Abstract
Key Message Transcriptome analysis of SA sensitive and tolerant lines indicates that SA enhances anthracnose resistance in sorghum by upregulating the expression of some immune-related genes and pathways.
Abstract Anthracnose caused by the hemibiotrophic pathogen Colletotrichum sublineolum is one of the most destructive diseases of sorghum, the fifth most important cereal crop in the world. Salicylic acid (SA) is a phytohormone essential for plant immunity; however, the role of SA in sorghum resistance to anthracnose has not been well explored. In this study, we found that Colletotrichum sublineolum infection induced the expression of SA-responsive genes and that exogenous SA enhanced resistance to anthracnose in the sorghum line BTx623. To rule out the possibility that SA triggers anthracnose resistance in sorghum by its direct toxic function on pathogen, an SA-tolerant line, WHEATLAND, was identified, and we found that SA treatment could not induce anthracnose resistance in WHEATLAND. Then, SA-induced transcriptome changes during Colletotrichum sublineolum infection in BTx623 and WHEATLAND were analyzed to explore the molecular mechanism of SA-triggered resistance. SA pretreatment regulated the expression of 2125 genes in BTx623 but only 524 genes in WHEATLAND during Colletotrichum sublineolum infection. The cutin, suberine and wax biosynthesis pathway involved in the plant immune response and the flavonoid biosynthesis pathway involved in anthracnose resistance were enriched in BTx623-specifically upregulated genes. Additionally, some immune-related genes, including multiple resistance genes, were differentially expressed in BTx623 and WHEATLAND. Taken together, our results revealed the mechanisms of SA-induced anthracnose resistance in sorghum at the transcriptional level and shed light on the possibility of enhancing sorghum resistance to anthracnose by activating the SA signaling pathway by molecular breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sorghum [Sorghum bicolor (L.) Moench] is an important grain crop and serves as the main cereal food for approximately 500 million people in Africa and Asia (Khoddami et al. 2021). Moreover, sorghum is a major source of forage, animal feed and biofuel production (Colombini et al. 2012; Rooney et al. 2007). As a diploid C4 crop, the small genome of sorghum (approximately 730 Mb) also makes it a candidate model to explore conserved molecular mechanisms among C4 crops (Calvino and Messing 2012; Paterson et al. 2009). However, as a sessile plant, the growth and production of sorghum are affected by environmental factors, including pathogens. Among all pathogens, Colletotrichum sublineolum (C. sublineolum), a hemibiotrophic fungal pathogen, causes anthracnose, which is one of the most devastating diseases of sorghum in most growing areas worldwide (Moore et al. 2008; Wharton et al. 2001). C. sublineolum can infect multiple tissues of sorghum, including leaves, stalks and panicles, and causes grain yield losses as high as 86% (Cota et al. 2017). Currently, this disease is generally controlled by fungicide application, which is environmentally unfriendly and could lead to the development of fungicide-resistant strains if used frequently (Acharya et al. 2019).
Improving the resistance of cultivated sorghum to anthracnose is a sustainable strategy to reduce yield loss and fungicide application. To this end, many efforts have been made to elucidate the molecular mechanism of the interaction between C. sublineolum and sorghum by sequencing the genome of C. sublineolum, detecting the transcriptome changes of sorghum after C. sublineolum inoculation, and exploring the resistance genes of sorghum. The draft genome of C. sublineolum was first reported in 2014 and later improved by using PacBio sequencing combined with RNA-seq (Baldrich et al. 2021; Baroncelli et al. 2014). The total genome size of C. sublineolum is approximately 68 Mb, harboring 31,251 genes (Baldrich et al. 2021). This reference genome provides new insight into the pathogenicity of C. sublineolum. Infection with C. sublineolum causes transcriptome changes in sorghum in both anthracnose-resistant and anthracnose-susceptible cultivars, with thousands of genes being up/downregulated (Fu et al. 2020; Natarajan et al. 2021). Differences were observed in the regulation of some genes between anthracnose-resistant and anthracnose-susceptible cultivars, suggesting that the expression of these genes plays a role in the difference in disease response between them (Natarajan et al. 2021). Moreover, some microRNAs are also differentially expressed between anthracnose-resistant and anthracnose-susceptible cultivars, indicating that microRNAs and their targets also have potential functions in the sorghum response to C. sublineolum (Fu et al. 2020). To explore the genes determining sorghum resistance to C. sublineolum, Hugo Cuevas et al. used a set of 335 accessions to perform genome-wide associated mapping and detected a locus constituted by three resistance (R) genes on chromosome 9 (Cuevas et al. 2019). Dozens of other marker loci associated with anthracnose resistance in sorghum have also been identified in different studies by association analysis, indicating the complicated interaction between C. sublineolum and sorghum (Ahn et al. 2019, 2021; Cuevas et al. 2021; Mengistu et al. 2021; Prom et al. 2019; Singh et al. 2006; Upadhyaya et al. 2013). Recently, by crossing the anthracnose-resistant genotype SC283 with the anthracnose-susceptible genotype TAM428, Sanghum Lee et al. found that the enhanced expression of an R gene, anthracnose resistance gene 1, is responsible for anthracnose resistance in SC283, which provides a direct application candidate for controlling anthracnose (Lee et al. 2022). Even with all these findings, knowledge about the interaction of C. sublineolum and sorghum is still scant.
In addition to R genes, phytohormones also play prominent roles in plant immunity. Among immune-related phytohormones, salicylic acid mediates plant resistance to biotrophic and hemibiotrophic pathogens (Boatwright and Pajerowska-Mukhtar 2013). SA is synthesized through two pathways in planta, the isochorismate pathway and the phenylalanine ammonia lyase pathway, and is perceived by nonexpressor of pathogenesis-related gene family proteins (Ding and Ding 2020). In sorghum, SA can be detected, and exogenous application of SA results in transcriptome changes, indicating that both SA synthesis and signaling components exist in sorghum (Grover et al. 2020; Hithamani and Srinivasan 2014; Lohani and Muthukumarappan 2020; Salzman et al. 2005). However, the SA synthesis genes or receptors in sorghum have not been identified. As C. sublineolum is a hemibiotrophic pathogen and SA treatment has been proven to alter sorghum growth and responses to environmental factors (Dehnavi et al. 2019; Gao et al. 2004; Hussain et al. 2014), we believe that SA should be involved in sorghum resistance to C. sublineolum.
In this study, we found that infection with C. sublineolum induced the expression of SA-responsive genes and that SA treatment enhanced sorghum resistance to anthracnose in the SA-sensitive line but not in the SA-tolerant line. By analyzing SA-induced transcriptional changes during C. sublineolum infection in both SA-sensitive and SA-tolerant lines, we identified two pathways and multiple immune-related genes that may play critical roles in SA-induced anthracnose resistance in sorghum. Our study indicates that enhancing SA signaling should be a new breeding strategy for obtaining anthracnose-resistant sorghum.
Materials and methods
Sorghum and pathogen growth conditions
Seeds of BTx623 (PI659985) and WHEATLAND (PI655975) were gifts from Dr. Qi Xie of the Institute of Genetics and Developmental Biology, Chinese Academy of Science. For infection or SA treatment, sorghum seeds were sterilized in 10% bleach for 10 min and rinsed with ddH2O three times. The sterilized seeds were sown in soil in 7 × 7 cm pots. Plants were gown in a growth chamber with a constant temperature of 26 °C and a 20 h/4 h light/dark cycle with a light intensity of 150 µE m-2 s-1. For seed collection, plants were grown in the field from May to October.
The pathogenic C. sublineolum was provided by Wenming Wang of Sichuan Agricultural University, China. C. sublineolum was cultured on potato dextrose agar plates (Potato infusion (boiled 200 g potato in one liter ddH2O and the supernatant was used for preparing PDA culture medium), 20 g/l dextrose and 20 g/l agar) at 25 °C for 7 days, and then the fungi were moved to 20% V8 liquid (20% V8 vegetable juice (Campbell soup company) and 80% ddH2O, pH 3.9) and shaken at 130 rpm for 7 days with a 12/12 light/dark cycle for spore production. The spores of C. sublineolum were collected by filtering the culture using filter paper and centrifugation. Then, spore suspensions were placed on a haemocytometer and quantified using light microscope at 400 x magnification (Tugizimana et al. 2018).
Pathogen infection
To measure the expression of SA-responsive genes during anthracnose in sorghum, two-week-old seedlings of BTx623 were sprayed with 5 × 106 ml− 1 C. sublineolum spores in water containing 2% Tween 20. After pathogen inoculation, the seedlings were covered with a plastic dome to create a high humidity condition to facilitate pathogen infection. Photos were taken before and four days after C. sublineolum infection. Samples were collected at the corresponding times for qRT–PCR. To quantify the disease symptoms, the third leaves of BTx623 and WHEATLAND seedlings were dropped with 5 µl water containing 5 × 106 ml− 1 C. sublineolum spores and 2% Tween 20. Ten leaves, with 10 drops on each, were used per line. Photos were taken at four days post-inoculation, and the infected lesion was quantified using ImageJ. Then, the inoculated spots were punched out, and DNA was extracted using CTAB buffer (0.1 M Tris-HCl (pH 8.0), 0.02 M EDTA (pH 8.0), 81.9 g/l NaCl, 20 g/l CTAB, and 20 g/l polyvinylpyrrolidone). qPCR was performed to measure the DNA level of ITS2 using the QuantStudio 5 Real-Time system and SYBR Green Master mix PCR kit (Mei5bio) as recommended by the manufacturer (Weihmann et al. 2016). Five leaf discs were collected as one sample, and 12 biological replicates were collected for each treatment per line. SbELF4α was used as an internal control (Jain et al. 2008).
For SA-induced anthracnose resistance in sorghum, two-week-old seedlings of BTx623 or WHEATLAND were treated with water or 2 mM SA for 1 day and then infected with C. sublineolum as described above.
BTH treatment
Two-week-old seedlings of different sorghum lines were pre-dried for 3 days and then watered with water (CK) or 1.5 mM BTH (Syngenta). 100 ml water or BTH solution was used for each 7 × 7 cm pot. 30 ~ 60 plants were used for each treatment per line. Two weeks after treatment, the height of each plant was measured using a ruler. This experiment was repeated three times with similar results.
Transcriptional profiling
For SA-induced transcriptome changes in BTx623 and WHEATLAND, two-week-old seedlings were sprayed with water (CK) or 2 mM SA for 1 day, and then leaves from 15 to 20 seedlings were collected as one biological replicate. Three biological replicates were collected for each treatment per line. Samples were frozen in liquid nitrogen and stored at -80 °C before RNA extraction. RNA extraction, RNA quality control, library construction and sequencing were performed by Biomarker Technologies Inc. Sequencing was performed on an Illumina platform.
For SA induced transcriptome changes during C. sublineolum infection, two-week-old seedlings were pretreated with water (CK) or 2 mM SA for 1 day and then inoculated with 5 × 106 ml− 1 C. sublineolum spores for 24 h. Samples were collected from leaves of 15–20 seedlings as one biological replicate, and three biological replicates were collected for each treatment per line. RNA extraction, library construction and sequencing were performed as described above.
Differentially expressed genes (DEGs) were identified between SA-treated and water-treated samples for each line with a fold change ≥ 1.5. Venn diagrams were created using online software (http://bioinformatics.psb.ugent.be/webtools/Venn), and KEGG analysis was performed using the analysis platform of Biomarker Technologies Inc. Heatmaps with DEG data were constructed using online software (http://www.ehbio.com/ImageGP) or the analysis platform of Biomarker Technologies Inc.
qRT-PCR
Total RNA was isolated using TRIzol (Takara) and treated with RQ1 DNase (Promega) to remove DNA, and then cDNA was synthesized by using the PrimeScript RT Master kit (Takara) according to the manufacturer’s instructions. The relative expression levels of target genes were checked by qPCR using the QuantStudio 5 Real-Time system and SYBR Green Master mix PCR kit (Mei5bio) as recommended by the manufacturer. The SbELF4α gene was used as an internal control to normalize the expression of target genes. The primers used are summarized in Supplemental Table 1. Each data point is the mean value of three replicates, and the significance was determined by Student’s t test using GraphPad Prism.
Results
SA-responsive genes are upregulated by C. sublineolum in sorghum
The phytohormone SA plays an essential role in plant defense against multiple pathogens, especially biotrophic and hemibiotrophic pathogens (Boatwright and Pajerowska-Mukhtar 2013). C. sublineolum is a hemibiotrophic pathogen whose infection causes anthracnose in sorghum (Wharton et al. 2001). To examine whether SA is involved in sorghum resistance to anthracnose, we first tested whether C. sublineolum infection activates SA signaling. Two-week-old seedlings of sorghum line BTx623 were sprayed with 5 × 106 ml-1 C. sublineolum spores (Fig. 1 A) and covered with a plastic dome after pathogen treatment to keep the seedlings in a high humidity condition. Four days after pathogen inoculation, infected leaves already showed clear disease symptoms (Fig. 1B). Then, C. sublineolum-infected samples were collected at 1, 2, 3 or 4 days post-inoculation (dpi) with uninfected samples (0 dpi) as a control, and RNA was extracted for qPCR analysis. Three SA-responsive genes, sorghum pathogen related 3 (SbPR3), sorghum pathogen related 10 (SbPR10) and sorghum water stressed 1–40 (SbWS1-40), chosen from published RNA-seq data, were used to represent the status of SA signaling (Salzman et al. 2005). We first confirmed that the expression of these SA-responsive genes was indeed induced by SA (Supplemental Fig. 1) and then tested their expression in C. sublineolum-infected samples. As shown in Fig. 1 C, the expression of all three SA-responsive genes was upregulated by C. sublineolum. While the induction of SbPR3 was transient at 1 dpi, the induction of SbPR10 and SbWS1-40 was consistently induced in all the samples, indicating that they may represent different SA signaling pathways in sorghum (Fig. 1 C). Our results indicate that SA signaling may be involved in the interaction of sorghum and anthracnose.
Colletotrichum sublineolum infection activates SA signaling in Sorghum. Two-week-old seedlings were sprayed with spores of C. sublineolum (5 × 106 spores/l) for 4 days, and the expression of SA-responsive genes was analyzed. A Representative seedlings used for C. sublineolum infection. The representative leaf is shown on the right. B Seedlings 4 days post-inoculation (4 dpi) with C. sublineolum. The representative infected leaf is shown on the right. C The expression of SA-responsive genes with or without C. sublineolum infection. The gene expression level was normalized to SbELF4α. Significant differences were detected using Student’s t test. Data are shown as the mean ± SD (n = 3). ns, no significant difference; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001
SA induces resistance to anthracnose in the sorghum line BTx623
According to our results in Fig. 1, SA may play a role in sorghum defense against anthracnose. Thus, we further treated BTx623 with 2 mM SA to determine whether the application of exogenous SA could induce sorghum resistance to anthracnose. To easily quantify the disease symptoms, we dropped 5 µl droplets of water containing C. sublineolum spores on the leaf surface of 2-week-old sorghum seedlings. Four days later, disease symptoms were monitored, and leaf discs of the infected sites were collected to quantify pathogen growth. Compared with water pretreatment, SA treatment significantly reduced the lesion size caused by C. sublineolum from 4.67 to 2.04 mm2 (Fig. 2 A, B). Pathogen growth was further quantified by analyzing the DNA level of C. sublineolum represented by the internally transcribed spacer 2 (ITS2) gene. Consistently, less pathogen DNA was detected in SA-pretreated samples than in water-pretreated samples (Fig. 2 C). Collectively, these data demonstrate that SA could induce sorghum resistance to anthracnose.
SA induces anthracnose resistance in BTx623. Two-week-old seedlings were treated with 2 mM SA for 1 day, and then 5 µl spores of C. sublineolum (5 × 106 spores/l) were dropped on the leaf surface for 4 days. A The disease symptoms of BTx623-treated water (Mock), water then pathogen (Water) and SA then pathogen (SA). B The lesion sizes were measured by ImageJ. C The DNA level of the pathogen internally transcribed spacer 2 (ITS2) gene, which is normalized to the DNA level of the sorghum gene SbELF4α. Significant differences were detected using Student’s t test. Data are shown as the mean ± SD (n = 3). ns, no significant difference; ***, p < 0.001; ****, p < 0.0001
Identification of the SA sensitive and tolerant lines
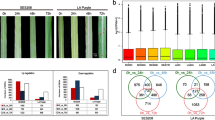
It has been reported that SA could influence spore germination of Colletotrichum spp. (Zhang et al. 2016). To rule out the possibility that SA triggers anthracnose resistance in sorghum by its direct toxic function on C. sublineolum, a negative sorghum control that cannot respond to SA is needed. As SA signaling in sorghum has rarely been studied and none of the key components in SA signaling have been identified in sorghum, we wondered if there is natural variation in sorghum lines that show different sensitivities to SA. To easily detect the sensitivity of sorghum lines to SA, we treated two-week-old seedlings of BTx623 with 1.5 mM benzothiadiazole (BTH), an SA ortholog that can inhibit the growth of Arabidopsis (Canet et al. 2010). After two weeks of treatment, BTH significantly inhibited the growth of BTx623 seedlings, confirming that BTx623 is an SA-sensitive line (Fig. 3 A). This result also indicates that BTH-triggered growth inhibition is an efficient strategy to screen the SA sensitivity of sorghum lines. Then, we checked the SA sensitivity of all sorghum lines available in our lab and found that WHEATLAND is a line that compromised the response to BTH (Fig. 3 A). BTH treatment inhibited the height of BTx623 from 31.62 to 15.96 cm but only inhibited WHEATLAND from 32.25 to 26.85 cm (Fig. 3B).
Identification of SA-sensitive and SA-tolerant lines. A The influence of BTH on plant growth of BTx623 and WHEATLAND. Two-week-old seedlings were treated with water or 1.5 mM BTH for 2 weeks. The representative plants are shown. B The height of water- and BTH-treated plants was measured by a ruler. Significant differences were detected using Student’s t test. Data are shown as the mean ± SD. ****, p < 0.0001. C The number of differentially expressed genes (DEGs) up/downregulated by SA in BTx623 and WHEATLAND. Two-week-old seedlings were treated with water or SA for 1 day. RNA was extracted for sequencing. D Venn diagrams of DEGs induced or repressed by SA in BTx623 and WHEATLAND. E Heatmap representing the transcript levels of genes that were co-up/downregulated in both BTx623 and WHEATLAND. The color gradient represents log2 (FPKM + 1)
To further confirm that BTx623 is sensitive and WHEATLAND is tolerant to SA, SA-induced transcriptome changes were examined in these two lines. Two-week-old seedlings were treated with water (CK) or 2 mM SA for 24 h, and samples were collected for RNA sequencing (RNA-seq) using Illumina high-throughput sequencing technology. A summary of the RNA-seq data and the correlated genome mapping information is shown in Supplemental Table 2. A total of 5355 differentially expressed genes (DEGs) were identified in BTx623, with 2462 SA upregulated DEGs and 2893 SA downregulated DEGs, while only 3444 DEGs were detected in WHEATLAND, with 1676 upregulated and 1768 downregulated DEGs (Fig. 3 C). The numbers of DEGs were significantly lower in WHEATLAND than in BTx623, further confirming that WHEATLAND is compromised in the SA response. To verify the RNA-seq results, we determined the abundance of 6 BTx623 specifically upregulated DEGs, Sobic.001G378300, Sobic.003G105800, Sobic.001G425100, Sobic.002G125700, Sobic.002G322900, and Sobic.002G275100, by qPCR assay. Consistent with the RNA-seq data, the expression of all these genes was induced by SA in BTx623 but not WHEATLAND (Supplemental Fig. 2). Comparing the DEGs in BTx623 and WHEATLAND, 1394 and 1755 nonredundant upregulated and downregulated DEGs were specifically identified in BTx623, respectively, representing 58.80% of the total SA-regulated genes in this line (Fig. 3D). Gene ontology (GO) analysis of DEGs specifically upregulated by SA in BTx623 enriched response to stimulus process, multi-organism process and immune system process et al. (Supplemental Fig. 3). A total of 1068 and 1138 DEGs were upregulated and downregulated in both BTx623 and WHEATLAND (Fig. 3D). We further analyzed the expression of these DEGs and found that their induction/repression was higher in BTx623 than in WHEATLAND (Fig. 3E). All of these results prove that BTx623 is an SA-sensitive sorghum line, while WHEATLAND is an SA-tolerant sorghum line. We speculate that a key regulator of SA signaling is mutated in WHEATLAND.
SA-triggered resistance to anthracnose is compromised in the SA-tolerant line
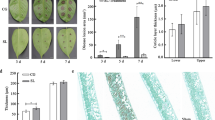
Based on the observation that the SA response was significantly dampened in WHEATLAND, we assume that SA could not induce anthracnose resistance in this line. To test this assumption, two-week-old seedlings of WHEATLAND were first treated with 2 mM SA for 1 day and then inoculated with C. sublineolum spores for another 4 days. Even though C. sublineolum caused similar disease symptoms in WHEATLAND and BTx623 in control samples, SA treatment significantly reduced the lesion size in BTx623 but had no effect in WHEATLAND (Fig. 4 A, B). The qPCR results, which examined the DNA level of C. sublineolum in the infected area, showed that the accumulation of C. sublineolum was not affected by SA in WHEATLAND, further confirming that SA could not trigger anthracnose resistance in this line (Fig. 4 C).
SA-induced anthracnose resistance is compromised in WHEATLAND. Experiments were performed as described in Fig. 2. A The disease symptoms of BTx623 and WHEATLAND treated with water (mock), water then pathogen (water) and SA then pathogen (SA). B The lesion sizes of samples were measured by ImageJ. C The DNA level of the pathogen ITS2 gene, which is normalized to the DNA level of the sorghum gene SbELF4α. Significant differences were detected by two-way ANOVA. Data are shown as the mean ± SD (n = 12). *, p < 0.05; ****, p < 0.0001
SA specifically activates two immune-related pathways in BTx623 during C. sublineolum infection
To explore the possible regulatory mechanism of SA on anthracnose resistance in sorghum, we carried out RNA-seq analysis on two different treatments, water pretreatment plus C. sublineolum inoculation (P) and SA pretreatment plus C. sublineolum inoculation (SA + P), in BTx623 and WHEATLAND. Supplemental Table 3 shows the sequencing metrics of the 12 RNA-seq libraries. A total of 905 DEGs were induced by SA pretreatment, and 1220 were repressed in BTx623, while only 317 upregulated and 207 downregulated DEGs in WHEATLAND (Fig. 5 A). The upregulated DEGs were involved in multiple pathways (Supplemental Fig. 4). Among all the DEGs in BTx623, 174 upregulated and 130 downregulated DEGs were shared with WHEATLAND DEGs, and 731 upregulated and 1090 downregulated DEGs were BTx623 specific (Fig. 5B). We further investigated which pathways were specifically induced in BTx623 after SA pretreatment. As shown in Fig. 5 C, genes involved in peroxisome, starch and sucrose metabolism, and tyrosine metabolism etc. could be enriched in KEGG analysis with the 731 BTx623-specific upregulated DEGs (Fig. 5 C). Among them, the cutin, suberine and wax biosynthesis pathway is involved in plant defense and the flavonoid biosynthesis pathway is known to positively regulate sorghum defense to C. sublineolum (Schreiber 2010; Serrano et al. 2014; Tugizimana et al. 2018). More interestingly, these two pathways were only enriched in BTx623, but not in WHEATLAND, upregulated DEGs (Supplemental Fig. 4). Thus, the activation of these two pathways may play roles in SA-induced anthracnose resistance. To verify the RNA-seq results, five genes that were specifically induced in BTx623 were selected for qPCR. In general, their relative expression patterns were consistent with the sequencing results (Fig. 5D, E).
Transcriptome changes during C. sublineolum infection induced by SA in BTx623 and WHEATLAND. A The number of DEGs up/downregulated by SA during pathogen infection in BTx623 and WHEATLAND. Two-week-old seedlings were treated with water or SA for 1 day and then inoculated with spores of C. sublineolum (5 × 106 spores/l). RNA was extracted for sequencing. B Venn diagrams of DEGs induced or repressed by SA during C. sublineolum infection in BTx623 and WHEATLAND. C Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment (P < 0.05) of the genes that were specifically upregulated in BTx623. D and E Validation of the expression of BTx623 specifically upregulated DEGs by qPCR in BTx623 (D) and WHEATLAND (E). The gene expression level was normalized to SbELF4α. Significant differences were detected using Student’s t test. Data are shown as the mean ± SD (n = 3). ns, no significant difference; *, p < 0.05; **, p < 0.01
Multiple immune-related genes were upregulated by SA
To further screen the potential genes that are essential for SA-induced anthracnose resistance in sorghum, the Top 30 DEGs that were exclusively upregulated in BTx623 SA + P samples were analyzed (Table 1). Their expression varied from 2.82- to 118.06-fold after SA pretreatment in BTx623 but was not detected or induced less than 1.5-fold by SA in WHEATLAND (Table 1). Among them, several genes were closely related to the plant immune response, such as Sobic. 001G0868800 (papain family cysteine protease), Sobic. 008G028600 (probable WRKY transcription factor 70) and Sobic. 003G064300 (glycine-rich cell wall structural protein). Papain family cysteine proteases are reported to be central hubs in the plant immune system and are required for full plant resistance to various pathogens (Misas-Villamil et al. 2016). WRKY transcription factors are essential components of SA signaling (Wang et al. 2006). WRKY70 and its orthologs are involved in plant defense in Arabidopsis, wheat and citrus (Deng et al. 2020; Hu et al. 2012; Wang et al. 2017). The cell wall is the physical barrier of plant cells to defend against pathogens. Modifying the cell wall acts as a hallmark of host basal defense and a known response of sorghum to C. sublineolum infection (Basavaraju et al. 2009; Malinovsky et al. 2014). SA may induce the expression of glycine-rich cell wall structural proteins to change the structure of the cell wall and then trigger defense responses (Bacete et al. 2018). These data indicate that SA induces the expression of multiple immune-related genes to trigger anthracnose resistance in sorghum.
It is well known that R proteins encoded by nucleotide binding-leucine rich repeat (NB-LRR) genes mediate the recognition of diverse effectors secreted by pathogens and initiate plant defense responses (Sun et al. 2020). Overexpression of R genes could enhance disease resistance in plants (Zhang et al. 2017; Zhu et al. 2017). More importantly, several identified sorghum anthracnose-resistant quantitative trait loci harbored R genes (Abreha et al. 2021). To explore whether SA functions by activating the expression of R genes to trigger anthracnose resistance, we analyzed the transcript levels of upregulated R genes in our RNA-seq data. In total, 62 R genes were upregulated by SA pretreatment (Fig. 6). Compared with the P samples, 48 R genes were activated in BTx623 SA + P samples, while only 23 R genes were induced in WHEATLAND SA + P samples. 9 R genes were upregulated by SA in both lines (Fig. 6 A), and 39 and 14 were only upregulated in BTx623 and WHEATLAND, respectively (Fig. 6B, C). SA induced more R genes in the SA-sensitive line, indicating that the upregulation of R genes may be another strategy used by SA to promote anthracnose resistance.
The relative expression levels of R genes that were upregulated in at least one line. A R genes that were upregulated in both BTx623 and WHEATLAND. B R genes that were specifically upregulated in BTx623. C R genes that were specifically upregulated in WHEATLAND. The color gradient represents log2 FC
Discussion
Anthracnose caused by Colletotrichum spp., one of the top 10 fungal pathogens in plant pathology, is a severe disease in a wide range of crops, including pear, pepper, maize and sorghum (Cruet-Burgos et al. 2020; Dean et al. 2012; Gorman et al. 2021; Li et al. 2022; Son et al. 2021). SA is a phytohormone that plays an essential role in plant immunity, including defense against anthracnose, and regulates anthracnose resistance in several plant species (Kubota and Nishi 2006; Lee et al. 2009; Sangpueak et al. 2021; Shi et al. 2019; Zhang et al. 2016). For example, a higher level of basal SA and faster SA induction after infection may be correlated with stronger anthracnose resistance, and exogenous SA treatment has been reported to trigger resistance to anthracnose in strawberry (Zhang et al. 2016). However, does SA regulate the Sorghum-C. sublineolum interaction has not been well explored. In this study, we proved that SA could trigger anthracnose resistance in sorghum. To exclude the possibility that SA induced resistance by influencing fungal viability, a SA-sensitive and a SA-tolerant sorghum lines were identified, and we deeply analyzed the potential molecular mechanism of how SA modulates anthracnose resistance in sorghum using the RNA-seq data of these two lines. Using an SA-tolerant line as a control could also accurately analyze the RNA-seq data.
Even SA treatment differentially regulated plant growth, transcription and defense in the SA-sensitive line BTx623 and the SA-tolerant line WHEATLAND; surprisingly, these two lines showed a similar disease phenotype after C. sublineolum infection in water-treated samples. This result indicates that the basal defense levels of BTx623 and WHEATLAND against anthracnose are comparable. We expect other signaling pathways to complement the deficiency of SA in WHEATLAND, as the plant immune system is very complex (Ngou et al. 2022). And not only SA but also jasmonic acid, abscisic acid and other immune-related pathways also regulate sorghum-C. sublineolum interaction (Abreha et al. 2021). Moreover, not all SA-insensitive mutants exhibit compromised basal defense. For example, pathogen accumulation in the triple mutant of TGA2/5/6, the key transcription factors in SA signaling, is comparable to that in the wild type when infected with the bacterial pathogen Pseudomonas syringe (Ding et al. 2018; Zhang et al. 2003).
We explored two KEGG pathways, the cutin, suberine and wax biosynthesis pathway and the flavonoid biosynthesis pathway, that may explain the potential molecular mechanism of SA-triggered anthracnose resistance in sorghum in this study. These two pathways have been reported to be involved in plant immunity and/or sorghum C. sublineolum interaction (Serrano et al. 2014; Tugizimana et al. 2018). Additionally, we also detected several genes that may be critical for SA-induced anthracnose resistance, such as orthologs of WRKY70 and cysteine protease, which have been identified as regulators of SA signaling and/or plant immune responses. Moreover, the regulation of R genes may also contribute to this process. All this funding indicates that we explored the correct pathways and genes that may be the targets of SA to induce anthracnose resistance in sorghum. We should check whether modifying these corresponding pathways and upregulating the potential target genes could enhance sorghum resistance to anthracnose in the future.
In summary, we clarified that SA-triggered anthracnose resistance in sorghum. By analyzing the RNA-seq data, the transcriptomic mechanisms for SA-induced anthracnose resistance in sorghum were explored. Our results identified several pathways and candidate genes that may be targets of sorghum breeding to improve anthracnose resistance through SA signaling.
Data Availability
The datasets generated during the current study are available from the corresponding author on request.
References
Abreha KB, Ortiz R, Carlsson AS, Geleta M (2021) Understanding the Sorghum-Colletotrichum sublineola Interactions for Enhanced Host Resistance. Front Plant Sci 12:641969
Acharya B, O’Quinn TN, Everman W, Mehl HL (2019) Effectiveness of Fungicides and Their Application Timing for the Management of Sorghum Foliar Anthracnose in the Mid-Atlantic United States. Plant Dis 103:2804–2811
Ahn E, Hu ZB, Perumal R, Prom LK, Odvody G, Upadhyaya HD, Magill C (2019) Genome wide association analysis of sorghum mini core lines regarding anthracnose, downy mildew, and head smut, vol 14. Plos One
Ahn E, Prom LK, Hu Z, Odvody G, Magill C (2021) Genome-wide association analysis for response of Senegalese sorghum accessions to Texas isolates of anthracnose. Plant Genome 14:e20097
Bacete L, Melida H, Miedes E, Molina A (2018) Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. Plant J 93:614–636
Baldrich P, Chaya T, Caplan JL, Meyers BC (2021) Genome Assembly and Transcriptome of Colletotrichum sublineola CsGL1, a New Resource to Study Anthracnose Disease in Sorghum. Mol Plant Microbe Interact 34:1209–1211
Baroncelli R, Sanz-Martin JM, Rech GE, Sukno SA, Thon MR (2014) Draft Genome Sequence of Colletotrichum sublineola, a Destructive Pathogen of Cultivated Sorghum. Genome Announc 2.
Basavaraju P, Shetty NP, Shetty HS, de Neergaard E, Jorgensen HJ (2009) Infection biology and defence responses in sorghum against Colletotrichum sublineolum. J Appl Microbiol 107:404–415
Boatwright JL, Pajerowska-Mukhtar K (2013) Salicylic acid: an old hormone up to new tricks. Mol Plant Pathol 14:623–634
Calvino M, Messing J (2012) Sweet sorghum as a model system for bioenergy crops. Curr Opin Biotech 23:323–329
Canet JV, Dobon A, Ibanez F, Perales L, Tornero P (2010) Resistance and biomass in Arabidopsis: a new model for salicylic acid perception. Plant Biotechnol J 8:126–141
Colombini S, Galassi G, Crovetto GM, Rapetti L (2012) Milk production, nitrogen balance, and fiber digestibility prediction of corn, whole plant grain sorghum, and forage sorghum silages in the dairy cow. J Dairy Sci 95:4457–4467
Cota LV, Souza AGC, Costa RV, Silva DD, Lanza FE, Aguiar FM, Figueiredo JEF (2017) Quantification of yield losses caused by leaf anthracnose on sorghum in Brazil. J Phytopathol 165:479–485
Cruet-Burgos CM, Cuevas HE, Prom LK, Knoll JE, Stutts LR, Vermerris W (2020) Genomic Dissection of Anthracnose (Colletotrichum sublineolum) Resistance Response in Sorghum Differential Line SC112-14. G3 (Bethesda). 10:1403–1412
Cuevas HE, Cruet-Burgos CM, Prom LK, Knoll JE, Stutts LR, Vermerris W (2021) The inheritance of anthracnose (Colletotrichum sublineola) resistance in sorghum differential lines QL3 and IS18760. Sci Rep 11:20525
Cuevas HE, Prom LK, Cruet-Burgos CM (2019) Genome-Wide Association Mapping of Anthracnose (Colletotrichum sublineolum) Resistance in NPGS Ethiopian Sorghum Germplasm. G3:2879–2885(Bethesda) 9
Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J et al (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430
Dehnavi AR, Zahedi M, Razmjoo J, Eshghizadeh H (2019) Effect of exogenous application of salicylic acid on salt-stressed sorghum growth and nutrient contents. J Plant Nutr 42:1333–1349
Deng B, Wang W, Ruan C, Deng L, Yao S, Zeng K (2020) Involvement of CsWRKY70 in salicylic acid-induced citrus fruit resistance against Penicillium digitatum. Hortic Res 7:157
Ding PT, Ding YL (2020) Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci 25:549–565
Ding Y, Sun T, Ao K, Peng Y, Zhang Y, Li X, Zhang Y (2018) Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 173:1454–1467e1415
Fu F, Girma G, Mengiste T (2020) Global mRNA and microRNA expression dynamics in response to anthracnose infection in sorghum. BMC Genomics 21:760
Gao XQ, Zeng XC, Xia K, Yoshihara T, Zhou X (2004) Interactive effects of methyl jasmonate and salicylic acid on floret opening in spikelets of Sorghum. Plant Growth Regul 43:269–273
Gorman Z, Tolley JP, Koiwa H, Kolomiets MV (2021) The Synthesis of Pentyl Leaf Volatiles and Their Role in Resistance to Anthracnose Leaf Blight. Front Plant Sci 12:719587
Grover S, Agpawa E, Sarath G, Sattler SE, Louis J (2020) Interplay of phytohormones facilitate sorghum tolerance to aphids. Plant Mol Biol, https://doi.org/10.1007/s11103-020-01083-y
Hithamani G, Srinivasan K (2014) Bioaccessibility of Polyphenols from Wheat (Triticum aestivum), Sorghum (Sorghum bicolor), Green Gram (Vigna radiata), and Chickpea (Cicer arietinum) as Influenced by Domestic Food Processing. J Agr Food Chem 62:11170–11179
Hu Y, Dong Q, Yu D (2012) Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae. Plant Sci 185–186; 288–297
Hussain B, War AR, Sharma HC (2014) Jasmonic and salicylic acid-induced resistance in sorghum against the stem borer Chilo partellus. Phytoparasitica 42:99–108
Jain M, Chourey PS, Li QB, Pring DR (2008) Expression of cell wall invertase and several other genes of sugar metabolism in relation to seed development in sorghum (Sorghum bicolor). J Plant Physiol 165:331–344
Khoddami A, Messina V, Vadabalija Venkata K, Farahnaky A, Blanchard CL, Roberts TH (2021) Sorghum in foods: Functionality and potential in innovative products.Crit Rev Food Sci Nutr;1–17
Kubota M, Nishi K (2006) Salicylic acid accumulates in the roots and hypocotyl after inoculation of cucumber leaves with Colletotrichum lagenarium. J Plant Physiol 163:1111–1117
Lee S, Fu F, Liao CJ, Mewa DB, Adeyanju A, Ejeta G, Lisch D, Mengiste T (2022) Broad spectrum fungal resistance in sorghum is conferred through the complex regulation of an immune receptor gene embedded in a natural antisense transcript. Plant Cell 34: 1641–1665
Lee S, Hong JC, Jeon WB, Chung YS, Sung S, Choi D, Joung YH, Oh BJ (2009) The salicylic acid-induced protection of non-climacteric unripe pepper fruit against Colletotrichum gloeosporioides is similar to the resistance of ripe fruit. Plant Cell Rep 28:1573–1580
Li C, Sun W, Cao S, Hou R, Li X, Ming L, Kan J, Zhao Y, Liu F (2022) The CfMK1 Gene Regulates Reproduction, Appressorium Formation, and Pathogenesis in a Pear Anthracnose-Causing Fungus. J Fungi (Basel) 8.
Lohani UC, Muthukumarappan K (2020) Influence of fermentation followed by ultrasonication on functional properties of sorghum extrudates.J Food Process Eng43
Malinovsky FG, Fangel JU, Willats WG (2014) The role of the cell wall in plant immunity. Front Plant Sci 5:178
Mengistu G, Shimelis H, Assefa E, Lule D (2021) Genome-wide association analysis of anthracnose resistance in sorghum [Sorghum bicolor (L.) Moench]. PLoS ONE 16:e0261461
Misas-Villamil JC, van der Hoorn RA, Doehlemann G (2016) Papain-like cysteine proteases as hubs in plant immunity. New Phytol 212:902–907
Moore JW, Ditmore M, TeBeest DO (2008) Pathotypes of Colletotrichum sublineolum in Arkansas. Plant Dis 92:1415–1420
Natarajan P, Ahn E, Reddy UK, Perumal R, Prom LK, Magill C (2021) RNA-Sequencing in Resistant (QL3) and Susceptible (Theis) Sorghum Cultivars Inoculated With Johnsongrass Isolates of Colletotrichum sublineola. Front Genet 12:722519
Ngou BPM, Ding P, Jones JD (2022) Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 34:1447–1478
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A et al (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457:551–556
Prom LK, Ahn E, Isakeit T, Magill C (2019) GWAS analysis of sorghum association panel lines identifies SNPs associated with disease response to Texas isolates of Colletotrichum sublineola. Theor Appl Genet 132:1389–1396
Rooney WL, Blumenthal J, Bean B, Mullet JE (2007) Designing sorghum as a dedicated bioenergy feedstock. Biofuel Bioprod Bior 1:147–157
Salzman RA, Brady JA, Finlayson SA, Buchanan CD, Summer EJ, Sun F, Klein PE, Klein RR, Pratt LH, Cordonnier-Pratt MM et al (2005) Transcriptional profiling of sorghum induced by methyl jasmonate, salicylic acid, and aminocyclopropane carboxylic acid reveals cooperative regulation and novel gene responses. Plant Physiol 138:352–368
Sangpueak R, Phansak P, Thumanu K, Siriwong S, Wongkaew S, Buensanteai N (2021) Effect of Salicylic Acid Formulations on Induced Plant Defense against Cassava Anthracnose Disease. Plant Pathol J 37:356–364
Schreiber L (2010) Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci 15:546–553
Serrano M, Coluccia F, Torres M, L’Haridon F, Metraux JP (2014) The cuticle and plant defense to pathogens. Front Plant Sci 5:274
Shi YL, Sheng YY, Cai ZY, Yang R, Li QS, Li XM, Li D, Guo XY, Lu JL, Ye JH et al (2019) Involvement of Salicylic Acid in Anthracnose Infection in Tea Plants Revealed by Transcriptome Profiling. Int J Mol Sci 20: 2439
Singh M, Chaudhary K, Boora KS (2006) RAPD-based SCAR marker SCA 12 linked to recessive gene conferring resistance to anthracnose in sorghum [Sorghum bicolor (L.) Moench]. Theor Appl Genet 114:187–192
Son S, Kim S, Lee KS, Oh J, Choi I, Do JW, Yoon JB, Han J, Choi D, Park SR (2021) Identification of the Capsicum baccatum NLR Protein CbAR9 Conferring Disease Resistance to Anthracnose. Int J Mol Sci 22: 12612
Sun Y, Zhu YX, Balint-Kurti PJ, Wang GF (2020) Fine-Tuning Immunity: Players and Regulators for Plant NLRs. Trends Plant Sci 25:695–713
Tugizimana F, Djami-Tchatchou AT, Steenkamp PA, Piater LA, Dubery IA (2018) Metabolomic Analysis of Defense-Related Reprogramming in Sorghum bicolor in Response to Colletotrichum sublineolum Infection Reveals a Functional Metabolic Web of Phenylpropanoid and Flavonoid Pathways. Front Plant Sci 9:1840
Upadhyaya HD, Wang YH, Sharma R, Sharma S (2013) Identification of genetic markers linked to anthracnose resistance in sorghum using association analysis. Theor Appl Genet 126:1649–1657
Wang D, Amornsiripanitch N, Dong X (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog 2:e123
Wang JJ, Tao F, An F, Zou YP, Tian W, Chen XM, Xu XM, Hu XP (2017) Wheat transcription factor TaWRKY70 is positively involved in high-temperature seedling plant resistance to Puccinia striiformis f. sp tritici. Mol Plant Pathol 18:649–661
Weihmann F, Eisermann I, Becher R, Krijger JJ, Hubner K, Deising HB, Wirsel SGR (2016) Correspondence between symptom development of Colletotrichum graminicola and fungal biomass, quantified by a newly developed qPCR assay, depends on the maize variety. BMC Microbiol 16, https://doi.org/10.1186/s12866-016-0709-4
Wharton PS, Julian AM, O’Connell RJ (2001) Ultrastructure of the Infection of Sorghum bicolor by Colletotrichum sublineolum. Phytopathology 91:149–158
Zhang C, Chen H, Cai T, Deng Y, Zhuang R, Zhang N, Zeng Y, Zheng Y, Tang R, Pan R et al (2017) Overexpression of a novel peanut NBS-LRR gene AhRRS5 enhances disease resistance to Ralstonia solanacearum in tobacco. Plant Biotechnol J 15:39–55
Zhang QY, Zhang LQ, Song LL, Duan K, Li N, Wang YX, Gao QH (2016) The different interactions of Colletotrichum gloeosporioides with two strawberry varieties and the involvement of salicylic acid. Hortic Res 3:16007
Zhang Y, Tessaro MJ, Lassner M, Li X (2003) Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15:2647–2653
Zhu X, Xiao K, Cui H, Hu J (2017) Overexpression of the Prunus sogdiana NBS-LRR Subgroup Gene PsoRPM2 Promotes Resistance to the Root-Knot Nematode Meloidogyne incognita in Tobacco. Front Microbiol 8:2113
Acknowledgements
We thank Qi Xie from the Institute of Genetics and Developmental Biology, Chinese Academy of Science, China, for providing the WHEATLAND and BTx623 seeds and Wenming Wang from Sichuan Agricultural University, China for sharing Colletotrichum sublineolum. This work was supported by the Natural Science Foundation of China (32000224), the Natural Science Foundation of Shandong Province (ZR2020MC026), the Qilu Scholarship from Shandong University (11200087963080), the Qingchuang Science and Technology Support Program of Shandong Provincial College (2020KJE002) for Lijing Liu, and the Fundamental Research Funds of Shandong University (61200079614090) for Aixia Li.
Funding
Lijing Liu was supported by the Natural Science Foundation of China (32000224), the Natural Science Foundation of Shandong Province (ZR2020MC026), the Qilu Scholarship from Shandong University (11200087963080), and the Qingchuang Science and Technology Support Program of Shandong Provincial College (2020KJE002). Aixia Li was supported by the Fundamental Research Funds of Shandong University (61200079614090).
Author information
Authors and Affiliations
Contributions
Lijing Liu conceived the study and supervised the research. Shuangyi Zhao co-supervised the research. Xue Sun performed all the experiments and data collection and analysis with help from Aixia Li and Guojing Ma. The first draft of the manuscript was written by Lijing Liu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We have no financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, X., Li, A., Ma, G. et al. Transcriptome analysis provides insights into the bases of salicylic acid-induced resistance to anthracnose in sorghum. Plant Mol Biol 110, 69–80 (2022). https://doi.org/10.1007/s11103-022-01286-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-022-01286-5