disease mechanisms and therapy. Acad Press, 38:119–149
Maehly AC, Chance BC (1954) The assay of catalases and peroxidases. In: Glick D (ed) Methods of biochemical analysis, vol 1. Intersci Publish, New York
Majer BJ, Tscherko D, Paschke A (2002) Effects of heavy metal contamination of soils on micronucleus induction in Tradescantia and on microbial enzyme activities: a comparative investigation. Mutat Res 515:111–124
Mandal C, Ghosh N, Maiti S, Das K, Gupta S, Dey N, Adak MK (2013) Antioxidative responses of Salvinia (Salvinia natans Linn.) to aluminium stress and it’s modulation by polyamine. Physiol Mol Biol Plants 19:91–103
Mandal C, Ghosh N, Dey N, Adak MK (2014) Effects of putrescine on oxidative stress induced by hydrogen peroxide in Salvinia natans L. J Plant Interact 9(1):550–558
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15(4):523–530
Moschou PN, Wu J, Cona A, Tavladoraki P, Angelini R, Roubelakis Angelakis KA (2012) The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J Exp Bot 63:5003–5015
Nazar R, Iqbal N, Masood A, Khan M, Syeed S, Khan N (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Amer J Plant Sci 3:1476–1489
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Oyaizu M (1986) Studies on the product of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Pál M, Csávás G, Szalai G, Tímea O, Khalil R, Yordanova R, Gell G, Birinyi Z, Németh E, Janda T (2017) Polyamines may influence phytochelatin synthesis during cd stress in rice. J Hazard Mater 340:272–280
Poduslo JF, Curran GL (1996) Increased permeability of superoxide dismutase at the blood-nerve and blood-brain barriers with retained enzymatic activity after modification with naturally occurring polyamine, putrescine. J Neurochem 67:734–741
Porebski S, Bailey LG, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Report 15:8–15
Pottosin I, Velarde-Buendía AM, Bose J, Zepeda-Jazo I, Shabala S, Dobrovinskaya O (2014) Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: implications for plant adaptive responses. J Exp Bot 65:1271–1283
Quartacci MF, Cosi E, Navari-Izzo F (2001) Lipids and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. J Exp Bot 52:77–84
Rady MM, Hemida KA (2015) Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol Environ Saf 119:178–185
Rady MM, Seif El-Yazal MA, Taie HAA, Ahmed SMA (2016a) Response of wheat growth and productivity to exogenous polyamines under lead stress. J Crop Sci Biotechnol 19(5):363–371
Rady MM, Seif El-Yazal MA, Taie HAA, Ahmed SMA (2016b) Response of Triticum aestivum (L.) plants grown under cadmium stress to polyamines pretreatments. Plant 4(5):29–36
Rady MM, Elrys AS, Abo El-Maati MF, Desoky EM (2019a) Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris plant. Plant Physiol Biochem 139:558–568
Rady MM, Kuşvuran A, Alharby HF, Alzahrani Y, Kuşvuran S (2019b) Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. J Plant Growth Regul https://doi.org/10.1007/s00344-018-9860-5
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Scoccianti V, Iacobucci M, Speranza A, Antognoni F (2013) Over accumulation of putrescine induced by cyclo hexylamine interferes with chromium accumulation and partially restores pollen tube growth in Actinidiadeliciosa. Plant Physiol Biochem 70:424–432
Semida WM, Rady MM, Abd El-Mageed TA, Howladar SM, Abdelhamid MT (2015) Alleviation of cadmium toxicity in common bean (Phaseolus vulgaris L.) plants by the exogenous application of salicylic acid. J Hortic Sci Biotechnol 90:83–91
Semida WM, Hemida KA, Rady MM (2018) Sequenced ascorbate-proline-glutathione seed treatment elevates cadmium tolerance in cucumber transplants. Ecotoxicol Environ Saf 154:171–179
Shevyakova NI, Il’ina EN, Stetsenko LA, Kuznetsov VIV (2010) Nickel accumulation in rape shoots (Brassica Napus L.) increased by putrescine. Int J Phytoremed 13(4):345–356
Singh Y, Malik CP (2011) Phenols and their antioxidant activity in Brassica juncea seedlings growing under HgCl2 stress. J Microbiol Biotech Res 1(4):124–130
Taneja SR, Sachar RC (1974) Introduction of polyphenol oxidase in germinating wheat seeds. Phytochem 13:2695–2702
Taylor RSL, Hudson JB, Manandhar NP, Towers GHN (1996) Antiviral activities of medicinal plants of southern Nepal. J Ethnopharmacol 53:97–104
Tiburcio AF, Kaur-Sawhney R, Galston AW (1990) Polyamine metabolism. In: Intermedatory nitrogen metabolism. 16, the biochemistry of plants. Miflin, B.J., Lea, P.J. (Ed). Academic Press, pp. 283–325
Tiburcio AF, Altabella T, Masgrau C (2002) Polyamines. In: Bisseling T, Schell J (eds) New developments in plant hormone research. Springer-Verlag, New York
Velarde-Buendía AM, Shabala S, Cvikrova M, Dobrovinskaya O, Pottosin I (2012) Salt-sensitive and salt-tolerant barley varieties differ in the extent of potentiation of the ROS-induced K+ efflux by polyamines. Plant Physiol Biochem 61:18–23
Voronova A, Belevich V, Jansons A, Rungis D (2014) Stress-induced transcriptional activation of retro transpos on-like sequences in the Scots pine (Pinus sylvestris L.) genome. Tree Genet Genomes 10:937–951
Walden R, Alexandra C, Tiburcio AF (1997) Polyamines: small molecules triggering pathways in plant growth and development. Plant Physiol 113:1009–1013
Wu J-W, Shi Y, Zhu Y-X, Wang Y-C, Gong H-J (2013) Mechanisms of enhanced heavy metal tolerance in plants by silicon: a review. Pedosphere 23(6):815–825
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Yin LM (1994) Effects of polyamines and kinetin on in vitro frond senescence in Lemna aequinoctialis. Acta Bot Sin 36:522–527 in Chinese with an English abstract
Zhao H, Yang H (2008) Exogenous polyamines alleviate the lipid peroxidation induced by cadmium chloride stress in Malus hupehensis. Rehd Sci Hortic 116:442–447
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taie, H.A.A., Seif El-Yazal, M.A., Ahmed, S.M.A. et al. Polyamines modulate growth, antioxidant activity, and genomic DNA in heavy metal–stressed wheat plant. Environ Sci Pollut Res 26, 22338–22350 (2019). https://doi.org/10.1007/s11356-019-05555-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05555-7