Abstract
Fructans represent the major component of water soluble carbohydrates (WSCs) in the maturing stem of temperate cereals and are an important temporary carbon reserve for grain filling. To investigate the importance of source carbon availability in fructan accumulation and its molecular basis, we performed comparative analyses of WSC components and the expression profiles of genes involved in major carbohydrate metabolism and photosynthesis in the flag leaves of recombinant inbred lines from wheat cultivars Seri M82 and Babax (SB lines). High sucrose levels in the mature flag leaf (source organ) were found to be positively associated with WSC and fructan concentrations in both the leaf and stem of SB lines in several field trials. Analysis of Affymetrix expression array data revealed that high leaf sucrose lines grown in abiotic-stress-prone environments had high expression levels of a number of genes in the leaf involved in the sucrose synthetic pathway and photosynthesis, such as Calvin cycle genes, antioxidant genes involved in chloroplast H2O2 removal and genes involved in energy dissipation. The expression of the majority of genes involved in fructan and starch synthetic pathways were positively correlated with sucrose levels in the leaves of SB lines. The high level of leaf fructans in high leaf sucrose lines is likely attributed to the elevated expression levels of fructan synthetic enzymes, as the mRNA levels of three fructosyltransferase families were consistently correlated with leaf sucrose levels among SB lines. These data suggest that high source strength is one of the important genetic factors determining high levels of WSC in wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water soluble carbohydrates (WSCs) in temperate cereal species (e.g. wheat, barley, oats and rye) are mainly composed of sucrose, glucose, fructose and fructans. WSCs can accumulate in the stem and leaf sheath of temperate cereals during the period from stem elongation to the early phase of grain filling and serve as a temporary carbohydrate reserve (Schnyder 1993; Wardlaw and Willenbrink 1994; Blum 1998; Gebbing 2003). WSCs in wheat are mobilised from the stem and leaf sheath during the later phase of grain filling and can potentially contribute to about 20 % of grain yield under normal conditions (Wardlaw and Willenbrink 2000). The grain yield of temperate cereal crops in terminal drought and heat-prone environments becomes more heavily dependent on the stem carbon reserve (van Herwaarden et al. 1998b; Wardlaw and Willenbrink 2000; Barnabás et al. 2008). In wheat crops under terminal drought stress conditions, stem WSCs could potentially contribute to >50 % of grain yield (Brooks et al. 1982; Aggarwal and Sinha 1984). This is because the carbon supply from photosynthesis is reduced during drought stress due to both stomatal closure in the leaves (Chaves et al. 2002) and coordinated down-regulation of genes involved in the Calvin cycle (Xue et al. 2008a). Variation in stem WSC concentrations among wheat genotypes is one of the genetic factors influencing grain weight and yield in terminal drought- and heat-prone environments (Xue et al. 2008b).
Fructans are the major component of WSCs in wheat stem (particularly in the lower internodes) at the developmental stage from anthesis to early grain filling (Ruuska et al. 2006, 2008; Xue et al. 2008b, 2011). Fructans are soluble linear or branched β-2,1- or β-2,6-linked fructosyl-oligosaccharides, that are present in 15 % of angiosperm species (Vijn and Smeekens 1999; Van Laere and Van den Ende 2002; Chalmers et al. 2003, 2005; Van den Ende et al. 2011). Fructans are synthesised from sucrose in the vacuole by a group of fructosyltransferses belonging to plant glycoside hydrolase family 32 enzymes (Ritsema and Smeekens 2003; Chalmers et al. 2005; Altenbach et al. 2009; Van den Ende et al. 2009). The 3D structure of a plant fructosyltransferase from Pachysandra terminalis has recently been determined (Lammens et al. 2012). Fructans in cereals are mainly the graminan type, that is predominantly β-2,6-linked fructosyl-units with short β-2,1-linked branches (Ritsema and Smeekens 2003; Chalmers et al. 2005). The enzymes involved in the synthesis of the β-2,6-linked fructan are sucrose:sucrose 1-fructosyltransferase (1-SST) and sucrose:fructan 6-fructosyltransferase (6-SFT). The enzyme responsible for the synthesis of β-2,1-linked branch in graminan is fructan:fructan 1-fructosyltransferase (1-FFT) (Kawakami and Yoshida 2005). Fructan:fructan 6G-fructosyltransferase (6G-FFT) present in some plant species has not been shown to exist in wheat and is known to be absent in barley (Lasseur et al. 2011). 1-SST, 6-SFT and 1-FFT cDNAs from wheat have been characterised (Kawakami and Yoshida 2002, 2005). The expression of 1-SST and 6-SFT genes in wheat has been shown to be transactivated by a R2R3-MYB transcription factor, which is tightly co-regulated with these fructosyltransferases (Xue et al. 2011).
Fructan accumulation in barley and wheat is influenced by both environmental and genetic factors (Blum 1998; van Herwaarden et al. 1998a; Ruuska et al. 2006, 2008; Ehdaie et al. 2006; Xue et al. 2008b; McIntyre et al. 2011). Genotypic ranking among wheat genotypes in stem WSC concentration, which is an indirect estimate for stem fructan level, is generally consistent across environments (Foulkes et al. 2002; Ruuska et al. 2006). Positive relationships between stem WSC concentration at anthesis and grain weight or yield in wheat have been observed in many studies, particularly under water-limited environments (Foulkes et al. 2002; Asseng and van Herwaarden 2003; Ruuska et al. 2006; Xue et al. 2008b; McIntyre et al. 2011). High WSC concentration is considered to be a potentially useful trait for improving grain weight and yield of wheat in water-limited environments (Blum 1998; Asseng and van Herwaarden 2003; Shearman et al. 2005; Ruuska et al. 2006; Foulkes et al. 2007).
In an attempt to elucidate the molecular and biochemical mechanisms that underlie genotypic variation in the WSC trait, we previously conducted a study using an integrated analysis of global transcript profiling and end product contents in the stems of wheat recombinant inbred lines (Xue et al. 2008b). The analyses of genes involved in WSCs and their associated metabolic pathways in wheat stem at the fructan accumulation phase suggest that the high stem WSC trait in wheat is associated with enhanced fructan deposition, reduced sucrose hydrolysis and reduced carbon partitioning into cell wall polysaccharides (Xue et al. 2008b). The inverse relationship between stem WSC concentration and cell wall polysaccharide content has also been observed in various wheat genotypes (Valluru et al. 2011). These data implicate potentially high carbon sink strength for storing this temporary carbon reserve in the stem of high WSC lines, which is effected through a combination of the enhanced transcript levels of fructan synthetic enzymes and a reduced amount of carbon partitioning into other metabolic pathways. However, the factors that lead to the elevated expression levels of fructosyltransferases in high WSC lines are still unknown.
Theoretically, genotypic variation in carbon reserve accumulation is determined by relative carbon availability and demand at the whole plant level. Carbon reserve accumulation such as starch in plants can be influenced by either sink strength or source strength or both (Herbers and Sonnewald 1998; Smith 2008; Börnke and Sonnewald 2011; Geigenberger 2011). The enhanced rate of stem fructan synthesis can result from the high carbon strength in the leaf, which supplies sucrose to the stem, or a genetic determinant (sink strength) independent on the source carbon availability. Fructan synthesis in plants is known to be regulated by sucrose, as several studies have indicated that the expression of fructosyltransferase genes in the excised leaves or stems of barley and wheat is strongly upregulated by exogenous sucrose (Müller et al. 2000; Martínez-Noël et al. 2001, 2006, 2009, 2010; Koroleva et al. 2001; Nagaraj et al. 2001, 2004; Lu et al. 2002; Ruuska et al. 2008, Ritsema et al. 2009; Xue et al. 2011), which lead to the elevated activity of fructosyltransferases as observed in excised wheat leaves supplemented with exogenous sucrose (Joudi et al. 2012). Sucrose-mediated upregulation of fructosyltransferase genes has also been shown in the hairy root cultures of Cichorium intybus (Kusch et al. 2009). However, genotypic variation in stem fructan levels in the recombinant inbred SB lines does not appear to be associated with sucrose levels in the stem (Xue et al. 2008b). The sucrose level in the sink organ (stem) is influenced by both influx from the leaf source organ and the rate of its utilisation. A high rate of fructan synthesis in the stem of high fructan level genotypes can exhaust the stem sucrose pool more rapidly than in low fructan level genotypes, which can lead to no association between sucrose and fructan levels in the sink organ.
In order to understand whether the source carbon availability is one of the important genetic factors in contributing to genotypic variation in the expression levels of fructosyltransferases and fructan accumulation, in this study we performed an investigation into carbohydrate metabolism in the flag leaf in relation to fructan accumulation in the stem. Analysis of the levels of individual WSC components revealed that sucrose levels in the flag leaf at the developmental stage from anthesis to prior to grain filling were positively correlated with stem WSC and fructan concentrations in the SB lines. The high leaf sucrose genotypes are positively associated with the high expression levels of a number of genes involved in the sucrose synthetic pathway and carbon fixation and genes involved in the protection of photosynthesis during environmental stress. Genes involved in starch and fructan synthesis in the leaf are upregulated in high leaf sucrose lines, likely through the sucrose signalling pathway.
Results
Genotypic variation in leaf sucrose levels and its positive association with WSC and fructan concentrations
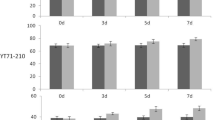
To examine the relationship between sugar levels in a source organ and fructan accumulation in a sink organ, the concentrations of individual WSC components (sucrose, glucose, fructose and fructans) in flag leaves at the fructan accumulation phase were determined in SB lines grown under field conditions. Sucrose is the major component of water soluble sugars in wheat leaf, accounting for about 75 % total water soluble sugars (data not shown). Leaf sucrose levels were highly correlated with leaf WSC levels among SB lines and correlation coefficients were 0.91 for 2005 trial, 0.96 for 2007 trial and 0.95 for 2008 trial (data not shown). There was considerable genotypic variation in flag leaf sucrose levels among SB lines (Fig. 1). Within 17 SB lines grown from the 2007 field trial under irrigated conditions, the flag leaf sucrose level in the highest line (SB189) was 73 % higher than the lowest line (SB165). Correlation analysis of leaf sucrose and stem WSC concentrations at anthesis or about a week after anthesis revealed that leaf sucrose levels were consistently correlated with stem WSC concentrations among SB lines in 3 years of field trials (Fig. 2). There was no association between stem WSC and leaf glucose or fructose levels (data not shown). Thin-layer chromatography analysis revealed that high leaf sucrose lines also showed high fructan concentration in the flag leaf (Fig. 3a). Subsequent quantitative analysis of leaf and stem fructan levels revealed significant correlations between the levels of leaf sucrose and leaf fructans or stem fructans among SB lines in two field trials analysed (Fig. 3b). These data indicate that the end product level of photo-assimilate in the leaf is one of the important factors contributing to the high stem WSC and fructan trait in wheat.
Correlation between flag leaf sucrose and stem WSC levels in SB lines grown in field. Growth conditions and sampling dates are rain-fed and at anthesis in 2005, irrigated and 6–8 days after anthesis in 2007 and rain-fed and 4–6 days after anthesis in 2008. Stem samples consisted of top two internodes with leaf sheath
Positive relationship between leaf sucrose levels and leaf and stem fructan concentrations. a Thin layer chromatogram illustrates that the high leaf sucrose lines have high leaf fructan concentrations. Samples are SB lines from the 2007 trial. Fru fructose, Suc sucrose, FM fructan markers from the WSC extract of Helianthus tuberosus. b Correlations between leaf sucrose levels and leaf fructan or stem fructan concentrations. Samples are SB lines from the 2007 or 2008 trial. **P < 0.01
Genome-wide expression profiling reveals positive correlation of genes involved in sucrose and fructan synthetic pathways with leaf sucrose level
To provide the molecular basis of high sucrose accumulation in wheat leaves and its association with fructan accumulation, Affymetrix genome array analysis was performed to determine the expression profiles of genes involved in major carbohydrate metabolism and photosynthesis using 8 SB lines with 2 field replicates selected from the 2007 field trial.
Correlation analysis between the transcript levels of individual genes and leaf sucrose concentrations was performed using both genotypic means (n = 8) and individual samples (8 genotypes × 2 field replicates, n = 16) [Supplementary Table S1 (S1.1-S1.7)]. As each carbohydrate metabolic enzyme in wheat usually consists of multiple genes (Xue et al. 2008a, b), the expression profile of some members within an enzyme gene family can differ or run into an opposite direction from other members, for example, sucrose synthase family (see Table S1.1). This makes it difficult to assess the association of a given enzyme with leaf sucrose concentration. Therefore, we used the total transcript level of an enzyme gene family to assess the relationship between leaf sucrose and a metabolic enzyme as used in our previous report (Xue et al. 2008b). At the first step, we examined whether the total transcript levels of the sucrose-phosphate synthase (SPS) family, which catalyses the critical step of sucrose synthesis, was associated with leaf sucrose levels. The expression of seven SPS genes was detected in the leaf by the Affymetrix Wheat Genome array (Table S1.1). The total hybridisation signals of TaSPS family transcripts in the flag leaf showed highly significant correlation (r = 0.78) with leaf sucrose concentrations among 16 samples used for the Affymetrix array expression analysis (Fig. 4). Furthermore, very close correlations (r values ranging from 0.74 to 0.91) between the total hybridisation signals of each fructan synthetic enzyme family (1-SST, 6-SFT or 1-FFT) and fructan concentrations in the flag leaf were observed among the 16 samples (Fig. 4).
Relationship between the expression levels of sucrose-phosphate synthase or fructosyltransferase genes and their product (sucorse or fructans) levels in the flag leaves of 16 biological samples from 8 SB lines with 2 field replicates from 2007 trial. Relative mRNA levels are the total hybridisation signal of each enzyme family in Affymetrix array data. **P < 0.01
Correlation analysis using the total transcript levels of each enzyme family showed that the expression levels of a number of enzyme gene families involved in major carbohydrate metabolic pathways were significantly correlated with leaf sucrose concentrations (Table 1). The expression data of individual members of each gene family and their correlations with leaf sucrose levels are presented in Table S1.1. The function of these enzymes in carbohydrate metabolic pathways is illustrated in Fig. 5. The total transcript levels of enzyme families that were positively correlated with leaf sucrose levels at both genotypic and individual sample levels were phosphoglycerate mutase, enzymes involved in the sucrose synthetic pathway [diphosphate-fructose-6-P 1-phosphotransferase (PFP) β-subunit, fructokinase, UDP-glucose pyrophosphorylase and SPS] and fructan synthetic enzymes (1-SST, 6-SFT and 1-FFT). In addition, the expression levels of a number of other sucrose synthetic pathway enzymes (triose-phosphate isomerise, fructose-bisphosphate aldolase and fructose bisphophatase) were also positively associated with leaf sucrose levels, but their correlations were statistically significant only at the individual sample level. The inversely correlated enzyme families include pyruvate dehydrogenase complex subunit E3, glucose-6-phosphate isomerise and apoplastic invertase [also called cell wall-bound invertase (CWInv)], as well as hexokinase (HxK). The expression correlation patterns of these carbohydrate metabolic enzymes in the leaf with leaf sucrose levels appear to be quite different from those in the stem (sink organ) with stem WSC concentrations (Table 1). However, the expression correlation patterns of the enzymes involved in fructan synthesis in the stem were in common with those in the leaf.
Illustration of major WSC metabolic pathways and leaf sucrose-correlated enzyme families. The total mRNA levels of individual enzyme families were determined by Affymetrix Genechip analysis as shown in Table 1. Red colour indicates enzyme families with the total mRNA levels in the leaf positively correlated with leaf sucrose concentrations; blue indicates enzyme families with the total mRNA levels inversely correlated with sucrose; black indicates enzyme families that showed no significant correlations. TPI triosephosphate isomerase, PFP Diphosphate-fructose-6-P 1-phosphotransferase, P phosphate. Asterisk significant correlation only at the individual sample level. † Hexokinase expression is inversely correlated with leaf sucrose levels in the Affymetrix data, but quantitative RT-PCR analysis in the extended members of SB lines showed no signification correlation
Positive association of expression of genes involved in photosynthetic process and chloroplast ATP production with leaf sucrose levels
The high-level sucrose accumulation in the mature leaf accompanied by the high-level expression of its synthetic enzyme indicates high source strength. One explanation for high sucrose accumulation in the mature leaf is a high photo-assimilation capacity. Therefore, we examined the transcript levels of genes involved in the Calvin cycle, electron transport and ATP synthesis in chloroplasts. The expression levels of three enzyme families [ribulose-1,5-bisphosphate carboxylase small subunit (rbcS), chloroplast triose-phosphate isomerase and transketolase (TK)] in the Calvin cycle in the leaf were found to be positively correlated with leaf sucrose levels (Table 2; Table S1.2; Figure S1), while only one enzyme (ribulose-phosphate 3-epimerase) in the stem was positively correlated with stem WSC concentrations.
A large number of genes are involved in light energy harvest and subsequent electron transport to produce NADPH and H+ for the generation of ATP. First, we examined the photosystem I and II subunits and the chlorophyll binding proteins of light harvesting complex (LHCA and LHCB) genes. No significant differences in the transcript levels of LHCA and LHCB genes were found among 8 SB lines in both Affymetrix expression datasets (Table S1.3). The expression levels of only two members (PsaP and PsbS) of photosystem I and II showed significantly positive correlation with leaf sucrose levels and one member (PsbR) was inversely correlated with leaf sucrose levels. For stem samples, expression levels of two members (PsaD and PsaL) of photosystems were positively correlated with stem WSC concentrations at both genotypic and individual sample levels (Table S1.3). Furthermore, there was no significant differential expression of genes involved in linear electron flow (photosynthetic electron transport) in the leaf (Table 3; Table S1.4; Fig. S2). However, significant positive correlation of ferredoxin-NADP oxidoreductase expression with stem WSC levels was found in the stem. Among these significantly correlated photosystem subunits, PsbS in photosystem II is known to be involved in non-photochemical quenching through the violaxanthin cycle (Fig. S3). The expression levels of two genes encoding violaxanthin de-epoxidase and zeaxanthin epoxidase involved in the violaxanthin cycle were also positively correlated with leaf sucrose levels, but were statistically significant only for violaxanthin de-epoxidase at the genotypic level (Table 3; Table S1.4).
In contrast, significant correlations of leaf sucrose or stem WSC concentrations with the expression levels of genes involved in cyclic electron flow were observed. The transcript levels of PGRL1 involved in the major cyclic electron flow pathway were positively correlated with both leaf sucrose and stem WSC concentrations (Table 3; Table S1.4; Fig. S2). The other pathway is via the chloroplast NADPH dehydrogenase-like complex and there were significant correlations of three subunits with leaf sucrose levels. NdhF showed the positive correlation, while NdhA and NdhE showed inverse correlation (Table 3; Table S1.4). This makes it difficult to assess the net outcome of the change. However, the expression levels of two nuclear-encoded subunits of the chloroplast NADPH dehydrogenase-like complex in the stem were positively correlated with stem WSC concentrations.
The transcript levels of two subunits (atpA and atpI) of chloroplast ATP synthase complex were found to be positively correlated with leaf sucrose levels with statistical significance or very close to it (Table 3; Table S1.5). The transcript levels of chloroplast ATP synthase subunit genes in the stem were in general positively associated with stem WSC concentrations, though not statistically significant at both genotypic and individual sample levels (Table 3).
Positive association of the transcript levels of chloroplast H2O2 scavenging genes with leaf sucrose levels
Photochemical transfer of electrons generated through split of water in the photosystem II during photosynthesis ultimately leads to production of reactive oxygen species (ROS) such as singlet oxygen and H2O2, which could lead to oxidative damage to photosynthetic apparatus and enzymes if they are not effectively removed. Therefore, the ROS scavenger systems in chloroplasts are a closely integrated part of photosynthesis. Two of the predominant ROS removal systems in the chloroplast of higher plants are the ascorbate peroxidase (APX) and peroxiredoxins (Prx) dependent pathways (Foyer and Shigeoka 2011). The total transcript levels of chloroplast APX (thylakoid-bound and stromal APX) were significantly correlated with leaf sucrose levels. The transcript levels of enzymes involved in regeneration of ascorbic acid (AsA) in chloroplasts showed no significant correlation (Table 4; Table S1.6; Fig. S4). Significant positive correlation of leaf sucrose levels with the expression levels of γ-glutamylcysteine synthetase that catalyses the first step of the glutathione synthetic pathway was also found, but not with glutathione synthetase gene. The total transcript levels of the chloroplast Prx gene family, involved in the other important H2O2 removal pathway in chloroplasts, also showed significantly positive correlation with leaf sucrose levels (Table 4; Table S1.6). The expression levels of NADPH-thioredoxin reductase (NTR) involved in regeneration of oxidised thioredoxin (Trx) were also positively correlated with leaf sucrose levels at both genotypic and individual sample levels. The total transcript levels of the chloroplast thioredoxin gene family were positively associated with leaf sucrose levels with statistical significance at individual sample level. No significant association of chloroplast FTR gene expression with leaf sucrose levels was observed. The expression levels of many of these antioxidant genes in the stem also showed positive association with stem WSC concentrations, but statistical significance at both genotypic and individual sample levels was seen only for the chloroplast thioredoxin gene family (Table 4; Table S1.6).
Expression levels of genes involved in the starch synthetic pathway are up-regulated in high leaf sucrose lines
Starch is considered to be an integrator of carbohydrate metabolism in many plant species and is strongly regulated by carbon availability in the leaf (Sulpice et al. 2009). High leaf sucrose levels together with high expression levels of SPS and some enzymes in the Calvin cycle may indicate enhanced carbon assimilation in high sucrose and WSC lines, which may be reflected by upregulation of starch synthetic enzymes in chloroplasts. The expression levels of almost all genes involved in the chloroplast starch synthetic pathway [phosphoglucomutase, glucose-1-phosphate adenylyltransferase small subunit, soluble starch synthase, granule-bound starch synthase (GBSS) and starch branching enzyme] were positively correlated with leaf sucrose levels (Table 5; Fig. 6; Table S1.7). Interestingly, the expression levels of α-glucan phosphorylase were also positively correlated with leaf sucrose levels (Table 5). Alpha-glucan phosphorylase can extend glucan chains using glucose-1-phosphate as a substrate in a reversible reaction and is present in the protein complexes containing starch synthases and branching enzymes in cereals (Kötting et al. 2010). Alpha-glucan phosphorylase was also a highly expressed gene in wheat leaves, with a comparable transcript level to glucose-1-phosphate adenylyltransferase and soluble starch synthase genes (Table S1.7). There was no significant correlation of leaf sucrose levels with the expression levels of starch debranching enzyme genes, isoamylase and pullulanase. The expression levels of enzymes directly involved in starch synthesis in the stem were also positively correlated with stem WSC concentrations (Table 5).
Illustration of enzymes involved in starch synthesis in the leaf chloroplast and leaf sucrose-correlated enzyme families. The total mRNA levels of individual enzyme families were determined by Affymetrix Genechip analysis as shown in Table 5. Red colour indicates enzyme families with the total mRNA levels in the leaf positively correlated with leaf sucrose concentrations
Validation of key differentially expressed genes among two sets of SB lines from two field trials using quantitative RT-PCR and positive association of leaf sucrose and fructan levels with the expression levels of their synthetic enzymes
The Affymetrix expression data provide candidate genes that are potentially linked with genotypic variation in leaf sucrose and stem fructan accumulation. We selected 14 representative differentially expressed genes from various pathways involved in carbon assimilation and photosynthesis for expression analysis among two sets of SB lines from two field trials using quantitative RT-PCR (Table 6). Significant positive correlation between TaSPSIa mRNA levels, a predominantly expressed SPS member in the leaf (Xue et al. 2008a), and leaf sucrose levels were observed in both 2007 (16 SB lines analysed) and 2008 (12 SB lines analysed) field trials (Fig. S5). Among 14 genes selected for analysis, 11 genes showed significant correlations of their mRNA levels with leaf sucrose concentrations; one gene (TaGBSS1) showed significant positive correlation only in 2007 trial samples with a correlation coefficient (r = 0.52) in 2008 trial samples that is close to statistical significance (Table 6). However, two genes (TaHxK1 and TaPGRL1) showed no significant correlation, but TaPGRL1 mRNA levels still showed positive association with leaf sucrose levels in both trials. The expression levels of fructokinase gene (TaFK1) were positively correlated with leaf sucrose levels in both trials. The cell-wall-bound invertase (TaCWInv1) was inversely correlated in expression with leaf sucrose levels in both trials. The expression levels of three fructosyltransferase genes (Ta1-SST1, Ta6-SFT1 and Ta1-FFT1) showed highly significant positive corrections not only with leaf sucrose levels, but also with the product of their catalytic reactions, fructans, in the leaf among SB lines in both field trials (Fig. 7). Although genotypic difference in leaf sucrose levels among SB lines was relatively small, marked differences in the expression levels of fructosyltransferase genes were observed in the leaves of these SB lines (Fig. S6).
Relationship between the expression levels of fructosyltransferase genes and fructan levels in the flag leaves. The mRNA levels were determined using quantitative RT-PCR. Mean values of two field replicates of each genotype for fructan and mRNA levels were used for correlation analysis. 16 SB lines were analysed in 2007 trial and 12 SB lines in 2008 trial. *P < 0.05; **P < 0.01
The expression levels of two Calvin cycle enzyme genes (TarbcS1 and TaTK1), TaPsbS1 and chloroplast H2O2 removal genes [thylakoid-bound APX (TatAPX1) and chloroplast Prx (TacPrx1)] showed significant correlations with leaf sucrose levels in both trials. Interestingly, co-expression analysis between the transcript levels of chloroplast TatAPX1 or TacPrx1 gene with TarbcS1, showed a highly significant positive correlation (Fig. S7), indicating that these genes are co-regulated.
High leaf sucrose levels linked with drought tolerance
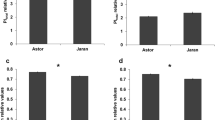
To examine whether SB lines with high leaf sucrose levels are associated with drought tolerance, we performed a comparative analysis of relative anthesis biomass production and grain yield of 17 SB lines grown under rain-fed and irrigated conditions in the 2005 field trial, which were planted in the same date with replicated plots. Plants under the rain-fed conditions in 2005 experienced pre-anthesis water deficit stress, which resulted in an average 30 % reduction (the value is the mean of 17 genotypes) in anthesis biomass and an average 47 % reduction in grain yield among these SB lines in comparison with plants under irrigated conditions. Therefore, ratios of anthesis biomass or grain yield of plants under rain-fed (water deficit stress) to irrigated conditions among genotypes represent relative agronomic performance of genotypes under drought stress conditions, hence providing a good assessment of genotypic ranking in drought tolerance under the field conditions. As shown in Fig. 8, in general genotypes with higher leaf sucrose levels showed less reduction in pre-anthesis biomass and grain yield under drought stress than those with lower leaf sucrose levels. Leaf sucrose levels of plants under rain-fed conditions were positively correlated with ratios of anthesis biomass (r = 0.48, P = 0.05) or grain yield (r = 0.56, P = 0.02) of plants under rain-fed to irrigated conditions among the 17 SB lines. These data indicate that high leaf sucrose levels are linked with drought tolerance in these SB lines.
Relationship between flag leaf sucrose levels and reduction in anthesis biomass or grain yield under water-deficit rain-fed conditions in comparison with irrigated conditions. Data obtained from the field trial in 2005. The leaf sucrose levels were determined from rain-fed samples. The water deficit-caused reduction of anthesis biomass or grain yield was expressed as ratios of rain-fed to irrigated conditions. Mean values of two field replicates of each genotype were used for correlation analysis. Replicated plots of each genotype at each treatment were planted at the same date (in early July, which is a late sowing season in Northern Australia)
Discussion
Contribution of source strength to genotypic variation in fructan accumulation
Fructans in the stem of temperate cereals at anthesis and the early grain-filling stage represent a major carbon sink. Genotypic variation in fructan accumulation is likely to be attributed to differences in either sink or source strength or both. This study showed that sucrose levels in the source leaf organ were positively correlated with WSC and fructan concentrations in the sink stem organ among SB lines in 2–3 years of field trials. Sucrose derived from photosynthetic CO2 fixation in the mature leaf of temperate cereals is the major form of carbon for exporting to sink organs via the phloem and is used for fructan synthesis in the stem.
The Affymetrix array expression analysis revealed that high leaf sucrose levels resulted in the markedly elevated expression levels of fructosyltransferase genes (1-SST, 6-SFT and 1-FFT) involved in fructan synthesis in the mature flag leaf of SB lines. The elevated expression levels of these fructosyltransferase genes in the high leaf sucrose lines indicates an enhanced rate of fructan synthesis, which is supported by the fact that the fructan concentrations in the flag leaf were positively correlated with the transcript levels of the fructosyltransferase genes. These data clearly demonstrate that a relatively small difference in the endogenous sucrose level among wheat genotypes can have a marked influence on fructosyltransferase gene expression and fructan synthesis in the leaf. Upregulation of fructosyltransferase genes by sucrose has been shown in excised barley or wheat leaves with exogenous sucrose treatment (Müller et al. 2000; Martínez-Noël et al. 2001, 2006; Koroleva et al. 2001; Nagaraj et al. 2001, 2004; Lu et al. 2002; Ruuska et al. 2008; Xue et al. 2011). However, marked upregulation of fructosyltransferase genes due to genotypic variation in endogenous sucrose levels in the leaf has not been reported previously. Therefore, this study provides experimental evidence on how leaf sucrose level control fructan accumulation under natural conditions.
A close positive association between the source leaf sucrose levels and sink stem WSC or fructan concentrations among these recombinant inbred lines suggests that the source carbon availability is one of the important factors contributing to genotypic variation in WSC and fructan accumulation in wheat. In the previous study we observed that high stem fructan concentrations are associated with high mRNA levels of fructosyltransferase genes (Xue et al. 2008b). It appears that the high leaf sucrose signal may also exert a positive influence on fructosyltransferase expression in the stem, as stem sucrose levels are not significantly correlated with fructan concentrations in the stem of SB lines (Xue et al. 2008b). The likely explanation for this is that the increased sucrose availability in the source leaf, which presumably enhances sucrose transport to the stem, is balanced by a high rate of sucrose utilisation for fructan synthesis in high leaf sucrose and stem fructan lines. In particular, fructan synthesis in wheat stems at anthesis and in the early grain filling period represents major sucrose consumption. In contrast, fructan synthesis activity in the leaf blade is relatively low.
It appears that high source strength in the high leaf sucrose and stem WSC lines is supported by the differential expression data of starch synthetic genes in the leaf among these recombinant inbred lines. There was a coordinated upregulation of genes involved in the starch synthetic pathway in the leaves of high leaf sucrose lines as well as the stems of high stem WSC lines. Starch synthesis is stimulated by an increase in carbon availability (Geigenberger et al. 2004). In the leaf, carbon for starch synthesis is derived from the Calvin cycle intermediates and photosynthesis activity is one of the factors determining starch accumulation in the leaf (Geigenberger 2011). High source carbon availability is likely to be a common factor for enhanced expression of both starch and fructan synthetic genes in these genotypes.
High leaf sucrose level is linked with enhanced expression of genes involved in carbon flow towards sucrose synthesis and carbon fixation
Analysis of the expression patterns of genes involved in glycolysis and gluconeogenesis in the source leaf of SB lines revealed that genes that showed positive correlation with leaf sucrose levels are generally involved in carbon flow towards sucrose synthesis, such as UDP-glucose pyrophosphorylase, fructokinase and SPS. In particular SPS is known as one of the major controlling factors in the sucrose synthetic pathway (Börnke and Sonnewald 2011). Interestingly, the transcript levels of apoplastic invertase (or cell wall-bound invertase) were inversely correlated with leaf sucrose levels. Apoplastic invertase catalyses the cleavage of sucrose in the apoplast into glucose and fructose. In wheat and rice, sucrose is the only sugar transported in the phloem (Hayashi and Chino 1986, 1990). It is likely that hexoses in the leaf apoplast return into mesophyll cells in the leaf (Chikov and Bakirova 2004; Kocal et al. 2008). Thus, apoplastic invertase can limit sucrose export. A decrease in apoplastic invertase expression in the source leaf organ of high leaf sucrose lines may reduce sucrose hydrolysis in the apoplast and thus favours sucrose export to sink organs, which would favour fructan accumulation in the stem. Interestingly, a concurrent decrease in apoplastic invertase gene expression was not observed in the sink stem organ among these SB lines, where a reduction in this enzyme expression would lead to a decrease in sucrose unloading in the stem. A decrease in leaf apoplastic invertase expression that could be potentially linked to enhanced sucrose export was accompanied by an increase in the sucrose concentration in the source leaf of these SB lines. These two observations indicate that the sucrose synthesis rate is likely to be increased in the high leaf sucrose lines.
A comparative analysis of the expression levels of genes involved around the metabolic pathways of gluconeogenesis and glycolysis and their association with sucrose and WSC accumulation showed marked differences between the source leaf and sink stem organs. In contrast to the leaf, the high WSC lines have the low expression levels of genes in the stem that divert carbon towards cell wall polysaccharide synthesis and carbon entering TCA cycle (Xue et al. 2008b). It is still unknown which is the driving force for this differential carbon partitioning favouring fructan accumulation in the stem of high WSC lines. The elevated expression levels of fructosyltransferases in high WSC lines, presumably influenced by high leaf sucrose, can potentially pull the carbon flow towards fructan synthesis. However, the argument can go to both ways.
High source leaf sucrose association with high sink stem demand for fructan synthesis indicates a potential high carbon assimilation rate in the leaves of high WSC lines. This assumption seems to be supported by the enhanced expression levels of a number of genes involved in the Calvin cycle: rbcS, triose phosphate isomerase and transketolase (TK). In particular, the significant positive corrections of the expression levels of TarbcS1 and TaTK1 with leaf sucrose levels were observed in two sets of SB lines derived from 2 years of field trials. RbcS appears to be one of the Calvin cycle genes are regulated by various factors that affect photosynthesis, such as glucose availability and drought stress (Pego et al. 2000; Xue et al. 2008a). Triose phosphate isomerase does not appear to be a rate-limiting enzyme in the Calvin cycle, as there is no report to date on variation in its activity influencing photosynthesis rate from the recent review by Raines (2011). Among these Calvin cycle enzymes, TK is probably one of the most influential enzymes in the rate of photosynthesis (Raines 2003). Chloroplast TK catalyses two reactions in the Calvin cycle (Fig. S1). A reduced level of TK activity leads to a reduction in photosynthesis, decreased sucrose level and starch accumulation (Henkes et al. 2001). A 20–40 % reduction of TK activity in the antisense tobacco plants inhibits ribulose-1,5-bisphosphate regeneration and photosynthesis (Henkes et al. 2001). In particular, in antisense plants sucrose level declines linearly with reduction in TK activity and even in plants with 75 % of wild-type TK activity a 25 % reduction in the sucrose level was observed (Henkes et al. 2001).
No significant genotypic variation was found in the expression levels of genes in the leaf involved in electron transfer pathways that lead to the production of NADPH and H+. However, Affymetrix data showed that the expression levels of two genes (atpA and atpI) involved in ATP production in chloroplasts were significantly, or very close to significantly, correlated with leaf sucrose levels. The proteins encoded by these two genes are components of the chloroplast ATP synthase complex. An increase in ATP synthesis would support the increasing ATP demand for higher carbon fixation through the Calvin cycle. However, the impact of an increase in the mRNA levels of atpA and atpI genes on the activity of the ATP synthase complex is unknown.
Potential role of photoprotection genes in contribution to genotypic variation in source strength in abiotic stress environments
A positive association between source leaf sucrose levels and the expression levels of genes involved in energy dissipation was observed among the SB lines, particularly PsbS that was verified by 2 years of field trials. The energy dissipation pathway provides a photoprotection mechanism under the conditions where light energy harvested is in excess, such as high light intensity in the mid-day or abiotic stresses. Excess light energy leads to production of the high levels of reactive oxygen species, which cause damages of proteins involved in photosynthesis and inhibition of photosystem repair (Takahashi and Badger 2010; Murchie and Niyogi 2011). PsbS is known to act as a pH sensor of non-photochemical quenching through the violaxanthin cycle (Li et al. 2004; Murchie and Niyogi 2011). Excess light energy in the form of over-excited chlorophyll pool can be dissipated as heat through the violaxanthin cycle, which prevents over-production of singlet oxygen from triplet chlorophyll (Müller et al. 2001). PsbS is a limiting factor in the capacity of qE, one component of the non-photochemical quenching. Li et al. (2002a, b) have shown that the more PsbS protein present, the higher the capacity for qE in Arabidopsis. In addition, the expression levels of two enzymes (violaxanthin de-epoxidase and zeaxanthin epoxidase) involved in the xanthophyll cycle also appear to be positively associated with source leaf sucrose levels and their correlation coefficients were at the levels very close to be statistically significant.
Along with the above notion, positive correlation of the expression levels of chloroplast antioxidant genes (ascorbate peroxidase and peroxiredoxin) with leaf sucrose levels provides another line of molecular evidence on that photoprotection capacity for preventing oxidative damage of photosynthesis machinery or repair system is likely to be one of the factors associated with the high leaf sucrose level. Thylakoid-bound ascorbate peroxidase (tAPX) is considered as one of the limiting factors of antioxidative system under photooxidative stress conditions (Yabuta et al. 2002). APX reduces H2O2 to water using ascorbic acid (AsA) as an electron donor. H2O2 is one of the potent inhibitors of photosynthesis and it can oxidise the thiol-modulated enzymes of the Calvin cycle. Even at a low concentration (10 µM) it can inhibit CO2 fixation by 50 % (Foyer and Shigeoka 2011). Transgenic plants over-expressing tAPX have been shown to have enhanced photoprotection (Yabuta et al. 2002; Murgia et al. 2004; Pang et al. 2011). The transcript levels of the enzyme involved in the first step of the glutathione synthetic pathway showed a significant positive correlation with leaf sucrose levels and glutathione is involved in AsA regeneration.
H2O2 generated by chloroplast superoxide dismutase can also be converted to water by chloroplast peroxiredoxin (Prx) or glutathione peroxidase. Chloroplast Prx transcript levels were found to be positively correlated with leaf sucrose levels. Prx is another important H2O2 scavenger in chloroplasts (Foyer and Shigeoka 2011), which functions together with thioredoxin and thioredoxin-like proteins in the chloroplast. Thioredoxin functions as disulfide reductase. Beside its role in reducing oxidised Prx, it is also involved in activating chloroplast enzyme activities or relieving them from inhibition, including the Calvin cycle enzymes, such as chloroplast glyceraldehyde-3-phosphate dehydrogenase and fructose bisphosphatase, and chloroplast ATP synthase γ-subunit (Ruelland and Miginiac-Maslow 1999). The expression levels of many chloroplast thioredoxin genes were significantly correlated with leaf sucrose levels. The positive correlation of the total transcript levels of the chloroplast thioredoxin gene family with leaf sucrose levels was statistically significant at the individual sample level and very close to statistically significant at genotypic level. Significant correlation of the total transcript levels of chloroplast thioredoxin with stem WSC concentrations was also observed in the stem.
The enhanced expression of photoprotection genes indicates the potential increased capacity of abiotic stress tolerance in the high leaf sucrose lines. Indeed, relative drought tolerance of these SB lines, measured as relative anthesis biomass and grain yield in the field, was positively associated with high leaf sucrose levels.
In summary, these gene expression data provide valuable molecular insights on genotypic variation in source leaf sucrose levels, which is likely to be one of the major factors underlying the WSC and fructan trait in wheat. Leaf sucrose levels appear to be an important factor that dictates fructan accumulation in both the leaf and stem. Positive expression association of some important genes involved in the sucrose synthetic pathway and photosynthesis with leaf sucrose levels further argues for the role of high source strength in high fructan accumulation in wheat. Consistent positive correlations of leaf sucrose and fructan concentrations with the expression levels of their synthetic enzyme genes (SPS and fructosyltransferases) suggests that regulation in stable mRNA levels (likely attributed to transcriptional regulation) plays an important role in contributing to genotypic variation in leaf sucrose and fructan levels among these recombinant inbred lines. Photosynthetic CO2 fixation rate can be limited either by the capacity of RBC to consume ribulose 1,5-bisphophate or by the capacity of the chloroplast electron transfer to generate ATP and NADPH or the activity of Calvin enzymes for ribulose 1,5-bisphophate regeneration (Farquhar et al. 1980; Yamori et al. 2011). In abiotic stress-prone environments (e.g. drought and heat stresses), the chloroplast antioxidant system is likely to have an important contribution in eliminating excessive reactive oxygen species and provide better maintenance of photosynthesis activity. Enhancing the expression levels of genes involved in these physiological processes, as observed in high leaf sucrose lines, would expect to have a potential positive impact on carbon fixation, which could lead to high leaf sucrose and stem fructan accumulation.
Materials and methods
Plant materials and field growth conditions
Recombinant inbred lines were derived from a cross between Triticum aestivum cultivars Seri M82 and Babax (SB) (Olivares-Villegas et al. 2007). SB lines were grown with two field replicates of each line under rain-fed or irrigated conditions in 2005, irrigated conditions in 2007 and rain-fed conditions in 2008 at the CSIRO Cooper Laboratory at Gatton, Queensland (latitude 27°34′S, longitude 152°17′E). The site was on a deep fertile prairie loam soil developed on alluvium with a plant available water holding capacity of about 250 mm to a depth of 1.5 m. Plot size in each field trial was 6 m × 1.76 m (8 rows) with an inter-row spacing of 22 cm. Plots were sprayed with herbicides to control weeds and fungicide to prevent foliar diseases. SB lines selected for this study were based on similarity in anthesis date, but differing in leaf sucrose or stem WSC concentrations.
The flag leaves and the top two internodes (peduncle and penultimate internode with leaf sheath attached) were sampled between 1:00 and 2:00 pm at anthesis in 2005, 6–8 days after anthesis in 2007 and 4–6 days after anthesis in 2008. All plants were well hydrated during sampling, as samples were taken about 2 days after heavy rain in 2005 and 2008 rain-fed trials (otherwise, prior irrigation would be required to relieve drought stress). Each sample contained 5–8 flag leaves or stems from main tillers that were randomly sampled from each plot, immediately dropped into liquid nitrogen, stored at −80 °C and used for RNA isolation and WSC analyses.
Measurements of water soluble carbohydrate levels
WSCs were extracted as described previously (Xue et al. 2008b). WSC concentrations in the extracts were measured using the modified anthrone procedure (Xue et al. 2009). Sucrose, glucose and fructose concentrations in WSC extracts were determined as described by Xue et al. (2008a). Fructan concentrations in WSC extracts were determined according to the method of Liu et al. (2011) using the procedure of fructan precipitation by 12 volume of acetone. Liu et al. (2011) achieved >95 % of fructan recovery and <5 % of sucrose coprecipitation using 12 volume of acetone for fructan precipitation. The fructan precipitation procedure was performed twice for each sample to reduce the amount of residual sucrose in the precipitate.
Total RNA extraction
Total RNA was isolated from samples using Plant RNA Reagent (Invitrogen, California, USA), according to the manufacturer’s instruction. RNA was further purified through a Qiagen RNeasy column (Qiagen, Australia) after pre-treatment with RNase-free DNase I (Xue and Loveridge 2004).
Expression analysis using Affymetrix GeneChip wheat genome array
In the Affymetrix GeneChip expression experiment, a set of 8 SB lines covering a range of flag leaf sucrose levels was selected (see Fig. 1). The wheat genome array (Affymetrix GeneChip) contains 61,127 probe sets representing 55,052 transcripts for all 42 chromosomes in the wheat genome. RNA quality check, cRNA preparation, labelling, hybridization, and data acquisition of Affymetrix wheat GeneChips were performed by the microarray service at the Australian Genome Research Facility (Melbourne, Australia). A total of 16 Affymetrix genechips for 16 RNA samples from the 8 SB lines with two field replicates of each line from the 2007 field trial were used in this study and the array data were deposited in the GEO website (GSE37675). The raw GeneChip data were normalized using GeneChip robust multiarray average (GC-RMA) developed by Wu et al. (2004) and the default settings for the Affymetrix package within Bioconductor, running within the R statistical programming environment (http://www.r-project.org/). The normalized expression data for genes (probe sets) from enzyme families related to major carbohydrate metabolism and photosynthesis (see Table S1) were retrieved and probe sets with normalized hybridization signals of <20 were discarded. The sequences of the retrieved probe sets were searched for corresponding tentative consensus (TC) sequences in Triticum aestivum Gene Index (TaGI) database version 12.0 (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=wheat). When multiple probe sets fall into the same TC sequence, the mean expression value was used for analysis if the difference in their values was less than twofold, otherwise, probe set with the highest value will be chosen for analysis. The sequences of these retrieved genes went through further bioinformatic analyses for checking correctness of their annotations against NCBI BLAST databases (http://www.ncbi.nlm.nih.gov/BLAST/) and prediction of potential sub-cellular location as described at the latter section. The re-annotated carbohydrate metabolic genes were then grouped into enzyme or protein families. Pearson correlation analysis was used for genes for their potential association with leaf sucrose accumulation. Correlations between mRNA levels and leaf sucrose concentrations in 8 SB lines were analysed at both genotypic (using the mean value of two field replicates of each line, n = 8) and genotype/field-replicates levels (8 genotypes × 2 field replicates, n = 16). Differences in mRNA and sucrose levels between two field replicates of each line represent both biological and environmental variation. Genes with correlation coefficients that were statistically significant (P < 0.05) at both levels were considered to be potentially associated with leaf sucrose accumulation.
Affymetrix wheat genome array GSE9767 data for genotypic variation in gene expression among 16 stem samples (top two internodes with leaf sheath) at anthesis from 8 recombinant inbred SB lines with two field replicates from the 2005 field trial were reported previously (Xue et al. 2008b). A different set of genotypes was used for the stem experiment except one genotype (SB165) that was used for both stem and flag leaf experiments. The array data were re-normalised using GC-RMA. Pearson correlation analysis was used for identification of candidate genes that were associated with stem WSC accumulation as described above.
Bioinformatic prediction of subcellular locations of wheat proteins
Prediction of the subcellular locations of wheat proteins was described previously using a combination of prediction tools (Xue et al. 2008a). Chloroplast location was predicted by ChloroP 1.1 and TargetP1.1, based on the presence of a chloroplast transit peptide. Mitochondrial location was predicted by TargetP 1.1 and MITOPROT, based on the presence of a mitochondrial targeting peptide. Extracellular location was predicted by SignalP4.0 (based on the presence of a secretory pathway signal peptide, but without a signal anchor sequence). Nuclear location was predicted using PredictNLS, based on the presence of nuclear localisation signal. Vacuolar location was predicted based on the presence of signal anchor sequences near the N-terminus with or without a signal peptide (SignalP4.0) and high homology with known vacuolar proteins in the SWISS-PROT database using WoLF PSORT. Many plant vacuolar invertases are known to have no N-terminal signal peptide, but contain a single hydrophobic sub-terminal transmembrane segment near the N-terminus (Ji et al. 2005). Fructan exohydrolases encoded by TaFEH genes listed in Table S1 all contain a hydrophobic N-terminal signal peptide with no signal anchor sequences, as predicted by SignalP. Although the presence of FEH enzymes and fructans in the apoplast has been demonstrated (Livingston and Henson 1998; Van den Ende et al. 2005), the vacuole is probably a primary subcellular location for most of FEH enzymes (Van den Ende et al. 2003; Lothier et al. 2007). Many proteins have experimentally been shown to have more than one subcellular locations, such as cytoplasmic and nuclear locations of a yeast hexokinase (Randez-gil et al. 1998). To date, no known vacuolar targeting signals have been identified in fructan 6-exohydrolases & 1-exohydrolases. Plasma membrane location and presence of transmembrane helices are predicted using WoLF PSORT and TMHMM. When a wheat TC sequence or EST was not full-length, the sequence of a highly homologous gene (>90 % amino acid similarities in the available sequence region) from other plant species, particularly full-length barley proteins, was used for prediction.
Expression analysis using quantitative RT-PCR
The transcript levels of wheat genes were quantified from cDNA samples synthesised from DNase I-treated total RNA using real-time PCR with an ABI Prism 7900 sequence detection system (Applied Biosystems) and SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer’s instructions. The sequences of primer pairs used for real-time PCR are listed in Table S2. The gene specificity of primers for each gene during primer designing was checked by blasting primer sequences in the TaGI database (using an expect value setting at 10,000), matching only with the sequence of the targeted gene. Primer pairs for carbohydrate metabolic enzymes and fructosyltransferase genes and some internal control genes were reported previously (Xue et al. 2006a, b, 2008a; Stephenson et al. 2007).
Wheat RNA polymerase (TaRP15, Xue et al. 2008a), ubiquitin 10 (TaUbi10) and elongation factor 1-α (TaEF1-α) were selected as internal reference genes for calculation of relative transcript levels of the genes under study. The PCR efficiency of each primer pair was determined by a dilution series of samples. The determination of the specificity of real-time PCR amplification and relative quantitation of mRNA levels were as described by Shaw et al. (2009).
Thin-layer chromatography of WSCs
To visualise the fructan fraction of WSCs, WSC extracts were fractionated by thin layer chromatography using a 0.2-mm thick silica gel plate (Merck, TLC Silica gel 60 F254) and a solvent system of 1-propanol:ethyl-ethanoate:water (5:3:2 by volume), as described by Incoll et al. (1989). The positions of sugars and fructans with various degrees of polymerisation were visualised by spraying with urea-phosphoric acid and heating the plate at 110 °C, as described by Wise et al. (1955). The WSC extract from Helianthus tuberosus was used as fructan markers.
References
Aggarwal PK, Sinha SK (1984) Effect of water stress on grain growth and assimilate partitioning in two cultivars of wheat contrasting in their yield stability in a drought-environment. Ann Bot 53:329–340
Altenbach D, Rudino-Pinera E, Olvera C, Boller T, Wiemken A, Ritsema T (2009) An acceptor-substrate binding site determining glycosyl transfer emerges from mutant analysis of a plant vacuolar invertase and a fructosyltransferase. Plant Mol Biol 69:47–56
Asseng S, Van Herwaarden AF (2003) Analysis of the benefits to wheat yield from assimilates stored prior to grain filling in a range of environments. Plant Soil 256:217–229
Barnabás B, Jäger K, Fehér A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38
Blum A (1998) Improving wheat grain filling under stress by stem reserve mobilization. Euphytica 100:77–83
Börnke F, Sonnewald S (2011) Biosynthesis and metabolism of starch and sugars. In: Ashihara H, Crozier A, Komamine A (eds) Plant metabolism and biotechnology. Wiley, London, pp 1–25
Brooks A, Jenner CF, Aspinall D (1982) Effect of water deficit on endosperm starch granules and grain physiology of wheat and barley. Aust J Plant Physiol 9:423–436
Chalmers J, Johnson X, Lidgett A, Spangenberg G (2003) Isolation and characterisation of a sucrose: sucrose 1-fructosyltransferase gene from perennial ryegrass (Lolium perenne). J Plant Physiol 160:1385–1391
Chalmers J, Lidgett A, Cummings N, Cao Y, Forster J, Spangenberg G (2005) Molecular genetics of fructan metabolism in perennial ryegrass. Plant Biotechnol J 3:459–474
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML, Carvalho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field? Photosynthesis and growth. Ann Bot 89:907–916
Chikov VI, Bakirova GG (2004) Role of the apoplast in the control of assimilate transport, photosynthesis, and plant productivity. Russian J Plant Physiol 51:420–431
Ehdaie B, Alloush GA, Madore MA, Waines JG (2006) Genotypic variation for stem reserves and mobilization in wheat: II. postanthesis changes in internode water-soluble carbohydrates. Crop Sci 46:2093–2103
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Foulkes MJ, Scott RK, Sylvester-Bradley R (2002) The ability of wheat cultivars to withstand drought in UK conditions: formation of grain yield. J Agric Sci 138:153–169
Foulkes MJ, Snape JW, Shearman VJ, Reynolds MP, Gaju O, Sylvester-Bradley R (2007) Genetic progress in yield potential in wheat: recent advances and future prospects. J Agric Sci 145:17–29
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Gebbing T (2003) The enclosed and exposed part of the peduncle of wheat (Triticum aestivum)—spatial separation of fructan storage. New Phytol 159:245–252
Geigenberger P (2011) Regulation of starch biosynthesis in response to a fluctuating environment. Plant Physiol 155:1566–1577
Geigenberger P, Stitt M, Fernie AR (2004) Metabolic control analysis and regulation of the conversion of sucrose to starch in growing potato tubers. Plant Cell Environ 27:655–673
Hayashi H, Chino M (1986) Collection of pure phloem sap from wheat and its chemical composition. Plant Cell Physiol 27:1387–1393
Hayashi H, Chino M (1990) Chemical composition of phloem sap from uppermost internode of the rice plant. Plant Cell Physiol 31:247–251
Henkes S, Sonnewald U, Badur R, Flachmann R, Stitt M (2001) A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. Plant Cell 13:535–551
Herbers K, Sonnewald U (1998) Molecular determinants of sink strength. Curr Opin Plant Biol 1:207–216
Incoll L, Bonnett G, Gott B (1989) Fructans in the underground storage organs of some australian plants used for food by Aborigines. J Plant Physiol 134:196–202
Ji X, Van den Ende W, van Laere A, Cheng S, Bennett J (2005) Structure, evolution, and expression of the two invertase gene families of rice. J Mol Evol 60:615–634
Joudi M, Ahmadi A, Mohamadi V, Abbasi A, Vergauwen R, Mohammadi H, Van den Ende W (2012) Comparison of fructan dynamics in two wheat cultivars with different capacities of accumulation and remobilization under drought stress. Physiol Plant 144:1–12
Kawakami A, Yoshida M (2002) Molecular characterization of sucrose: sucrose 1-fructosyltransferase and sucrose: fructan 6-fructosyltransferase associated with fructan accumulation in winter wheat during cold hardening. Biosci Biotechnol Biochem 66:2297–2305
Kawakami A, Yoshida M (2005) Fructan:fructan 1-fructosyltransferase, a key enzyme for biosynthesis of graminan oligomers in hardened wheat. Planta 223:90–104
Kocal N, Sonnewald U, Sonnewald S (2008) Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv vesicatoria. Plant Physiol 148:1523–1536
Koroleva OA, Tomos AD, Farrar JF, Gallagher J, Pollock CJ (2001) Carbon allocation and sugar status in individual cells of barley leaves affects expression of Sucrose: fructan 6-Fructosyltransferase gene. Ann Appl Biol 138:27–32
Kötting O, Kossmann J, Zeeman SC, Lloyd JR (2010) Regulation of starch metabolism: the age of enlightenment? Curr Opin Plant Biol 13:320–328
Kusch U, Greiner S, Steininger H, Meyer AD, Corbière-Divialle H, Harms K, Rausch T (2009) Dissecting the regulation of fructan metabolism in chicory (Cichorium intybus) hairy roots. New Phytol 184:127–140
Lammens W, Le Roy K, Yuan S, Vergauwen R, Rabijns A, Van Laere A, Strelkov SV, Van den Ende W (2012) Crystal structure of 6-SST/6-SFT from Pachysandra terminalis, a plant fructan biosynthesizing enzyme in complex with its acceptor substrate 6-kestose. Plant J 70:205–219
Lasseur B, Lothier J, Wiemken A, Van Laere A, Morvan-Bertrand A, Van den Ende W, Prud’homme M-P (2011) Towards a better understanding of the generation of fructan structure diversity in plants: molecular and functional characterization of a sucrose:fructan 6-fructosyltransferase (6-SFT) cDNA from perennial ryegrass (Lolium perenne). J Exp Bot 62:1871–1885
Li X-P, Gilmore AM, Niyogi KK (2002a) Molecular and global time-resolved analysis of a psbS gene dosage effect on pH- and xanthophyll cycle-dependent non-photochemical quenching in photosystem II. J Biol Chem 277:33590–33597
Li X-P, Müller-Moulé P, Gilmore AM, Niyogi KK (2002b) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA 99:15222–15227
Li X-P, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279:22866–22874
Liu Z, Mouradov A, Smith KF, Spangenberg G (2011) An improved method for quantitative analysis of total fructans in plant tissues. Anal Biochem 418:253–259
Livingston DP, Henson CA (1998) Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening. Plant Physiol 116:403–408
Lothier J, Lasseur B, Le Roy K, Van Laere A, Prud’homme MP, Barre P, Van den Ende W, Morvan-Bertrand A (2007) Cloning, gene mapping, and functional analysis of a fructan 1-exohydrolase (1-feh) from Lolium perenne implicated in fructan synthesis rather than in fructan mobilization. J Exp Bot 58:1969–1983
Lu C, Koroleva OA, Farrar JF, Gallagher J, Pollock CJ, Tomos AD (2002) Rubisco small subunit, chlorophyll a/b-binding protein and sucrose:fructan-6-fructosyltransferase gene expression and sugar status in single barley leaf cells in situ. Cell type specificity and induction by light. Plant Physiol 130:1335–1348
Martinez-Noël GA, Tognetti JA, Salerno GL, Pontis HG (2010) Sugar signaling of fructan metabolism: new insights on protein phosphatases in sucrose-fed wheat leaves. Plant Signal Behav 5:311–313
Martínez-Noël G, Tognetti JA, Pontis HG (2001) Protein kinase and phosphatase activities are involved in fructan synthesis initiation mediated by sugars. Planta 213:640–646
Martínez-Noël G, Tognetti J, Nagaraj V, Wiemken A, Pontis H (2006) Calcium is essential for fructan synthesis induction mediated by sucrose in wheat. Planta 225:183–191
Martínez-Noël GA, Tognetti JA, Salerno GL, Wiemken A, Pontis HG (2009) Protein phosphatase activity and sucrose-mediated induction of fructan synthesis in wheat. Planta 230:1071–1079
McIntyre CL, Casu RE, Rattey A, Dreccer MF, Kam JW, van Herwaarden AF, Shorter R, Xue GP (2011) Linked gene networks involved in nitrogen and carbon metabolism and levels of water soluble carbohydrate accumulation in wheat stems. Func Integr Genomics 11:585–597
Müller J, Aeschbacher RA, Sprenger N, Boller T, Wiemken A (2000) Disaccharide-mediated regulation of sucrose: fructan-6-fructosyltransferase, a key enzyme of fructan synthesis in barley leaves. Plant Physiol 123:265–274
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Murchie EH, Niyogi KK (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 155:86–92
Murgia I, Tarantino D, Vannini C, Bracale M, Carrabvieri S, Soave C (2004) Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J 38:940–953
Nagaraj VJ, Riedla R, Bollera T, Wiemken A, Meyer AD (2001) Light and sugar regulation of the barley sucrose: fructan 6-fructosyltransferase promoter. J Plant Physiol 158:1601–1607
Nagaraj VJ, Altenbach D, Galati V, Lüscher M, Meyer AD, Boiler T, Wiemken A (2004) Distinct regulation of sucrose: sucrose-1-fructosyltransferase (1-SST) and sucrose: fructan-6-fructosyltransferase (6-SFT), the key enzymes of fructan synthesis in barley leaves: 1-SST as the pacemaker. New Phytol 161:735–748
Olivares-Villegas JJ, Reynolds MP, McDonald GK (2007) Drought-adaptive attributes in the Seri/Babax hexaploid wheat population. Func Plant Biol 34:189–203
Pang C-H, Li K, Wang B (2011) Overexpression of SsCHLAPXs confers protection against oxidative stress induced by high light in transgenic Arabidopsis thaliana. Physiol Plant 143:355–366
Pego JV, Kortstee AJ, Huijser C, Smeekens SCM (2000) Photosynthesis, sugars and the regulation of gene expression. J Exp Bot 51:407–416
Raines CA (2003) The Calvin cycle revisited. Photosynth Res 75:1–10
Raines CA (2011) Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol 155:36–42
Randez-gil F, Herrero P, Sanz P, Prieto JA, Moreno F (1998) Hexokinase II has a double cytosolic-nuclear localisation in Saccharomyces cerevisiae. FEBS Lett 425:475–478
Ritsema T, Smeekens S (2003) Fructans: beneficial for plants and humans. Curr Opin Plant Biol 6:223–230
Ritsema T, Brodmann D, Diks SH, Bos CL, Nagaraj V, Pieterse CMJ, Boller T, Wiemken A, Peppelenbosch MP (2009) Are small GTPases signal hubs in sugar-mediated induction of fructan biosynthesis? PLoS ONE 4:e6605
Ruelland E, Miginiac-Maslow M (1999) Regulation of chloroplast enzyme activities by thioredoxins: activation or relief from inhibition? Trends Plant Sci 4:136–141
Ruuska SA, Rebetzke GJ, van Herwaarden AF, Richards RA, Fettell NA, Tabe L, Jenkins CLD (2006) Genotypic variation in water-soluble carbohydrate accumulation in wheat. Funct Plant Biol 33:799–809
Ruuska SA, Lewis DC, Kennedy G, Furbank RT, Jenkins CLD, Tabe LM (2008) Large scale transcriptome analysis of the effects of nitrogen nutrition on accumulation of stem carbohydrate reserves in reproductive stage wheat. Plant Mol Biol 66:15–32
Schnyder H (1993) The role of carbohydrate storage and redistribution in the source-sink relations of wheat and barley during grain filling. New Phytol 123:233–245
Shaw LM, McIntyre CL, Gresshoff PM, Xue GP (2009) Members of the Dof transcription factor family in Triticum aestivum are associated with light-mediated gene regulation. Funct Integr Genomics 9:485–498
Shearman VJ, Sylvester-Bradley R, Scott RK, Foulkes MJ (2005) Physiological processes associated with wheat yield progress in UK. Crop Sci 45:175–185
Smith AM (2008) Prospects for increasing starch and sucrose yields for bioethanol production. Plant J 54:546–558
Stephenson TJ, McIntyre CL, Collet C, Xue GP (2007) Genome-wide identification and expression analysis of the NF-Y family of transcription factors in Triticum aestivum. Plant Mol Biol 65:77–92
Subbaiah CC, Palaniappan A, Duncan K, Rhoads DM, Huber SC, Sachs MM (2006) Mitochondrial localization and putative signaling function of sucrose synthase in maize. J Biol Chem 281:15625–15635
Sulpice R, Pyl ET, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques MC, Korff MV, Steinhauser MC, Keurentjes JJB, Guenther M, Hoehne M, Selbig J, Fernie AR, Altmann T, Stitt M (2009) Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci USA 106:10348–10353
Takahashi S, Badger MR (2010) Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16:53–60
Valluru R, Link J, Claupein W (2011) Natural variation and morpho-physiological traits associated with water-soluble carbohydrate concentration in wheat under different nitrogen levels. Field Crops Res 124:104–113
Van den Ende W, Clerens S, Vergauwen R, Van Riet L, Van Laere A, Yoshida M, Kawakami A (2003) Fructan 1-exohydrolases. β-(2,1)-trimmers during graminan biosynthesis in stems of wheat? Purification, characterization, mass mapping, and cloning of two fructan 1-exohydrolase isoforms. Plant Physiol 131:621–631
Van den Ende W, Yoshida M, Clerens S, Vergauwen R, Kawakami A (2005) Cloning, characterization and functional analysis of novel 6-kestose exohydrolases (6-KEHs) from wheat (Triticum aestivum). New Phytol 166:917–932
Van den Ende W, Lammens W, Van Laere A, Schroeven L, Le Roy K (2009) Donor and acceptor substrate selectivity among plant glycoside hydrolase family 32 enzymes. FEBS J 276:5788–5798
Van den Ende W, Coopman M, Clerens S, Vergauwen R, Le Roy K, Lammens W, Van Laere A (2011) Unexpected presence of graminan- and levan-type fructans in the evergreen frost-hardy eudicot Pachysandra terminalis (Buxaceae): purification, cloning, and functional analysis of a 6-SST/6-SFT enzyme. Plant Physiol 155:603–614
Van Herwaarden AF, Angus JF, Richards RA, Farquhar GD (1998a) `Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertilizer II. Carbohydrate and protein dynamics. Aust J Agric Res 49:1083–1093
Van Herwaarden AF, Richards RA, Farquhar GD, Angus JF (1998b) `Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertilizer III. The influence of water deficit and heat shock. Aust J Agric Res 49:1095–1110
Van Laere A, Van den Ende W (2002) Inulin metabolism in dicots: chicory as a model system. Plant Cell Environ 25:803–813
Vijn I, Smeekens S (1999) Fructan: more than a reserve carbohydrate? Plant Physiol 120:351–360
Wardlaw IF, Willenbrink J (1994) Carbohydrate storage and mobilisation by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Aust J Plant Physiol 21:255–271
Wardlaw IF, Willenbrink J (2000) Mobilization of fructan reserves and changes in enzyme activities in wheat stems correlate with water stress during kernel filling. New Phytol 148:413–422
Wise C, Dimler R, Davis H, Rist C (1955) Determination of easily hydrolyzable fructose units in dextran preparations. Anal Chemi 27:33–36
Wu Z, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F (2004) A model-based background adjustment for oligonucleotide expression arrays. J Am Stat Asso 99:909–917
Xue GP, Loveridge CW (2004) HvDRF1 is involved in abscisic acid-mediated gene regulation in barley and produces two forms of AP2 transcriptional activators, interacting preferably with a CT-rich element. Plant J 37:326–339
Xue GP, Bower NI, McIntyre CL, Riding GA, Kazan K, Shorter R (2006a) TaNAC69 from the NAC superfamily of transcription factors is up-regulated by abiotic stresses in wheat and recognises two consensus DNA-binding sequences. Funct Plant Biol 33:43–57
Xue GP, McIntyre CL, Chapman S, Bower NI, Way H, Reverter A, Clarke B, Shorter R (2006b) Differential gene expression of wheat progeny with contrasting levels of transpiration efficiency. Plant Mol Biol 61:863–881
Xue GP, McIntyre CL, Glassop D, Shorter R (2008a) Use of expression analysis to dissect alterations in carbohydrate metabolism in wheat leaves during drought stress. Plant Mol Biol 67:197–214
Xue GP, McIntyre CL, Jenkins CLD, Glassop D, van Herwaarden AF, Shorter R (2008b) Molecular dissection of variation in carbohydrate metabolism related to water soluble carbohydrate accumulation in stems of wheat (Triticum aestivam L.). Plant Physiol 146:441–454
Xue GP, McIntyre CL, Rattey AR, van Herwaarden AF, Shorter R (2009) Use of dry matter content as a rapid and low-cost estimate for ranking genotypic differences in water soluble carbohydrate concentrations in the stem and leaf sheath of Triticum aestivum. Crop Pasture Sci 60:51–59
Xue GP, Kooiker M, Drenth J, McIntyre CL (2011) TaMYB13 is a transcriptional activator of fructosyltransferase genes involved in β-2,6-linked fructan synthesis in wheat. Plant J 68:857–870
Yabuta Y, Motoki T, Yoshimura K, Takada T, Ishikawa T, Shigeoka S (2002) Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J 32:912–925
Yamori W, Nagai T, Makino A (2011) The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ 34:764–777
Acknowledgments
This work was supported by funding from the Australian Grain Research & Development Corporation. Authors are grateful to Drs Ray Shorter, Allan R. Rattey, Fernanda Dreccer, Mr Greg Roberts and Mr. Philip van Drie for their help in field trials.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xue, GP., Drenth, J., Glassop, D. et al. Dissecting the molecular basis of the contribution of source strength to high fructan accumulation in wheat. Plant Mol Biol 81, 71–92 (2013). https://doi.org/10.1007/s11103-012-9983-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-012-9983-1