Abstract
Key message
Rice leucine-rich repeat extensin-like protein OsPEX1 mediates the intersection of lignin deposition and plant growth.
Abstract
Lignin, a major structural component of secondary cell wall, is essential for normal plant growth and development. However, the molecular and genetic regulation of lignin biosynthesis is not fully understood in rice. Here we report the identification and characterization of a rice semi-dominant dwarf mutant (pex1) with stiff culm. Molecular and genetic analyses revealed that the pex1 phenotype was caused by ectopic expression of a leucine-rich repeat extension-like gene, OsPEX1. Interestingly, the pex1 mutant showed significantly higher lignin content and increased expression levels of lignin-related genes compared with wild type plants. Conversely, OsPEX1-suppresssed transgenics displayed low lignin content and reduced transcriptional abundance of genes associated with lignin biosynthesis, indicating that the OsPEX1 mediates lignin biosynthesis and/or deposition in rice. When OsPEX1 was ectopically expressed in rice cultivars with tall stature that lacks the allele of semi-dwarf 1, well-known green revolution gene, the resulting transgenic plants displayed reduced height and enhanced lodging resistance. Our study uncovers a causative effect between the expression of OsPEX1 and lignin deposition. Lastly, we demonstrated that modulating OsPEX1 expression could provide a tool for improving rice lodging resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice, wheat, and maize are the world’s three major crops. Of these three crops, rice is the most important food crop in Asian, especially in Southeast Asian. Lodging resistance is an important trait for increasing rice grain yield. Lodging resistance is closely related to plant height and culm composition. The broad use of dwarf rice varieties resulted in the Green Revolution in the 1960s. However, lodging resistance of rice with the semi-dwarf 1 (sd1) gene alone is limited (Yano et al. 2015), and many varieties with sd1 allele remain lodging-sensitive (Zhang et al. 2014). Another approach for improving lodging resistance is enhancing mechanical strength of the culm, which is determined by culm diameter, wall thickness and composition of cell wall at the basal internodes including third and fourth internodes from the tops of the plants. Secondary wall, which is primarily composed of cellulose, hemicellulose, and lignin, provides a major mechanical strength for rice culm (Zhong et al. 2011). The stiffness of the basal stem is positively correlated with the content of lignin in the secondary wall (Zhang et al. 2014). Lignin is a major structural component of secondary cell walls in vascular plants; however, mechanisms underlying the regulation of lignin synthesis remain elusive, in spite of extensive research on lignin metabolism (Yoon et al. 2015).
Cell walls in plants protect and restrict cell movement and enlargement. As a result, strict regulation of cell wall expansion is crucial for plant growth and development. The primary cell wall is composed of cellulose and hemi-cellulose intermingled with xyloglucan. In addition to these structural cell wall components, plant cells have evolved a rich array of complex wall-associated proteins with diverse extracellular domains for the purpose of sensing, signaling, and cytoskeleton reconfiguration functions. At least three main classes of cell wall proteins have been described: proline-rich-proteins (PRP)s, glycine-rich-proteins (GRPs), and hydroxyproline-rich-glycoproteins (HRGPs). Extensins (EXTs) are a diverse plant-specific family of HRGPs characterized by the repeated occurrence of serine (Ser) followed by several consecutive prolines (Pro) or Tyr-X-Tyr motifs (X: any amino acid). They require several post-translational modifications (PTMs) including proline Hydroxylation that is catalyzed by prolyl 4-hydroxylases (P4Hs), hydroxyproline and serine O-glycosylation by glycosyltransferases, and tyrosine (Tyr) crosslinking by type-III peroxidases, before becoming functional in plant cell walls (Marzol et al. 2018). The Tyr crosslinking is responsible for intramolecular or intermolecular cross-linking with other EXT molecules. These cross-linking properties contribute to the extracellular matrix and play roles in plant development and defense mechanisms (Borassi et al. 2016).
LRXs (leucine-rich repeat extensins) are a class of chimeric EXTs which have signal peptides at the N terminus, followed by leucine-rich repeat (LRR) domains, and extensin domain (harboring Ser-Pro(3–5) repeated motif) near the C terminus, which is probably involved in cross-linking to cell wall components. Since LRR domain is known for protein–protein interactions, the LRXs are candidates for regulatory functions on the cell surface. In Arabidopsis, LRX proteins have been implicated in root hair morphogenesis, cell wall composition modifications, and plant growth (Baumberger et al. 2001, 2003; Draeger et al. 2015).
Plants have evolved cell wall signaling pathways to senses the state of the cell wall and transmit the information in plant growth and development and in response to environmental stress. In agreement with this notion, it is becoming clear that any disturbance in plant cell wall has a direct impact on cell membrane systems; and likewise, disruption of any intracellular component also could influence plant cell wall (Kohorn and Kohorn 2012; Borassi et al. 2016). Extensins are abundant structural proteins of the cell walls, but their relatively simple structure suggests that they have a role in cross linking components within the cell wall rather than signaling (Bedinger 2018). Recently, several members of the Catharanthus roseus receptor-like kinase (CrRLK1L) subfamily such as FERONIA (FER) ANXURs (ANX1/ANX2) and BUDDHA’S PAPER SEAL1 and 2 (BUPS1/BUPS2) have been identified as potential cell wall sensors (Marzol et al. 2018). These members of the CrRLK1L subfamily are located at the interface between the apoplast and the cytoplasmic side of the plasma membrane and have pleiotropic functions in a variety of cellular processes, including root cell and pollen tube growth. FER functions as a receptor of RALF (RAPD ALKALINIZATION FACTOR) peptides to regulate cell expansion (Haruta et al. 2014). Recently, it was reported that the Arabidopsis cell-wall-localized LRX3/4/5 interact with RALF22/23, which in turn interact with the plasma membrane-localized receptor-like protein kinase FER (Zhao et al. 2018). In addition, pollen-expressed ANX1/2 and BUPS1/2 interact with AtRALF4/19 peptides, which show a physical interaction with AtLRX8/9 (Ge et al. 2017; Mecchia et al. 2017). Moreover, it was suggested that LRR domain of LRX4 physically interacts with the FER, suggesting a direct connection between the LRX LRR domain and the cell surface CrRLK1L sensors (Dunser et al. 2017). Together these observations suggest that LRX proteins might link the extensin network to the cell surface integrity sensors (Nissen et al. 2016; Marzol et al. 2018).
According to a recent large-scale genome survey, LRXs have been identified in all flowering plants, with eudicot genomes encoding more LRXs in general (Liu et al. 2016). Several LRXs in Arabidopsis have been studied in detail (Baumberger et al. 2001, 2003; Draeger et al. 2015; Mecchia et al. 2017; Fabrice et al. 2018; Sede et al. 2018; Wang et al. 2018). The rice genome encodes at least eight LRX proteins (Baumberger et al. 2003a, b), compared to 11 proteins encoded by the Arabidopsis genome (Baumberger et al. 2003a, b; Liu et al. 2016). Here we report the characterization of a rice mutant that affects plant stature and lodging resistance due to ectopic expression of the rice LRX gene OsPEX1. Our study also uncovered an important role of OsPEX1 in lignin biosyntheis and deposition in rice.
Materials and methods
Plant materials and growth conditions
Rice cultivar ‘Zhonghua 11’ was used as the wild type. The pex1 mutant described in this paper was derived from an activator/dissociator (Ac/Ds) transposon-tagging population in the japonica rice variety Zhonghua 11 (Liu et al. 2007). All rice seeds in this study were propagated in the paddy field in Guangzhou, China.
Isolation of the Ds flanking sequences
Genomic sequences flanking the Ds insertions in pex1 were identified by TAIL-PCR (Liu and Whittier 1995). Specific tertiary PCR fragments were sequenced. The presence of the Ds insertion was verified according to the methods described previously (Zhang et al. 2016). The primers used for TAIL-PCR were listed in Supplementary Table S1.
Molecular cloning and rice transformation
To suppress OsPEX1 expression in rice plants, rice OsPEX1 cDNA fragment was amplified with the primers LRX-RNAiF and LRX-RNAiR (Supplementary Table S1), and cloned into a binary pCUbi1390 vector in antisense orientation under the control of the maize (Zea mays) Ubiquitin promoter. To produce OsPEX1 overexpression transgenic plants, the full-length OsPEX1 coding sequence was amplified with the primers LRX-1BF and LRX-4200SR. The amplified sequence was cloned into the binary vector pCUbi1390 for OsPEX1 overexpression in rice. For each construct, about twenty transgenic lines were produced by Agrobacterium-mediated transformation (strain EHA105) and their transgenic seeds were planted individually to obtain T1 generation.
Reverse transcription (RT)-PCR, quantitative RT-PCR
Total RNA was extracted from frozen samples with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Purified RNA was pre-treated with DNase I, and first-strand cDNA was generated using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). Quantitative RT-PCRs were performed using a SYBR Premix Ex Taq™ RT-PCR kit (Takara) following the manufacturer’s instructions. The relative expression level of a target gene was normalized to that of rice ACTIN1. The primers used for RT-PCR were listed in Supplementary Table S1.
Lignin staining and autofluorescence of rice stems
The internodes of each sample were numbered consecutively from the top to the base of the stem. Sections were cut at the center of each internode and stained with either phloroglucine-HCl according to the procedure described by Hirano et al. (2012) or directly examined for autofluorescence. Sections were viewed and photographed with a light microscope (Zeiss Axioimager with UV excitation).
Measurement of lignin content
Lignin contents were determined using the method of Moreira-Vilar et al (Moreira-Vilar et al. 2014) with modifications and expressed as a percentage of the cell wall residue (%). Briefly, samples were ground to fine powder using a pestle and mortar containing liquid nitrogen and dried in an oven at 30 °C until a constant weight was achieved. Dry samples (20 mg) were placed into screw-cap centrifuge tubes containing 2 ml of 25% acetyl bromide (v/v in glacial acetic acid) and incubated at 70 °C for 30 min. After complete digestion, partial samples (0.2 ml) were mixed with 0.45 ml of 2 M NaOH, 0.05 ml of 7.5 M hydroxylamine-HCl, and 3.3 ml of glacial acetic acid. After centrifugation (1400×g, 5 min), the absorbance of the supernatant was measured at 280 nm. The results were expressed as mg lignin g− 1 dry samples.
Measurement of physical properties of rice stems
Breaking force was referred to as the force required for breaking a culm segment. The breaking force of the third internode of rice culms 20 days after heading was measured using a single column testing machine (HDE-500N). The bending load at breaking was measured at a distance of 5 cm between two supporting points.
Gene accession number
LOC_Os11g43640 (OsPEX1), LOC_Os06g04090 (OsSWN1), LOC_Os08g01330 (OsSWN3), LOC_Os06g01480 (OsSWN7), LOC_Os04g43800 (OsPAL6), LOC_Os05g35290 (OsPAL7), LOC_Os09g08720 (OsCCR), LOC_Os02g09490 (OsCAD2), LOC_Os12g02080 (OsPRX1), LOC_Os11g02130 (OsPRX2).
Results
Isolation and characterization of a rice semi-dominant dwarf mutant
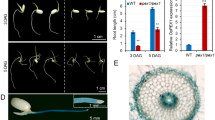
A dwarf mutant, designated as pex1, was obtained from a two-component activator/dissociation (Ac/Ds) transposon-tagging population in the japonica rice variety Zhonghua 11 (Liu et al. 2007). To determine the inheritance of pex1, we examined the phenotypes of 135 progeny of self-pollinated pex1 plants. Three distinct phenotypes were observed. Among them, 40 were normal looking (wild type) plants, 65 were semi-dwarf, and 30 exhibited dwarf phenotype (Fig. 1a). The ratio of wild type to semi-dwarf to dwarf plants was 1:1.63:0.75 (for 1:2:1 ratio, χ2 = 1.67, p > 0.05). In addition, 35 F1 plants derived from the cross between pex1 and Zhonghua11 showed a near 1:1 ratio of wild-type vs semi-dwarf progeny (Data not shown). These results demonstrated that the pex1 mutant is a heterozygous allele controlled by a single, semi-dominant locus. Thus, the semi-dwarf progeny is referred to as the pex1 heterozygotes (pex1/+), and the dwarf progeny as pex1homozygotes (pex1/pex1).
Phenotypes of the pex1 mutant. a Mature plant phenotype of wild type (left), pex1 heterozygote (middle), and homozygote (right). b Shortened internodes resulted in semi-dwarfism in pex1. The length of the upper five internodes of pex1on the main culms was proportionally diminished compared with WT. c Cross-sections of the first (I, panicle-neck internode) down to the fifth (V, basal intermode) of the WT and pex1 mutants. In addition to dwarfism throughout its growth, the pex1 mutant exhibited the dramatic reduction of stem diameters. d Comparison of culm diameter and culm thickness and the ratio of wall thickness to culm diameter of the 3rd internode in WT and pex1 mutants at 20 days after heading. Each column represents mean ± SD (n = 6). Asterisks represent significant difference determined by Student’s t-test at **p value < 0.01, not significant (n.s.)
The reduced height of pex1 was the result of uniformly shortened internodes in the mutant culms, determined by comparing the length of each internode between mutant and wild-type plants (Fig. 1b). In addition, the pex1 mutant showed decreased culm diameter, but with an increased ratio of wall thickness to culm diameter compared with WT (Fig. 1c, d). Interestingly, in spite of slightly shortened panicle length (Supplementary Fig. S1a), average grain number per panicle/plant in heterozygous pex1 is comparable to that in wild type plants, whereas homozygous pex1 has a significantly reduced grain number due to reduced panicle length (Supplementary Fig. S1b). Moreover, tiller number of the pex1 is slightly increased (Supplementary Fig. S1b). However, other agronomic traits, such as heading date, blade length of flag leaf and blade width of flag leaf, in the pex1 mutant are similar to wide-type plants (Supplementary Fig. S1b, c).
Molecular cloning of PEX1
To determine the Ds insertion site in the pex1 mutant, we isolated genomic flanking sequences of the Ds insertion by thermal asymmetric interlaced (TAIL)-PCR (Liu and Whittier 1995). Analysis of flanking sequences revealed that the Ds element is inserted into the promoter of the OsPEX1 (LOC_Os11g43640) gene on rice chromosome 11, generating an 8-bp duplication at the target site expected for a Ds insertion (Fig. 2a, b). All plants showing the mutant phenotype were heterozygous or homozygous for the Ds insertion (Fig. 2c). We next investigated the expression level of OsPEX1. Compared with wild-type stems, OsPEX1 showed elevated expression in pex1/+ and pex1/pex1 stems (Fig. 2d, e). The results suggested that OsPEX1 overexpression in pex1 plants is due to the Ds insertion.
Molecular cloning of the OsPEX1 gene. a Location of Ds insertion and the schematic structure of OsPEX1. The Ds insertion was mapped to rice genomic PAC clone AC120888 on chromosome 11. The Ds element was inserted about 3.2 kb upstream of the OsPEX1 gene. The untranslated regions are shown in gray rectangles, the exons in black rectangles. Ds 5′ and Ds 3′, the 5′ and 3′ end of the Ds transposon, respectively; Bar, the BASTA resistant gene. b Identification of Ds insertion footprint in the pex1 mutant. The 8 base-pairs (bp) sequence, ACTAGGCA (in red), is duplicated upon Ds insertion. c PCR-based analysis on the genomic DNAs of the wild-type control, the pex1 heterozygous (pex1/+) and homozygous (pex1/pex1) mutants. Expression analysis of OsPEX1 by reverse transcription-PCR (RT-PCR, d) and real-time reverse transcription-PCR (qRT-PCR, e) in the wild type (WT), pex1 heterozygous (pex1/+) and homozygous (pex1/pex1) mutants. f Morphology of WT, and transgenic plants overexpressing OsPEX1 (OE). Bar = 10 cm. g qRT-PCR analysis showing the transcript levels of OsPEX1 in overexpressing transgenic plants. h Morphology of transgenic rice plants with antisense-mediated knock-down of OsPEX1 expression (KD). Bar = 10 cm. i qRT-PCR analysis showing the transcript levels of OsPEX1 in the knock-down transgenic plants. j Expression pattern of OsPEX1 in wild-type plants. k Comparsion of OsPEX1 expression in various tissues between wild-type and pex1 plants. For qRT-PCR analysis, the samples in g and i were derived from the second internode of rice stem at heading stage; Total RNA in j and k was isolated from roots, leaf blade, the second internode of rice stem and panicle at heading stage. Values are means ± SD of three biological replicates. Asterisks represent significant difference determined by Student’s t-test at **p value < 0.01, not significant (n.s.)
Analysis of amino acids sequences revealed that OsPEX1 protein includes a C-terminal extensin-like domain containing numerous copies of Ser-(Pro)2−4 repeats (Supplementary Fig. S2). Analysis of the N terminus of OsPEX1 by Petersen et al method (Petersen et al. 2011) confirmed the presence of a signal sequence, suggesting that the protein is secreted. In addition to the extensin-like domain, it has a distinct N-terminal domain containing eight LRR repeats (LRRs). LRRs are frequently implicated in protein–protein interactions, pathogen recognition and defense in plants (Borassi et al. 2016). The most prevalent amino acids in this domain are leucine, phenylalanine, and aspartic acid (10.6%, 9.7%, and 8.2%, respectively). By contrast, the extensin-like domain has an entirely different composition of amino acids, with a very high ratio (> 40%) of proline.
To confirm that overexpression of OsPEX1 is responsible for the developmental defects in pex1, we overexpressed OsPEX1 in wild type plant. All positive transformants displayed dwarf phenotypes characteristic of the pex1 mutant plants (Fig. 2f, g). Thus, we concluded that the abnormal phenotype in the pex1 mutant results from overexpression of OsPEX1. Interestingly, specific knock-down of OsPEX1 in wild type plants (Supplementary Fig. S3) also resulted in dwarf phenotype with uniformly shortened internodes (Fig. 2h, I, and Supplementary Fig. S4a, b), which resulted from reduced cell size as in the pex1 mutants (Supplementary Fig. S5). In addition, the KD (knock-down) plants showed decreased stem diameter and culm wall thickness compared with WT (Supplementary Fig. S4c, d). These results suggest that OsPEX1 gene is required for normal plant growth.
In wild-type plants, OsPEX1 transcripts are detected in roots, leaf blades, stems and panicles, with expression level varies significantly among tissues examined (Fig. 2j). For example, compared to leaf, OsPEX1 expression is significantly elevated in root, stem, and panicle tissues, highlighting a special requirement of its activity in these tissues. Interestingly, the expression of OsPEX1 is even further induced in pex1 mutant compared to wild-type in roots, stems, and panicles, but not in leaves (Fig. 2k). This is consistent with the pex1 mutant exhibiting normal leaf morphology (Fig. 1 and Supplementary Fig. S1c).
Lignin content increase in pex1 stem
Autofluorescence examination under UV light excitation is a sensitive and effective technique for total lignin distribution in plant cell wall (De Micco and Aronne 2007). As shown in Fig. 3a, autofluorescence was clearly visible in cell walls of wild-type stems. By contrast, enhanced autofluorescence was observed in parenchyma cells of the pex1, which have smaller cell size with thickened cell wall compared with those of WT. Moreover, phloroglucinol staining for lignin showed that strong phloroglucinol staining (a red–violet colour) were detected in pex1compared to WT (Fig. 3b and Supplementary Fig. S6), suggesting elevated lignin contents in pex1 mutant. We quantified the total lignin content of stems of wild-type and pex1 plants using the acetyl-bromide method (Moreira-Vilar et al. 2014). Stem from the pex1 plants have higher lignin content (% dry cell wall residues) than the WT (Supplementary Table S2) and the difference is more profound in older stems (Fig. 3c). We also observed a slight reduction in cellulose content in pex1 plants, which might be a consequence due to their increased lignin content (Supplementary Fig. S7). We next analyzed the expression of lignin pathway genes in the second internode at 10 days before heading (Fig. 3d). Compared to wild-type plants, the pex1/pex1 showed increased expression in all the lignin biosynthesis genes examined, including PHENYLALANINE AMMONIA-LYASE (OsPAL6, OsPAL7), CINNAMOYL-CoA REDUCTASE (OsCCR), CINNAMYL ALCOHOL DEHYDROGENASE (OsCAD2), PEROXIDASE (OsPRX1 and OsPRX2), which showed at least sixfold increase in abundance. The rice secondary wall-associated NAC (NAM, ATAF and CUC2) transcription factors (namely the OsSWNs) are master transcriptional activators of the secondary wall biosynthetic program (Zhong et al. 2011). The expression of OsSWNs (OsSWN1, OsSWN3, and OsSWN7) in the pex1/pex1 mutants is induced more than fourfold compared to wild-type plants, consistent with elevated transcriptional levels of lignin biosynthesis genes in the pex1 mutants. In contrast to the pex1 mutants, OsPEX1-knowndown (KD) transgenic lines showed a substantial reduction in lignin content and down-regulation of lignin-associated genes expression (Fig. 3e, f and Supplementary Fig. S8). These findings provide empirical evidence that modulating OsPEX1 expression affects lignin biosynthesis in rice, either directly or indirectly.
OsPEX1 promotes lignin deposition. a Autofluoresence micrographs of transverse stem sections of the third internode under UV light for visualizing lignin distribution in cell walls of wild type and pex1 stems. b Phloroglucinol staining of cross sections of the third internode from the WT and pex1 plants at 20 days after heading. c Lignin content of stem from the plants of WT and pex1 at 20 days after heading. d Relative expressive levels of lignin-related genes in the second internodes of wild-type and pex1/pex1 plants at 10 days before heading. e Lignin content of stem in WT and knock-down transgenic plants (KD) at 20 days after heading. f qRT-PCR analysis of lignin-related genes in the second internodes of WT and KD plants at 10 days before heading. Each column represents the means of three biological samples ± SD. Asterisks represent significant difference determined by Student’s t-test at **p value < 0.01, not significant (n.s.)
OsPEX1 is involved in lignin accumulation in developing rice internodes
The rice internode is a suitable organ for studying secondary cell wall formation, because after cell elongation has ceased, the secondary cell wall accumulates at the maturation zone, resulting in a continuing developmental stages of secondary wall thickening from the lower (the young stage) to the upper (the developed stage) part of the internode (Hirano et al. 2013). To further understand the relationship between OsPEX1 expression and lignin metabolism in a natural physiological tissue, we analyzed the lignin deposition and OsPEX1 expression in different section of at the second internode from wild-types plants at 10 days before heading. The second internode was divided into five sections from the bottom up (stage I to V, representing from younger to more developed stage) (Fig. 4a). Wiesner staining with phloroglucinol-HCl revealed that lignin deposition gradually increased in the internode from the bottom up (Fig. 4b). Gene expression analysis showed that transcript abundance of OsPEX1 gradually increased from section 1 to 5 (Fig. 4c). This is concomitant with a rapid increase in lignin accumulation toward upper sections of internode (Fig. 4d). These results and our earlier observations support that OsPEX1 may function in lignin biosynthesis and deposition.
Effects of OsPEX1 expression on lignin accumulation in developing rice internodes. a Schematic structure of the second internode at 10 days before heading, tentatively divided into five zones (from lower to upper, with I being the youngest stage). Bar = 1 cm. b Fresh hand cut cross-sections of the segments in a stained with phloroglucine-HCl. Bar = 100 µm. c Relatively OsPEX1 expressive level in the five segments of the second internode. The expression levels of OsPEX1in segment I was assigned value of 1. d Lignin content of the five segments of the second internode. Values are means ± SD. (n = 3). Asterisks represent significant difference determined by Student’s t-test at **p value < 0.01
OsPEX1 overexpression confers rice dwarfism and lodging resistance
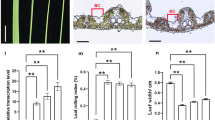
Lodging resistance is an agronomically important trait for crop productivity, which is closely related to plant height and culm strength. The loss-of-function mutations in GA 20-oxidase, namely sd1 alleles known as “Green Revolution Gene”, have been widely used in rice dwarfism breeding. Thus, the use of semi-dwarf trait to increase lodging resistance by reducing plant height has been effectively exploited and is proven beneficial to a certain degree (Yano et al. 2015). Another potential strategy for improving lodging resistance is to explore traits with enhanced culm strength. It has been reported that lignin content positively correlated with lodging resistance (Peng et al. 2014). Given the fact that OsPEX1 play a crucial role in lignin deposition in rice stem, we examined the effect of OsPEX1 overexpression on rice lodging resistance. The entire OsPEX1 coding sequence driven by a maize Ubiquitin promoter was introduced into two representative cultivars (japonica variety Nanyangzhan, W10; indica variety Basmati 370, W11) with functional SD1 allele encoding gibberellin 20-oxidase (OsGA20ox2), a key enzyme in the gibberellin biosynthesis pathway. We found that the transgenic plants W10-T and W11-T showed reduced plant height (Fig. 5a), reduced diameter of clum (Fig. 5b), elevated transcriptional level of OsPEX1 (Fig. 5c) and increased lignin content (Fig. 5d), resulting in high stem breaking resistance (Fig. 5e), indicating increased lodging resistance. The findings suggest that overexpression of OsPEX1 can significantly decrease the risk of rice lodging by increasing culm strength and reducing plant height independent of the sd1allele.
Effects of overexpressing OsPEX1 on rice lodging resistance. a Morphological characteristics of transgenic plants overexpression OsPEX1 gene at 20 days after heading. b Transverse sections of the fourth internodes 20 days after heading from corresponding cultivars in a. c Relative OsPEX1expressive level of the second internode in the corresponding cultivars at 10 days before heading. The expression level of OsPEX1 in W10 was assigned as the value of 1. d Lignin content of the fourth internodes 20 days after heading from corresponding cultivars in a. e Breaking resistance of fourth internodes 20 days after heading from corresponding cultivars in a. W10 (japonica variety Nanyangzhan) and W11 (indica variety Basmati 370) were two representative cultivars with functional SD1 allele encoding gibberellin 20-oxidase (OsGA20ox2); W10-T and W11-T represent the resulting transgenic plants overexpression OsPEX1 gene. Each column represents mean ± SD (n = 3). **p < 0.01 based on Student’s t-test
Discussion
Ds insertion induced ectopic expression of the pex1 allele
In this study, we characterized a rice semi-dominant dwarf mutant line caused by a Ds insertion in the promoter of the rice LRX gene OsPEX1. Our analyses revealed that the semi-dominant dwarf phenotype was a result of OsPEX1 ectopic expression induced by the Ds insertion. Ectopic expression of the mutant allele could be caused by a loss of negative regulation of OsPEX1 transcription due to the Ds insertion. Alternatively, Ds transposon insertion could provide new cis-elements and alter the transcriptional regulation of the inserted genes. The latter is particularly evident in maize where many KNOX genes were originally identified as dominant or semi-dominant mutants where ectopic expression of the transposon-inserted genes is responsible for the mutant phenotypes (Hake et al. 1989; Vollbrecht et al. 1991; Schneeberger et al. 1995; Muehlbauer et al. 1999). Furthermore, a rice mutant allele, OSH6-Ds, was generated by inserting a Ds element into the OSH6 gene (an ortholog of the maize Liguleless3 KNOX gene) also resulted in ectopic expression of a truncated novel transcript caused by the Ds insertion (Park et al. 2007). Based on these reports, we favor the model that ectopic OsPEX1 expression in pex1 is likely due to transposon induced new transcriptional regulation (Lisch 2013).
OsPEX1 affects rice lodging resistance
Increasing evidences have suggested that a hierarchical transcriptional network is involved in the coordinated regulation of the biosynthesis of secondary walls, in which several NACs (NAM, ATAF, and CUC2) are located at the top tier of regulatory hierarchy and orchestrate a cascade of downstream transcription factors, leading to the activation of secondary wall biosynthetic genes (Zhong and Ye 2007; Demura and Ye 2010; Huang et al. 2015). It has been shown that three rice secondary wall-associated NACs (namely OsSWN1, OsSWN3 and OsSWN7) are master transcriptional activators of the secondary wall biosynthetic program (Zhong et al. 2011). When overexpressed in Arabidopsis, OsSWNs can activate a number of secondary wall-associated transcription factors and biosynthetic genes, and ectopic deposition of cellulose, xylan and lignin (Zhong et al. 2011). In this study, we found that in OsPEX1 overexpressing lines, the expression of the rice SWNs were substantially up-regulated, while knockdown of OsPEX1 significantly reduced their expression (Fig. 3), consistent with the alterations of lignin content in the pex1 mutants.
Significantly, overexpression of OsPEX1 can substantially decrease the risk of lodging occurrence not only by altering the plant height but also by increasing the physical strength of the culm internode through altering the lignin accumulation (Fig. 5), suggesting that OsPEX1 could be a promising candidate gene for novel breeding strategy.
Coordinated regulation of growth and lignin deposition
The formation of cell wall is necessary for plant growth and development. It is well known that the processes of cell expansion and elongation are tightly correlated with the regulation of lignification (Rogers and Campbell 2004). As shown in Fig. 4, during cell elongation, the mRNA level of OsPEX1 is lower, thus avoiding excessive accumulation of lignin and permitting cell expansion and elongation. By contrast, at the mature zone the expression of OsPEX is up-regulated, promoting lignin deposition and enhancing the physical strength of stem. Therefore, it is proposed that OsPEX1 is involved in the regulation of secondary cell wall biosynthesis in response to plant growth and development.
Cell expansion cannot occur smoothly unless cell wall is sufficiently softened to allow cell expansion but rigid enough to resist turgor pressure. Lignin is a major component of secondary cell wall and the deposition of lignin is closely correlated with cell expansion (Rogers and Campbell 2004). Not surprisingly, given the fact that OsPEX1 plays a vital role in lignin deposition, overexpression and down-regulation of OsPEX1 could lead to a significant change in lignin deposition and results in diminished plant growth. In fact, it has been reported that mutation of lignin-related genes leads to a significant change in lignin deposition as well as reduced plant growth (Rogers and Campbell 2004; Yoon et al. 2015), supporting the idea that proper lignin deposition is essential for appropriate cell expansion and plant development.
Our results indicate that OsPEX1 plays an important role in lignin deposition, thus affecting cell expansion and altering plant stature in rice. However, the regulatory mechanisms of lignin biosynthesis and deposition by OsPEX1 remain to be elucidated. Increasing evidences suggest that LRXs could potentially be involved in the regulation of cell wall expansion in response to signals (Baumberger et al. 2001; Borassi et al. 2016). More recently, it has been shown that Arabidopsis LRX8/9, an orthologue of OsPEX1, can bind small peptides, which function as signal molecules (Mecchia et al. 2017). Thus, it is possible that rice OsPEX1 may also have a signaling capacity in regulating lignin biosynthesis and deposition.
References
Baumberger N, Ringli C, Keller B (2001) The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes Dev 15:1128–1139
Baumberger N, Doesseger B, Guyot R, Diet A, Parsons RL, Clark MA, Simmons MP, Bedinger P, Goff SA, Ringli C, Keller B (2003a) Whole-Genome comparison of leucine-rich repeat extensins in Arabidopsis and Rice. A conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol 131:1313–1326
Baumberger N, Steiner M, Ryser U, Keller B, Ringli C (2003b) Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J 35:71–81
Bedinger P (2018) Coordinating cell walls and cell growth: A role for LRX extensin chimeras. Plant Physiol 176:1890
Borassi C, Sede AR, Mecchia MA, Salgado Salter JD, Marzol E, Muschietti JP, Estevez JM (2016) An update on cell surface proteins containing extensin-motifs. J Exp Bot 67:477–487
De Micco V, Aronne G (2007) Combined histochemistry and autofluorescence for identifying lignin distribution in cell walls. Biotech Histochem 82:209–216
Demura T, Ye Z (2010) Regulation of plant biomass production. Curr Opin Plant Biol 13:298–303
Draeger C, Ndinyanka Fabrice T, Gineau E, Mouille G, Kuhn BM, Moller I, Abdou M, Frey B, Pauly M, Bacic A, Ringli C (2015) Arabidopsis leucine-rich repeat extensin (LRX) proteins modify cell wall composition and influence plant growth. BMC Plant Biol 15:1–11
Dunser K, Gupta S, Ringli C, Kleine-Vehn J (2017) LRX- and FER-dependent extracellular sensing coordinates vacuolar size for cytosol homeostasis. bioRxiv. https://doi.org/10.1101/231043
Fabrice TN, Vogler H, Draeger C, Munglani G, Gupta S, Herger AG, Knox P, Grossniklaus U, Ringli C, Ciereszko I (2018) LRX proteins play a crucial role in pollen grain and pollen tube cell wall development. Plant Physiol 176:1981–1992
Ge Z, Bergonci T, Zhao Y, Zou Y, Du S, Liu M, Luo X, Ruan H, García-Valencia LE, Zhong S, Hou S, Huang Q, Lai L, Moura DS, Gu H, Dong J, Wu H, Dresselhaus T, Xiao J, Cheung AY, Qu L (2017) Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358:1596–1600
Hake S, Vollbrecht E, Freeling M (1989) Cloning knotted, the dominant morphological mutant in maize using Ds2 as a transposon tag. EMBO J 8:15–22
Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343:408–411
Hirano K, Aya K, Kondo M, Okuno A, Morinaka Y, Matsuoka M (2012) OsCAD2 is the major CAD gene responsible for monolignol biosynthesis in rice culm. Plant Cell Rep 31:91–101
Hirano K, Aya K, Morinaka Y, Nagamatsu S, Sato Y, Antonio BA, Namiki N, Nagamura Y, Matsuoka M (2013) Survey of genes involved in rice secondary cell wall formation through a co-expression network. Plant Cell Physiol 54:1803–1821
Huang D, Wang S, Zhang B, Shang-Guan K, Shi Y, Zhang D, Liu X, Wu K, Xu Z, Fu X, Zhou Y (2015) A Gibberellin-mediated della-nac signaling cascade regulates cellulose synthesis in rice. Plant Cell 27:1681–1696
Kohorn B, Kohorn S (2012) The cell wall-associated kinases, WAKs, as pectin receptors. Front Plant Sci 3:88. https://doi.org/10.3389/fpls.2012.00088
Lisch D (2013) How important are transposons for plant evolution? Nat Rev Genet 14:49–61
Liu Y, Whittier R (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674–681
Liu F, Zhang X, Zhang Z, Chen Z, Zhu H, Wang J, Zhang J, Zhang G (2007) Transpositional behaviour of the Ds element in the Ac/Ds system in rice. Chin Sci Bull 52:2789–2796
Liu X, Wolfe R, Welch LR, Domozych DS, Popper ZA, Showalter AM (2016) Bioinformatic identification and analysis of extensins in the plant kingdom. PLoS ONE 11:e150177
Marzol E, Borassi C, Bringas M, Sede A, Rodríguez Garcia DR, Capece L, Estevez JM (2018) Filling the gaps to solve the extensin puzzle. Mol Plant 11:645–658
Mecchia MA, Santos-Fernandez G, Duss NN, Somoza SC, Boisson-Dernier A, Gagliardini V, Martínez-Bernardini A, Fabrice TN, Ringli C, Muschietti JP, Grossniklaus U (2017) RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 358:1600–1603
Moreira-Vilar FC, Siqueira-Soares RDC, Finger-Teixeira A, Oliveira DMD, Ferro AP, Da Rocha GJ, Ferrarese MDLL, Dos Santos WD, Ferrarese-Filho O (2014) The acetyl bromide method is faster, simpler and presents best recovery of lignin in different herbaceous tissues than klason and thioglycolic acid methods. PLOS ONE 9:e110000
Muehlbauer GJ, Fowler JE, Girard L, Tyers R, Harper L, Freeling M (1999) Ectopic expression of the maize homeobox gene liguleless3 alters cell fates in the leaf. Plant physiol 119:651–662
Nissen KS, Willats WGT, Malinovsky FG (2016) Understanding CrRLK1L function: cell walls and growth control. Trends Plant Sci 21:516–527
Park SH, Kim CM, Je BI, Park SH, Park SJ, Piao HL, Xuan Y, Choe MS, Satoh K, Kikuchi S, Lee KH, Cha YS, Ahn BO, Ji HS, Yun DW, Lee MC, Suh S, Eun MY, Han C (2007) A Ds-insertion mutant of OSH6 (Oryza sativa Homeobox 6) exhibits outgrowth of vestigial leaf-like structures, bracts, in rice. Planta 227:1–12
Peng D, Chen X, Yin Y, Lu K, Yang W, Tang Y, Wang Z (2014) Lodging resistance of winter wheat (Triticum aestivum L.): lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crop Res 157:1–7
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786
Rogers LA, Campbell MM (2004) The genetic control of lignin deposition during plant growth and development. New Phytol 164:17–30
Schneeberger RG, Becraft PW, Hake S, Freeling M (1995) Ectopic expression of the knox homeo box gene rough sheath1 alters cell fate in the maize leaf. Genes Dev 9:2292–2304
Sede AR, Borassi C, Wengier DL, Mecchia MA, Estevez JM, Muschietti JP (2018) Arabidopsis pollen extensins LRX are required for cell wall integrity during pollen tube growth. FEBS Lett 592:233–243
Vollbrecht E, Veit B, Sinha N, Hake S (1991) The developmental gene KNOTTED-1 is a member of a maize homeobox gene family. Nature 350:241–243
Wang X, Wang K, Liu X, Liu M, Cao N, Duan Y, Yin G, Gao H, Wang W, Ge W, Wang J, Li R, Guo Y (2018) Pollen-expressed leucin-rich-repeat extensins are essential for pollen germination and growth. Plant Physiol 176:1993–2006
Yano K, Ookawa T, Aya K, Ochiai Y, Hirasawa T, Ebitani T, Takarada T, Yano M, Yamamoto T, Fukuoka S, Wu J, Ando T, Ordonio RL, Hirano K, Matsuoka M (2015) Isolation of a novel lodging resistance QTL gene involved in strigolactone signaling and its pyramiding with a QTL gene involved in another mechanism. Mol Plant 8:303–314
Yoon J, Choi H, An G (2015) Roles of lignin biosynthesis and regulatory genes in plant development. J Integr Plant Biol 57:902–912
Zhang J, Li G, Song Y, Liu Z, Yang C, Tang S, Zheng C, Wang S, Ding Y (2014) Lodging resistance characteristics of high-yielding rice populations. Field Crops Res 161:64–74
Zhang X, Zheng X, Ke S, Zhu H, Liu F, Zhang Z, Peng X, Guo L, Zeng R, Hou P, Liu Z, Wu S, Song M, Yang J, Zhang G (2016) ER-localized adenine nucleotide transporter ER-ANT1: an integrator of energy and stress signaling in rice. Plant Mol Biol 92:701–715
Zhao C, Zayed O, Yu Z, Jiang W, Zhu P, Hsu C, Zhang L, Tao WA, Lozano-Durán R, Zhu J (2018) Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc Natl Acad Sci USA 115:13123
Zhong R, Ye Z (2007) Regulation of cell wall biosynthesis. Curr Opin Plant Biol 10:564–572
Zhong R, Lee C, McCarthy RL, Reeves CK, Jones EG, Ye Z (2011) Transcriptional activation of secondary wall biosynthesis by rice and maize nac and myb transcription factors. Plant Cell Physiol 52:1856–1871
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 30900884, 31622041, 31471788 and 31671594); by Natural Science Foundation of Guangdong Province, China (Grant Nos. 2014A030313457 and 2015A020209118); by the Hatch Project of National Institute of Food and Agriculture, U.S.D.A (Grant No. 02413).
Author information
Authors and Affiliations
Contributions
SK and XL performed experiments and conducted fieldwork. JL and YHH worked on the transgenic lines. TFH and XQZ designed the experiments and analyzed the data; TFH supervised and complemented the writing; XQZ conceived the project and wrote the article with contributions of all the authors.
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ke, S., Luan, X., Liang, J. et al. Rice OsPEX1, an extensin-like protein, affects lignin biosynthesis and plant growth. Plant Mol Biol 100, 151–161 (2019). https://doi.org/10.1007/s11103-019-00849-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-019-00849-3