Abstract
Key message
This is the first report that GLP gene (OsGLP2-1) is involved in panicle blast and bacterial blight resistance in rice. In addition to its resistance to blast and bacterial blight, OsGLP2-1 has also been reported to co-localize with a QTLs for sheath blight resistance in rice. These suggest that the disease resistance provided by OsGLP2-1 is quantitative and broad spectrum. Its good resistance to these major diseases in rice makes it to be a promising target in rice breeding.
Abstract
Rice (Oryza sativa) blast caused by Magnaporthe oryzae and bacterial blight caused by Xanthomonas oryzae pv. oryzae are the two most destructive rice diseases worldwide. Germin-like protein (GLP) gene family is one of the important defense gene families which have been reported to be involved in disease resistance in plants. Although GLP proteins have been demonstrated to positively regulate leaf blast resistance in rice, their involvement in resistance to panicle blast and bacterial blight, has not been reported. In this study, we reported that one of the rice GLP genes, OsGLP2-1, was significantly induced by blast fungus. Overexpression of OsGLP2-1 quantitatively enhanced resistance to leaf blast, panicle blast and bacterial blight. The temporal and spatial expression analysis revealed that OsGLP2-1is highly expressed in leaves and panicles and sub-localized in the cell wall. Compared with empty vector transformed (control) plants, the OsGLP2-1 overexpressing plants exhibited higher levels of H2O2 both before and after pathogen inoculation. Moreover, OsGLP2-1 was significantly induced by jasmonic acid (JA). Overexpression of OsGLP2-1 induced three well-characterized defense-related genes which are associated in JA-dependent pathway after pathogen infection. Higher endogenous level of JA was also identified in OsGLP2-1 overexpressing plants than in control plants both before and after pathogen inoculation. Together, these results suggest that OsGLP2-1 functions as a positive regulator to modulate disease resistance. Its good quantitative resistance to the two major diseases in rice makes it to be a promising target in rice breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa) blast caused by Magnaporthe oryzae and bacterial blight caused by Xanthomonas oryzae pv. oryzae are two of the most destructive rice diseases leading to severe yield losses in rice production worldwide (Hu et al. 2008; Liu et al. 2014). Thus far, though many approaches, such as chemical controls, biological controls, cultivation and disease forecasting, have been widely applied to control these diseases, the most economical and environmentally friendly method is still the application of host resistance (Hu et al. 2008). The host resistance in plants was usually classified into two categories: qualitative (complete) resistance and quantitative (partial) resistance (Kou and Wang 2010; Fu et al. 2011). In most cases, qualitative resistance mediated by major disease resistance (R) genes is specific to pathogen race and is lifetime limited due to the strong selection pressure and the rapid evolution of the pathogen (McDonald and Linde 2002). On the contrary, quantitative resistance conferred by quantitative trait loci (QTLs) is presumably race non-specific and considered to be more durable (Roumen 1994). Thus, there is increasing interest in the development of variety with quantitative and race non-specific resistance in plants (Hayashi et al. 2010).
For the past decades, considerable progress has been made in the study of rice host resistance for blast and bacterial blight. So far, more than 100 R genes for blast resistance and 37 R genes for bacterial blight resistance have been identified and mapped, 23 R genes for blast resistance and 6 R genes for bacterial blight resistance have been cloned (Liu et al. 2014). Marker-assisted selection (MAS) has been successfully used to improve qualitative blast resistance in rice (Jia et al. 2009; Roychowdhury et al. 2012). Furthermore, more than 300 QTLs for quantitative blast resistance (Ballini et al. 2008) and more than 50 QTLs for quantitative bacterial blight resistance (Li et al. 2006) have been identified. However, although quantitative disease resistance is the preferred strategy for sustainable control of plant disease and numerous QTLs have been identified, there are rare successful cases in MAS for quantitative disease resistance. This issue is attributed to the complex genetic control of quantitative resistance and unknown functional genes underlying resistance QTLs. Therefore, isolation of the genes underlying resistance QTLs is the key for effective use of quantitative resistance for disease control.
Map-based cloning has been commonly used for isolation of R genes. However, this strategy is difficult to be used for isolation of the genes for quantitative disease resistance due to their polygenic nature and smaller effect of each QTL (Hu et al. 2008). Therefore, a new strategy should be employed. Recently, increasing evidences have demonstrated the close correlation between defense responsive (DR) genes and quantitative disease resistance in plants. DR genes are predicted to function in plant disease resistance in a quantitative manner, and frequently recognized based on their increased expression pattern during plant defense response (Ramalingam et al. 2003; Hu et al. 2008). A large number of DR genes have been identified in rice and mapping of these DR genes on molecular linkage maps has indicated that some of the genes co-localize with disease resistance QTLs (Ramalingam et al. 2003; Liu et al. 2004; Wu et al. 2004; Fu et al. 2009; Fukuoka et al. 2009). Our previous research indicated that the five DR genes which encode putative germin-like protein (GLP), dehydrin, PR-1, chitinase, and 14-3-3 protein, accounted for 30.0, 23.0, 15.8, 6.7, and 5.5 % of blast diseased leaf area (DLA) variation, respectively and co-localized with resistance QTL identified by interval mapping (Liu et al. 2004). These findings imply that some of the DR genes could be the candidates underlying disease resistance QTLs. Using this strategy, Hu et al. (2008) have characterized four candidate genes and shown that they could influence the disease phenotypes in the interaction with Xoo or M. oryzae (Hu et al. 2008), suggesting that the candidate DR gene approach is a good strategy for isolation of disease resistance QTLs and characterization of the contributed DR genes will enhance our understanding of the mechanisms underlying quantitative disease resistance.
GLP gene family is one of the important defense gene families and has been considered to play an important role in several aspects of plant development or stress tolerance (Knecht et al. 2010). It has been considered that GLP genes can function as a cofactor for reinforcement of the cell wall by cross-linking of plant cell wall proteins at the infection site through the production of H2O2 due to their OXO or SOD activity (Olson and Varner 1993; Wei et al. 1998). Its product H2O2 can also acts as a signal molecule to induce a range of defense responses in a direct or indirect manner (Lane 1994; Zhou et al. 1998; Knecht et al. 2010). Thus, GLPs are expected to play important roles in disease resistance in plants. Indeed, nowadays, more and more evidence have indicated that GLPs are involved in plant basal host resistance (Lane 2002), including the observation that expression of several GLPs are highly induced by pathogen attack, or application of disease resistance-associated chemicals such as hydrogen peroxide (H2O2), salicylic acid (SA), and ethylene (Lou and Baldwin 2006; Zimmermann et al. 2006; Godfrey et al. 2007; Manosalva et al. 2009; Himmelbach et al. 2010). Transgenic plants ectopically expressing GLPs have provided direct evidence for their defense role in basal resistance. For example, transient overexpression of TaGLP4 and HvGLP4 enhanced resistance against B. graminis in wheat and barley, whereas transient silencing the two genes reduced basal resistance in both cereals (Christensen et al. 2004). Similarly, silencing of a GLP gene in Nicotiana attenuata improved the performance of native herbivores (Lou and Baldwin 2006).
In rice plants, GLP protein family has many members which are scattered on chr 1, 2, 3, 4, 5, 8, 9, 11 and 12, respectively (Carrillo et al. 2009). Our previous report has indicated that the GLPs on chr 8 were involved in leaf blast resistance (Liu et al. 2004; Manosalva et al. 2009). Moreover, siRNA-mediated gene silencing of OsGLP1 (Os08g35760) also exhibited increased susceptibility to fungal diseases (Banerjee and Maiti 2010). These results suggest that rice GLP proteins may play important roles in rice disease resistance. However, the functions of the other GLP family members on rice disease resistance are still unknown. Leaf blast and panicle blast are different types of blast disease in rice. In terms of yield loss, panicle blast is more destructive compared to leaf blast (Liu et al. 2016). Inconsistent results between leaf blast resistance and panicle blast resistance were observed in rice production (Zhuang et al. 2002), implying that there may be different mechanisms between leaf and panicle blast resistance. Although the panicle blast resistance is emphasized, the effect of GLPs on panicle blast has never investigated.
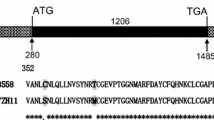
Four GLP genes were located on chromosome 2, and they are Os02g0491600, Os02g0491700, Os02g0491800 and Os02g0532500, respectively. In the present study, we identify that the member, Os02g0532500, was significantly induced by panicle blast infection and leaf blast infection. We designated this gene as OsGLP2-1. To confirm its function on leaf and panicle blast resistance and look insight into its mechanism on blast resistance, we have performed spatio-temporal expression and subcellular localization analysis. Transgenic plants were developed to confirm its functions on blast resistance and bacterial blight resistance as well. The putative molecular mechanisms of OsGLP2-1 were also investigated in this study.
Materials and methods
Plant materials
Rice cultivars, Nipponbare (ssp. japonica) and the resistant advanced backcross line BC10 (ssp. indica) were used in this study. All the plants were grown in soil in a greenhouse.
Chemicals treatments
The experiments were performed in wild-type Nipponbare plants using the same method as indicated previously with minor modifications (Liu et al. 2016). Mature seeds of Nipponbare were incubated at 49 °C for 4 days prior to germination, and then seeds were soaked in distilled water for 2 days in room temperature. Next, the seeds were embedded in wet towel for another 2 days in 32 °C for germination. Germinated seeds were then placed in gauze and transferred to salver for incubation in a growth chamber at 26 °C, 75 % relative humidity, 16,000 Lux and a 16 h/light and 8 h/dark interval. Rice seedlings at three- to four-leaf stage were sprinkled onto leaves with different plant hormone solutions in a concentration of 100 μM. For hormone treatment on the panicle tissue at the heading stage, the same cotton-wrapping inoculation method was used as for panicle blast inoculation. All the treatment experiments were repeated thrice.
Subcellular localization of GLP protein
The protein coding region of OSGLP2-1 was amplified from BC10 using the following primers. Forward primer, 5′-ATGGCGCATCGTCGTCGTT-3′; reverse primer, 5′-ATCCTCCATCTCCGCCTTCTT-3′. Then the product was cloned into the pGY1-mcherry vector to generate the GLP-mcherry fusion gene. The empty pGY1-mcherry plasmid was used as the control. For the transient expression assay, 1 μg of plasmid DNA was introduced into onion epidermal cells using the PDS-1000/He particle delivery system (BioRad, Hercules, CA, USA). After 24 h incubation in the dark, onion epidermal cells were observed under a laser confocal microscopy (Zeiss LSM710, Germany).
Gene transcription analysis
Plant tissues were grinded with liquid nitrogen and total RNA was extracted using RNAiso Plus (Takara, Japan). Then, 1 μg total RNA was reverse-transcribed to cDNA using the primescript™ RT reagent kit (Takara, Japan). Real-time PCR was performed using SYBR Premix ExTaq™ (Takara, Japan) according to the manufacturer’s instructions. EF1α gene was used as an internal control. The resulting melting curves were analyzed to ensure specificity of product detection. Each Experiment was performed in triplicate, and the result was represented by the mean ± standard derivation (SD). The Gene-specific primers that were used are listed in Supplemental Table 1.
Vector constructs and rice transformation
For constructing the OsGLP2-1 overexpressing (OXGLP2-1) plants, the cDNA sequence of OsGLP2-1 was amplified from a rice blast resistant cultivar BC10 by RT-PCR using the following primers. Forward primer, 5′-AGTGACAGAACGAGCGTAGAAT-3′; reverse primer, 5′-TCACTAACGGGGAGTAACCTAA-3′. The cDNA product was then inserted into pHQSN (modified from pCAMBIA1390) which harbors a CaMV35S promoter, and the constructed vector was electroporated into Agrobacterium tumefaciens EHA105. Rice transformation was performed as described with minor modifications (Toki et al. 2006).
Southern blot analysis
Genomic DNA was isolated from 1 g of 2-week-old rice seedlings using the CTAB method. After digested overnight by EcoR1, DNA samples (5–10 μg) were separated by electrophoresis on 0.8 % agarose gel, followed by transferring to Hybond nylon membrane (Amersham, GE Healthcare Limited, Buckinghamshire, UK). Labeling of hygromycin probe and detection of the signals were carried out using the DIG high prime DNA labeling and detection starter kit 1 (Cat. No: 11745832910; Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s protocol.
Pathogen inoculations
The control and transgenic plants were grown in soil in greenhouse. Leaf blast and panicle blast inoculations were conducted using the same method as described previously with some modifications (Liu et al. 2016). Concisely, seedlings at three- to four-leaf stage were inoculated by spraying with spore suspension of 1 × 106 spores/ml of a Magnaporthe oryzae isolate GD08-T13. Disease was assessed 6 days after inoculation by measuring the DLA. For panicle blast inoculation, the upper-middle part of a panicle was wrapped by cotton in 1 to 2 days after heading and 2 ml spore suspension of 1 × 106 spores/ml of GD08-T13 was injected into the cotton and the cotton was then wrapped by foil. Each inoculated panicles were sprayed with water for 2–3 min every 2 h to maintain the humidity. Disease was assessed in 3 weeks after inoculation by measuring the percent infected main axis length. Each treatment was repeated twice.
To evaluate bacterial blight disease, plants were inoculated with X. oryzaepv. Oryzae Chinese Xoo race 4 at the booting stage by the leaf-clipping method (Kauffman et al. 1973). Disease was assessed by measuring the percent lesion length (diseased leaf length/total leaf length) at 2 weeks after inoculation.
Measuring the level of H2O2 and detection of SOD enzyme activity
The concentration of H2O2 was measured using the hydrogen peroxide assay kit (Beyotime Institute of Biotechnology, China). The leaves of control and OXGLP2-1 plants at three- to four-leaf stage both before and after blast inoculation were collected and grinded with liquid nitrogen. Then 200 mL of the lysis buffer solution was added to per 10 mg dry powder. After mixing well, the supernatants were collected by centrifuging at 12,000g for 5 min. Next, 50 μL of the supernatants and 100 μL of test solutions were immediately added to the test-tubes and placed at room temperature for 30 min. H2O2 production was monitored by measuring the absorbance at 560 nm using a Thermo Scientific Multiskan Spectrum (Thermo, USA).
The same samples that used for H2O2 quantification were used for SOD activity analysis. Total SOD activity was measured with the WST-8 method using the detection kit according to the manual (Cat. NO: S0101, Beyotime Institute of Biotechnology, China).
Cis-elements analysis for the promoter
About 1500 bp sequence upstream of OsGLP2-1 was downloaded from MSU Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/) for cis-elements analysis. The sequence was scanned by PLACE (http://www.dna.affrc.go.jp/PLACE/) which is a database of nucleotide sequence motifs found in plant cis-acting regulatory DNA elements.
Jasmonic acid (JA) quantification by liquid chromatography tandem mass spectrometry (LC-MS)
The JA was extracted from the rice leaf according to Pan et al. (2010), then 10 μL of each JA sample solution was injected onto a C18 column (AQUITY UPLC BEH 130, 1.7 μm, 2.1 by 100 mm, Waters) at a flow rate of 0.1 mL/min, and the column was maintained at 30 °C. The JA sample solution was separated by reversed phase ultra-fast LC (Shimadzu, Kyoto) with a multi-step linear gradient elution using solution A (water with 5 mM ammonium formate and 0.1 % formic acid) and solution B (Methanol, with 5 mM ammonium formate and 0.1 % formic acid) over 30 min, as follows: 30 % solution B at 0–2 min, 30–100 % solution B at 2–20 min; 100 % solution B at 20–22 min, 100–30 % solution B at 22–25 min; and 30 % solution B at 25–30 min. The eluate was then introduced into the electrospray ion source of a tandem triple quadrupole MS analyser (API4000, AB SCIEX, Foster City, CA), and the JA compound was quantified in multiple reaction monitoring (MRM) mode using optimized MS/MS conditions, which were listed in Supplemental Table 2. The MS conditions were as follows: source, Turbo IonSpray; ion polarity, negative; IonSpray voltage, −4500 V; source temperature, 550 °C; gas, nitrogen; curtain gas, 30 psi; nebulizing gas (GS1), 55 psi; Collision gas (GS2), 55 psi; scan type, MRM; Q1 resolution: unit; Q3 resolution: unit. The Analyst 1.5.2 software (AB SCIEX, Foster City, CA) was used to control the instrument and to acquire and process all of the MS data.
Results
Transcription of OsGLP2-1 is induced after rice blast infection
Our previous microarray-assisted gene expression profiling of a blast resistant advanced backcross line (BC10) inoculated with blast isolate revealed that the GLP gene, Os02g0532500, designated as OsGLP2-1 was significantly induced by both leaf blast infection and panicle blast infection within a period of 48 h (data not shown). In the present study, real-time PCR was adopted to confirm this result. Our result showed that the transcription level of OsGLP2-1 was significantly increased at 6 and 12 h both in the leaf blast inoculation and panicle blast inoculation, whereas it returned to the basal level at 24 h during the panicle blast inoculation and remained highly induced in leaf blast inoculation (Fig. 1). These results suggest that OsGLP2-1 is responsive to leaf and panicle blast infection and may play an important role in rice blast disease resistance.
Expression pattern of the OsGLP2-1 gene after inoculation with blast disease as assessed by Quantitative RT-PCR; CK = water treatment, LB = leaf blast inoculation and PB = panicle blast inoculation. 0, 6, 12, 24 and 48 h indicate the time after inoculation. Error bars indicate the standard deviation (SD) from three biological replicates, and asterisks indicate statistically significant differences compared with water treatment (t test, **P < 0.01). a Expression pattern of the OsGLP2-1 gene after inoculation with leaf blast. b Expression pattern of the OsGLP2-1 gene after inoculation with panicle blast
OsGLP2-1 is highly expressed in leaves and seedlings, and the protein is localized in the cell wall
To understand the biological functions of the OsGLP2-1 gene in rice, we examined its temporal and spatial transcription in different rice tissues using the rice EF1α gene as the internal control. We identified that OsGLP2-1 was expressed throughout the entire life cycle of rice plant and in almost all rice tissues examined, with a relatively higher expression levels in leaves and panicles (Fig. 2a). To determine the subcellular localization of OsGLP2-1 in rice cells, we fused the coding region of OsGLP2-1 with the red fluorescent protein (mcherry) fragment under the control of the cauliflower mosaic virus 35S promoter and expressed it transiently in onion epidermal cells via the particle bombardment method (Shaner et al. 2004). Laser confocal microscopy showed that the red fluorescent signal was localized in the cell wall of the transfected onion epidermal cells, whereas the red fluorescent signal in the mcherry control vector was distributed both in the nucleus and the cytoplasm (Fig. 2b).
Tissue expression of OsGLP2-1 in rice plants and subcellular localization of this protein. a Quantitative RT-PCR analysis of OsGLP2-1 transcription in different tissues of Nipponbare plants. Error bars indicate the SD from three biological replicates. b Laser confocal microscopy images deriving from mcherry of onion epidermal cells transiently expressing mcherry or mcherry–OsGLP2-1 fusion protein. Bar 2 μm. Arrow indicates the nucleus. Cell plasmolysis was induced by a 30 % sucrose solution for 7 min before observation
Overexpression of OsGLP2-1 in transgenic rice quantitatively enhances resistance to blast disease
To elucidate the putative function of OsGLP2-1 on rice blast disease, transgenic rice plants constitutively overexpressing OsGLP2-1 were produced in Nipponbare, which was highly susceptible to blast isolate GD08-T13 in both leaf and panicle blast. Eighteen transgenic lines were obtained. The transgenic plants (OXGLP2-1) showed no morphological changes and were fertile. We used the resistance gene hygromycin to screen for putative overexpressing lines (Supplemental Fig. 1) and southern hybridization was used to confirm OsGLP2-1 transgene integration in rice genome (Supplemental Fig. 2). The evaluation of blast resistance showed that the T0 plants showed significantly enhanced resistance to panicle blast, with lower percentage of infected main axis length compared with wild type (Supplemental Fig. 3). The real-time PCR experiment indicated that the enhanced resistance was associated with the increased expression of OsGLP2-1 (R2 = 0.8115; Supplemental Fig. 1). To validate that the overexpression of OsGLP2-1 accounts for the increased resistance of the transgenic plants, three independent homozygous lines (OX-2, OX-11, OX-15; T2 generation) were further analyzed individually for their resistance to GD08-T13 and the OsGLP2-1expression levels (Fig. 3b). Our result showed that the enhanced resistance could be stably inherited in these plants, with the infected main axis length ranging from 34.3 to 57.8 % (average of 43 %) in the transgenic plants, compared with 79.4 % for the empty vector transformed plants (PHQSN) (Fig. 3a, c). The transcript level analysis showed that the enhanced resistance was associated with overexpression of OsGLP2-1 in these transgenic lines (R2 = 0.9387; Fig. 3d).
Phenotypes of the OsGLP2-1 overexpressing (OXGLP2-1) plants to panicle blast infection. OX-2, OX-11 and OX-15 are three independent homozygous transgenic lines of OXGLP2-1 in T2 generation. a Disease phenotypes of the OXGLP2-1 plants and PHQSN (control) plants after inoculation with M. oryzae isolate GD08-T13 using the cotton-wrapping method at the heading stage. b Transcription analysis of the OXGLP2-1 plants by quantitative RT-PCR. Error bars indicate the SD from three biological replicates, and asterisks indicate statistically significant differences compared with control (PHQSN) plants (t test, **P < 0.01). c Relative diseased main axis length in the OXGLP2-1 plants and control plants after panicle blast infection. Error bars indicate the SD from at least ten biological replicates, and asterisks indicate statistically significant differences compared with control plants (t test, **P < 0.01; *P < 0.05). d The enhanced resistance co-segregated with the increased level of OsGLP2-1 in transgenic plants (T2 generation) after panicle blast inoculation. The correlation coefficient was calculated by linear regression
The three transgenic lines (OX-2, OX-11, OX-15) were also subjected to blast inoculation at the three- to four-leaf stage. The DLA ranged from 29.61 to 56.02 % (average of 39.23 %) in transgenic plants, compared with 77.35 % for PHQSN control plants (Fig. 4a, b). The enhanced resistance of the plants correlated with over-presented expression of OsGLP2-1 (R2 = 0.9343; Fig. 4c; Supplemental Table 3). Taken together, these results showed that overexpression of OsGLP2-1 quantitatively enhances resistance to both leaf blast and panicle blast.
Phenotypes of the OsGLP2-1 overexpressing (OXGLP2-1) plants to leaf blast infection. a Disease phenotypes of the OXGLP2-1 plants (T2 generation) and control plants after spray inoculation with M. oryzae isolate GD08-T13 for 7 days at the three- to four-leaf stage. b Relative diseased leaf area (DLA) in the OXGLP2-1 plants and control plants after leaf blast inoculation. Error bars indicate the SD from at least ten biological replicates, and asterisks indicate statistically significant differences compared with control plants (t test, *P < 0.05, **P < 0.01). c The enhanced resistance co-segregated with the increased level of OsGLP2-1 in transgenic plants (T2 generation) after leaf blast inoculation. The correlation coefficient was calculated by linear regression
Overexpression of OsGLP2-1 in transgenic rice quantitatively enhanced resistance to bacterial blight
Bacterial blight caused by Xanthomonas oryzae pv. oryzae (Xoo) is another devastating rice disease worldwide. Previous research has indicated that fungal blast resistance and bacterial blight resistance might share common pathways to some extent (Hu et al. 2008). To see if OsGLP2-1 gene also functions on bacterial blight, we evaluated the bacterial blight resistance of the OXGLP2-1 (OsGLP2-1 overexpressing) plants by inoculation with an isolate from Chinese Xoo race 4. Our results showed that the OXGLP2-1 plants showed significantly enhanced resistance to bacterial blight, with lesion length ranging from 17 to 27 % (average of 21 %), compared with 44 % for PHQSN control plants (Fig. 5a, b). The growth rate of bacteria in OXGLP2-1 plants was 2.4- to 3.2-fold lower than that in control plants (Fig. 5c). These results suggest that overexpression of OsGLP2-1 can also quantitatively enhances resistance to bacterial blight.
Phenotypes of the OXGLP2-1 plants to bacterial blight infection. a Disease phenotypes of the OXGLP2-1 plants and control plants after Xoo inoculation. b Relative lesion length in the OXGLP2-1 plants and control plants after Xoo inoculation. Error bars indicate the SD from at least ten biological replicates, and asterisks indicate statistically significant differences compared with control plants (t test, **P < 0.01). c Growth of Xoo race 4 in leaves of OXGLP2-1 plants and control plants. Bacterial populations were determined from three leaves 16 days after inoculation by counting colony-forming units (cfu). Similar results were obtained in two independent biological experiments. Error bars indicate the SD from six biological replicates, and asterisks indicate statistically significant differences compared with control plants (t test, **P < 0.01)
Overexpression of OsGLP2-1 results in accumulation of H2O2 in transgenic rice
GLPs have been proposed to exhibit superoxide dismutase (SOD) activity leading to hydrogen peroxide (H2O2) production, which is often correlated with plant defense responses (Lane 1994; Christensen et al. 2004; Laloiet al. 2004; Zimmermann et al. 2006). To confirm if the enhanced disease resistance by OsGLP2-1 is due to the accumulation of H2O2, we determined the H2O2 content using the xylenol orange method (Kim and Hwang 2014). An accumulation of H2O2 was identified at 48 h after pathogen inoculation both in the control and OXGLP2-1 plants (Fig. 6). However, the H2O2 content was significantly higher in OXGLP2-1 plants than in control plants both before and after pathogen inoculation (Fig. 6a). These results suggest that overexpression of OsGLP2-1 results in H2O2 accumulation in OXGLP2-1 plants. To further validate that if the generation of H2O2 in the transgenic plants was due to the SOD activity of OsGLP2-1, the SOD enzyme activity was analyzed in the leaves of the same plants that used for H2O2 quantification. As shown in Fig. 6b, the SOD activity was higher in OsGLP2-1 overexpressing plants than in control plants both before and after blast inoculation, indicating that OsGLP2-1 harbors SOD activity.
Analysis of the hydrogen peroxide (H2O2) content and SOD activity in the leaves of OXGLP2-1 plants and control plants. Error bars indicate the SD from at least ten biological replicates, and asterisks indicate significant differences between transgenic and control plants before blast inoculation (CK) and at 24 and 48 h after inoculation with M. oryzae (t test, **P < 0.01 and *P < 0.05). FW fresh weight. a The H2O2 content in the leaves of OXGLP2-1 plants and control plants. b The SOD activity in the leaves of OXGLP2-1 plants and control plants
OsGLP2-1 modulates the transcription of defense-related genes that are involved in JA dependent pathway in rice
To identify the regulatory components and understand the mechanism of OsGLP2-1 in regulation of disease resistance in rice, about 1500 bp promoter sequences from the translational start site of OsGLP2-1 were analyzed for cis-elements. We observed three GCCCOREs (Supplemental Table 4), which has been shown to function as a jasmonic acid (JA) responsive element that is necessary for the regulation of transcription by JA (Brown et al. 2003). These response elements of JA in the promoter region of OsGLP2-1 imply that this hormone may act upstream of OsGLP2-1 to modulate its activity and then ultimately regulate rice blast resistance. To confirm this, the wild-type Nipponbare plants were treated with exogenous JA at the seedling stage and heading stage respectively. The expression levels of OsGLP2-1 at 3, 6, 12 and 24 h after treatment were analyzed using RT-PCR. The results indicated that the transcription levels of OsGLP2-1 were increased at the four time points after JA treatment in Nipponbare both at the leaf and panicle tissue (Fig. 7). These observations indicate that JA may act upstream of OsGLP2-1 to induce its transcription.
Expression analysis of OsGLP2-1 after JA (jasmonic acid) treatment in Nipponbare plants. Error bars indicate the SD from three biological replicates, and asterisks indicate statistically significant differences compared with water treatment (t test, **P < 0.01; *P < 0.05). a Time-course transcription analysis of OsGLP2-1 after JA and water (CK) treatments by quantitative RT-PCR analysis at the seedling stage in Nipponbare plants. b Time-course transcription analysis of OsGLP2-1 after JA and water treatments by quantitative RT-PCR analysis at the heading stage in OXGLP2-1 plants
To further validate if OsGLP2-1 mediated disease resistance is involved in JA signaling pathway, we analyzed the expression patterns of some well-characterized defense-related genes which are involved in JA-dependent pathway, including the lipoxygenases (LOX1 and LOX11), an allene oxide synthase 2 (AOS2) gene and a pathogenesis-related [PR] gene PR10 (Deng et al. 2012; Liu et al. 2016; Mei et al. 2006). The pathogen infection strongly induced the expression of LOX1, AOS2 and PR10 in both the control (PHQSN) and OsGLP2-1 overexpressing plants both in the seedling and panicle tissue but the expression levels of LOX1, AOS2 and PR10 were significantly higher in OXGLP2-1 plants than in control plants after inoculation (Fig. 8a; Supplemental Fig. 4). Only significant difference was identified for LOX1 between the control and OXGLP2-1 plants at the seedling stage before pathogen inoculation (Fig. 8a), and there was no obvious difference in expression level of LOX11 between transgenic plants and control plants either before or after pathogen inoculation (data not shown). These results suggest that OsGLP2-1 mediated disease resistance is involved in the JA signaling pathway.
OsGLP2-1 mediated disease resistance is involved in the JA signaling pathway. Asterisks indicate significant differences between transgenic and control plants before blast inoculation (CK) and at 48 h after inoculation with M. oryzae (t test, **P < 0.01; *P < 0.05). Expression data are the means of three replicates ± SD. a Overexpressing of OsGLP2-1 influences the expression of a set of defense-related genes at the seedling stage. b Overexpressing of OsGLP2-1 leads to the accumulation of endogenous JA
OsGLP2-1 influences the accumulation of JA
To gain further insight into the relationship between OsGLP2-1 and the JA signaling pathway, we quantified the concentrations of the endogenous JA in the leaves of control and OXGLP2-1 plants both before and after inoculation with blast isolate GD08-T13. The endogenous level of JA was significantly induced by pathogen inoculation in both the control and OXGLP2-1 plants, but the endogenous JA level was significantly higher (P < 0.01) in OXGLP2-1 plants than that in control plants both before and after pathogen inoculation (Fig. 8b). These results further indicate that OsGLP2-1 mediated disease resistance is indeed associated with the JA signaling pathway.
Discussion
OsGLP2-1 quantitatively enhances disease resistance in rice and the resistance is broad spectrum
GLPs encoded by a heterogeneous group of genes present in many land plants including monocots, dicots, gymnosperms and moss (Davidson et al. 2009). Many GLP genes have been reported to play important roles in disease resistance in plants (Christensen et al. 2004; Lou and Baldwin 2006; Zimmermann et al. 2006; Godfrey et al. 2007; Manosalva et al. 2009; Himmelbach et al. 2010). In rice, there are 41 GLPs that are scattered on different chromosomes, respectively (Carrillo et al. 2009). However, only the GLPs on chr 8 have been confirmed their functions on leaf blast resistance and sheath blight resistance (Manosalva et al. 2009). In this study, we have demonstrated that one member of GLPs on chr 2, OsGLP2-1, was significantly induced by leaf blast and panicle blast infection (Fig. 1). By overexpressing OsGLP2-1, we were able to generate enhanced resistance to leaf blast, panicle blast and bacterial blight in rice (Figs. 3, 4, 5). These results demonstrate that OsGLP2-1 functions as a positive regulator in blast and bacterial blight resistance in rice. To our knowledge, this study provides the first evidence that GLP genes are also involved in panicle blast and bacterial blight resistance. Furthermore, our results showed that the enhanced disease resistance correlated with increasing transcription levels of this gene (Figs. 3, 4). The OsGLP2-1 overexpressing rice plants displayed less DLA and lower percent infected main axis length during leaf and panicle blast infection, and lower percent lesion length and fewer spores during bacterial blight infection compared with the control plants (Figs. 4, 5), exhibiting the nature of quantitative resistance. In addition to its resistance to blast and bacterial blight, OsGLP2-1 has been reported to co-localize with a QTLs for sheath blight resistance in rice (Davidson et al. 2009). Taken together, these results suggest that the disease resistance provided by OsGLP2-1 is quantitative and broad spectrum. These characteristics make OsGLP2-1 to be a good candidate in rice improvement for disease resistance in rice plants.
The broad spectrum of disease resistance of OsGLP2-1 is associated with the generation of H2O2 and the gene expression patterns
H2O2 has been proved to kill pathogens directly and also plays a key role in reinforcement of the cell wall by cross-linking of plant cell wall proteins in papillae at the infection sites (Olson and Varner 1993; Wei et al. 1998). The previous study has demonstrated that the enhanced resistance by overexpressing BvGLP-1 in Arabidopsis was correlated with the elevated H2O2 content (Knecht et al. 2010). Moreover, the enhanced resistance of GF14e silenced plants was attributed to the higher expression of peroxidase gene (POX22.3), and accumulation of reactive oxygen species (ROS), such as H2O2 (Manosalva et al. 2011). Based on these results, a possible explanation for the function of OsGLP2-1 on disease resistance in rice could be the generation of H2O2 through its enzymatic activity (OxO or SOD) in plant cells. Indeed, in the present study, accumulation of H2O2 was observed in the OsGLP2-1 overexpressing plants duing to its SOD activity (Fig. 6), and our sub-cellular localization experiment also indicated that OsGLP2-1 localized in the cell wall (Fig. 2b). Furthermore, we also observed a drastic reduction in the amount of spores of Xoo in the infected leaves of the OsGLP2-1 overexpressing plants compared to the control plants (Fig. 5c), indicating the strong inhibition of the bacterial propagation in transgenic rice leaves. From these results,it can be deduced that the accumulated H2O2 may contribute to the reinforcement of cell wall to protect the cell from pathogen penetration and may also act as a toxin to prevent pathogen propagation. Moreover, the expression pattern analysis revealed that OsGLP2-1 was expressed throughout the life cycle of the rice plants and in almost all rice tissues examined (Fig. 2a). The expression pattern of OsGLP2-1 agrees with its resistance to both leaf blast and panicle blast. Together, we can conclude that the broad spectrum of disease resistance conferred by OsGLP2-1 is associated with the generation of H2O2 and its expression pattern.
OsGLP2-1 mediated disease resistance is involved in JA signaling pathway
JA and SA are the well-known endogenous signal molecules that involved in the regulation of resistance against different pathogens. In the present study, the cis-element analysis revealed that there were JA response elements in the promoter region of OsGLP2-1 (Supplemental Table 4) and the expression of OsGLP2-1 was induced by JA treatment in Nipponbare plants (Fig. 7). In contrast, the transcription of OsGLP2-1 exhibited no obvious difference when subjected to exogenous SA treatment in Nipponbare plants (Supplemental Fig. 5). These results imply that OsGLP2-1 mediated disease resistance may be regulated by JA but not SA. Furthermore, our results showed that the expression levels of the defense-related genes, LOX1, AOS2 and PR10 which are involved in JA-dependent pathway, were significantly higher in OsGLP2-1 transgenic plants than in control plants after pathogen infection, which results in a more active JA signaling pathway in OsGLP2-1 over-expressing plants (Fig. 8). Contrastingly, the expression levels of NH1 and PAL1, which are associated with SA-dependent pathway, were not significantly different between transgenic plants and control plants after pathogen infection (Supplemental Fig. 6). These observations are consistent with the results from hormone treatments, suggesting that OsGLP2-1 mediated disease resistance is involved in JA signaling pathway but not the SA-dependent pathway.
Generally, plant resistance to necrotrophic pathogens is frequently regulated by the JA-dependent pathway, whereas resistance to biotrophic and hemibiotrophic pathogens is frequently controlled by the SA-dependent pathway (Bari and Jones 2009). Blast fungus is a hemibiotrophic pathogen and Xoo is a biotrophic pathogen. Thus, we expect that OsGLP2-1 mediated resistance to blast and bacterial blight is modulated by the SA-dependent pathway. However, the results presented here suggest that OsGLP2-1 mediated resistance to blast and bacterial blight was involved in the activation of JA-dependent pathway instead of SA-dependent pathway.
In summary, in this study, we have confirmed the functions of OsGLP2-1 on disease resistance through differential expression and transgenic method and investigated its possible regulatory mechanism. Our results suggest that OsGLP2-1 positively regulate leaf blast, panicle blast and bacterial blight resistance in rice. OsGLP2-1 mediated disease resistance is partially due to the accumulation of H2O2, and is also associated with activation of the JA-dependent pathway. However, because there are numerous GLP genes in rice, we do not know whether other GLP proteins (in addition to GLPs on chr 8 and OsGLP2-1) are involved in plant resistance to M. oryzae and Xoo in rice. Furthermore, we still do not know whether there are transcription factors acting upstream of OsGLP2-1 and how OsGLP2-1 coordinates with the other GLP genes to contribute to disease resistance in rice. Further study is still needed to address these issues.
References
Ballini E, Morel JB, Droc G, Price A, Courtois B, Notteghem JL, Tharreau D (2008) A genome-wide meta-analysis of rice blast resistance genes and quantitative trait loci provides new insights into partial and complete resistance. Mol Plant Microbe Interact 21:859–868
Banerjee J, Maiti MK (2010) Functional role of rice germin-like protein1 in regulation of plant height and disease resistance. Biochem Biophys Res Commun 394:178–183
Bari R, Jones JD (2009) Role of plant hormones in plant defense responses. Plant Mol Biol 69:473–488
Brown RL, Kazan K, McGrath KC, Maclean DJ, Manners JM (2003) A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol 132:1020–1032
Carrillo MGC, Goodwin PH, Leach JE, Leung H, Vera Cruz CM (2009) Phylogenomic relationships of rice oxalate oxidases to the cupin superfamily and their association with disease resistance QTL. Rice 1:67–69
Christensen AB, Thordal-Christensen H, Zimmermann G, Gjetting T, Lyngkjaer MF, Dudler R, Schweizer P (2004) The germinlike protein GLP4 exhibits superoxide dismutase activity and is an important component of quantitative resistance in wheat and barley. Mol Plant Microbe Interact 17:109–117
Davidson RM, Manosalva PM, Snelling J, Bruce M, Leung H, Leach J (2009) Rice germin-like proteins: allelic diversity and relationships to early stress responses. Rice 3:43–55
Deng H, Liu H, Li X, Xiao J, Wang S (2012) A CCCH-type zinc finger nucleic acid-binding protein quantitatively confers resistance against rice bacterial blight disease. Plant Physiol 158:876–889
Fu D, Uauy C, Distelfeld A, Blechl A, Epstein L, Chen X, Sela H, Fahima T, Dubcovsky J (2009) A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 323:1357–1360
Fu J, Liu H, Li Y, Yu H, Li X, Xiao J, Wang S (2011) Manipulating broad-spectrum disease resistance by suppressing pathogen-induced auxin accumulation in rice. Plant Physiol 155:589–602
Fukuoka S, Saka N, Koga H, Ono K, Shimizu T, Ebana K, Hayashi N, Takahashi A, Hirochika H, Okuno K, Yano M (2009) Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 325:998–1001
Godfrey D, Able A, Dry I (2007) Induction of a grapevine germin-like protein (VvGLP3) gene is closely linked to the site of Erysiphe necator infection: a possible role in defense? Mol Plant Microbe Interact 20:1112–1125
Hayashi N, Inoue H, Kato T, Funao T, Shirota M, Shimizu T, Kanamori H, Yamane H, Hayano-Saito Y, Matsumoto T, Yano M, Takatsuji H (2010) Durable panicle blast-resistance gene Pb1 encodes an atypical CC-NBS-LRR protein and was generated by acquiring a promoter through local genome duplication. Plant J 64:498–510
Himmelbach A, Liu L, Zierol U, Altschmied L, Maucher H, Beier F, Muller D, Hensel G, Heise A, Schutzendubel A, Kumlehn J, Schweizer P (2010) Promoters of the barley germin-like GER4 gene cluster enable strong transgene expression in response to pathogen attack. Plant Cell 22:937–952
Hu K, Qiu D, Shen X, Li X, Wang S (2008) Isolation and manipulation of quantitative trait loci for disease resistance in rice using a candidate gene approach. Mol Plant 1:786–793
Jia Y, Liu G, Costanzo S, Lee S, Dai Y (2009) Current progress on genetic interactions of rice with rice blast and sheath blight fungi. Front Agric China 3:231–239
Kauffman HE, Reddy APK, Hsieh SPY, Merca SD (1973) An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Dis Rep 57:537–541
Kim DS, Hwang BK (2014) An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J Exp Bot 65:2295–2306
Knecht K, Seyffarth M, Desel C, Thurau T, Sherameti I, Lou B, Oelmüller R, Cai D (2010) Expression of BvGLP-1 encoding a germin-like protein from sugar beet in Arabidopsis thaliana leads to resistance against phytopathogenic fungi. Mol Plant Microbe Interact 23:446–457
Kou Y, Wang S (2010) Broad-spectrum and durability: understanding of quantitative disease resistance. Curr Opin Plant Biol 13:181–185
Laloi C, Apel K, Danon A (2004) Reactive oxygen signalling: the latest news. Curr Opin Plant Biol 7:323–328
Lane B (1994) Oxalate, germin, and the extracellular matrix of higher plants. FASEB J 5:294–301
Lane B (2002) Oxalate, germins, and higher-plant pathogens. IUBMB Life 53:67–75
Li ZK, Arif M, Zhong DB, Fu BY, Xu JL, Domingo-Rey J, Ali J, Vijauakumar CHM, Yu SB, Khush GS (2006) Complex genetic networks underlying the defensive system of rice (Oryza sativa L.) to Xanthomonas oryzae pv. oryzae. Proc Natl Acad Sci USA 103:7994–7999
Liu B, Zhang S, Zhu X, Yang Q, Wu S, Mei M, Mauleon R, Leach J, Mew T, Leung H (2004) Candidate defense genes as predictors of quantitative blast resistance in rice. Mol Plant Microbe Interact 17:1146–1152
Liu W, Liu J, Triplett L, Leach J, Wang G (2014) Novel insights into rice innate immunity against bacterial and fungal pathogens. Annu Rev Phytopathol 52:213–241
Liu Q, Yang J, Zhang S, Zhao J, Feng A, Yang T, Wang X, Mao X, Dong J, Zhu X, Leung H, Leach JE, Liu B (2016) OsGF14b positively regulates panicle blast resistance, but negatively regulates leaf blast resistance in rice. Mol Plant Microbe Interact 29:46–56
Lou Y, Baldwin I (2006) Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores. Plant Physiol 140:1126–1136
Manosalva P, Davidson R, Liu B, Zhu X, Hulbert S, Leung H, Leach J (2009) A Germin-Like protein gene family functions as a complex QTL conferring broad-Spectrum disease resistance in rice. Plant Physiol 149:286–296
Manosalva P, Bruce M, Leach J (2011) Rice 14-3-3 protein (GF14e) negatively affects cell death and disease resistance. Plant J 68:777–787
McDonald BA, Linde C (2002) Pathogen population genetics, evolutionary potential, and durable resistance. Annu Rev Phytopathol 40:349–379
Mei C, Qi M, Sheng G, Yang Y (2006) Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact 19:1127–1137
Olson P, Varner J (1993) Hydrogen peroxide and lignifications. Plant J 4:887–892
Pan X, Welti R, Wang X (2010) Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc 5:986–992
Ramalingam J, Vera Cruz CM, Kukreja K, Chittoor JM, Wu JL, Lee SW, Baraoidan M, George ML, Cohen MB, Hulbert SH, Leach J, Leung H (2003) Candidate defense genes from rice, barley, and maize and their association with qualitative and quantitative resistance in rice. Mol Plant Microbe Interact 16:14–24
Roumen EC (1994) In: A strategy for accumulating genes for partial resistance to blast disease in rice within a conventional breeding program. In: Zeigler RS, Leong SA, Teng PS (eds) Rice Blast Disease. CAB International, Cambridge, pp 245–265
Roychowdhury M, Jia Y, Jia MH, Fjellstrom R, Cartwright RD (2012) Identification of the rice blast resistance gene Pib in the National Small Grains Collection. Phytopathology 102:700–706
Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature Biotechnol 22:1567–1572
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47:969–976
Wei Y, Zhang Z, Andersen C, Schmelzer E, Gregersen P, Collinge D, Smedegaard-Petersen V, Thordal-Christensen H (1998) An epidermis/papilla-specific oxalate oxidase-like protein in the defense response of barley attacked by the powdery mildew fungus. Plant Mol Biol 36:101–112
Wu JL, Sinha PK, Variar M, Zheng KL, Leach J, Courtois B, Leung H (2004) Association between molecular markers and blast resistance in an advanced backcross population of rice. Theor Appl Genet 108:1024–1032
Zhou F, Zhang Z, Gregersen PL, Mikkelsen JD, de Neergaard E, Collinge DB, Thordal-Christensen H (1998) Molecular characterization of the oxalate oxidase involved in the response of barley to the powdery mildew fungus. Plant Physiol 117:33–41
Zhuang JY, Ma WB, Wu JL, Chai RY, Lu J, Fan YY, Jin MZ, Leung H, Zheng KL (2002) Mapping of leaf and neck blast resistance genes with resistance gene analog, RAPD and RFLP in rice. Euphytica 128:363–370
Zimmermann G, Bäumlein H, Mock HP, Himmelbach A, Schweizer P (2006) The multigene family encoding germin-like proteins of barley. Regulation and function in basal host resistance. Plant Physiol 142:181–192
Acknowledgments
This research was supported partially by National Natural Science Foundation of China (NSFC)-International Rice Research Institute (IRRI) project (31461143019), the 973 project of Ministry of Science and Technology, China (2006BFD33320), the National Natural Science Foundation of China (30771392), the Presidential Foundation of the Guangdong Academy of Agricultural Sciences, China (201201), the Earmarked Fund for Modern Agro-Industry Technology Research System (CARS-01-24). We thank professor Jan Leach in Colorado State University, USA and Dr Hei Leung in International Rice Research Institute for their valuable discussion and suggestions on this study and during manuscript preparation. We also thank Jia Jian for technical support of sub-localization. We are grateful to Zhang Weina (Center for Agrobiological Gene Research, Guangdong Academy of Agricultural Sciences) for help with laser confocal microscopy.
Author contributions
QL conducted the quantitative qRT-PCR assay, transgenic functional confirmation and chemical treatment experiments, drafting the manuscript and proposal writing. JY and WJ evaluated the blast and Xoo resistance of the control and transgenic plants. SY conducted the quantitative analysis of the endogenous JA. SZ, JZ, TY, XW, XM, and JD participated in RNA extraction and quantitative qRT-PCR assays. XZ and BL conceived of the study, drafted proposal and corrected manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Bin Liu is the first corresponding author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Q., Yang, J., Yan, S. et al. The germin-like protein OsGLP2-1 enhances resistance to fungal blast and bacterial blight in rice. Plant Mol Biol 92, 411–423 (2016). https://doi.org/10.1007/s11103-016-0521-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0521-4