Abstract
Maintaining photosynthetic performance and remobilization of assimilates stored in vegetative tissues are strategies of superior wheat genotypes under drought stress conditions. To better understand the response of vegetative tissues to drought stress at the grain filling period, transcript profiling of genes encoding fructan and sucrose metabolism were studied in the stem (penultimate internode) and root of two drought-tolerant genotypes. Based on a preliminary screening, the cultivars T-65-7-1 (the mutant line) and Tabasi (wild type) were selected for further study with respect to the parameters associated with photosynthesis and stem remobilization under rain-fed conditions. The expression of photosynthetic genes, chlorophyll content and relative water content were sharply reduced in the T-65-7-1 compared to Tabasi, as a result of drought-induced leaf senescence. Under drought stress, fructan remobilization in the stem and root of T-65-7-1 was significantly higher than Tabasi, which was due to the over-expressed genes involved in the synthesis and hydrolysis of fructan, as well as the synthesis, hydrolysis and transport of sucrose. The stem and root tissues depicted similar assimilate remobilization behaviours under drought stress. The grain yield reduction was less in T-65-7-1 than Tabasi under drought stress during the grain filling period, therefore, the remobilization of assimilates to the grains was a more effective strategy than maintenance of photosynthesis under drought stress conditions during the grain filling period. This research provides valuable molecular indicators for selecting drought-tolerant wheat genotypes with high fructan content and increased remobilization in wheat breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of the world’s wheat (Triticum aestivum L.) is produced under rain-fed conditions, especially in the Mediterranean climate, where the crop faces drought stress during the grain filling period (Blum 1998). Current photosynthesis of leaves (mainly flag leaf) and the remobilization of stored materials in vegetative tissues (that is, stem, leaves and roots) are the two main sources of carbon supply for the filling of grains (Wardlaw and Willenbrink 2000; Zhang et al. 2016). Plant varieties employ different strategies when facing drought, either via maintaining photosynthetic performance by using photoassimilates or through the remobilization of assimilates stored in vegetative tissues to grains (Yang and Zhang 2006; Bazargani et al. 2011; Farooq et al. 2014). Drought stress can induce early senescence and increase the remobilization of assimilates to grains (Yang and Zhang 2006; Bazargani et al. 2012; Distelfeld et al. 2014). The most important event that occurs during leaf senescence is degradation of the photosynthetic apparatus. Despite the decrease of photosynthesis in plants under drought stress, a large amount of water-soluble carbohydrates (WSCs), including glucose, fructose, sucrose and fructan, accumulates in vegetative tissues (Yang et al. 2004; Joudi et al. 2012). WSCs may temporarily accumulate in the stem, leaf sheath and roots of wheat from stem elongation to the early stages of grain filling and might be utilized for grain filling (Gebbing 2003; Xue et al. 2013). The WSCs of wheat in vegetative tissue are mostly comprised of fructan (Chalmers et al. 2005; Van den Ende and El-Esawe 2014). Wheat fructans are made in vacuoles and are mainly graminan-types (Verspreet et al. 2013). Fructans are derived from sucrose, linear or branched polysaccharides based on fructose with β-(2–1) and β-(2–6) linkages (Verspreet et al. 2013).
The biosynthesis of fructan is done by the action of sucrose:sucrose 1-fructosyltransferase (1-SST), sucrose:fructan 6-fructosyltransferase (6-SFT) and fructan:fructan 1fructosyltransferase (1-FFT) (Van den Ende et al. 2003; Xue et al. 2008a, b). First, 1-SST produces trisaccharide 1-kestotriose, and then 1-kestotriose acts as a substrate for 6-SFT and produces the branched trisaccharide bifurcose (1 and 6 kestotetraose). The enzyme 6-SFT also produces 6-kestotriose from sucrose. 1-Kestotriose also acts as a substrate of the enzyme 1-FFT and leads the production of 1,1-kestotetraose (Ritsema and Smeekens 2003; Valluru and Van den Ende 2008; Xue et al. 2013). Furthermore, fructan is hydrolyzed by fructan 1-exohydrolases (1-FEHs) and fructan 6-exohydrolases (6-FEHs) and is transformed into sucrose and fructose (Van den Ende et al. 2003; Zhang et al. 2009).
Sucrose is synthesized from fructose-6-phosphate and uridine diphosphate glucose (UDP-Glc) in plant tissues by sequential actions of sucrose phosphate synthase (SPS) and sucrose6Fphosphate phosphohydrolase (SPP). In addition, sucrose can be synthesized from the combination of UDP-Glu and fructose by the action of SuS (Xue et al. 2008a, b). The hydrolysis of sucrose to ɑ-d-glucose and fructose is facilitated by different invertase enzymes, of which vacuolar invertase (INV) plays the most important role (Königshofer and Löppert 2015). Sucrose is the most important sugar in the phloem, and is transiently stored in the vacuole (Lemoine et al. 2013). Sucrose transport (SUT) from vacuoles to the cytosol is done by SUT2, which is located in the tonoplast (Deol et al. 2013). Moreover, during phloem loading, the transport of sucrose across the plasma membrane occurs through SUT1 (Aoki et al. 2004).
Studies have been performed on the expression of some genes involved in the biosynthesis, hydrolysis and transport of important carbohydrates in wheat during the grain filling period under terminal drought stress (Xue et al. 2008a, b, 2013; Zhang et al. 2009; Khoshro et al. 2014). However, the gene expression patterns have been studied in the leaf and stem, but there have been no studies related to the genes involved in carbohydrate metabolism and remobilization under drought stress in the root system of wheat. In this study, two drought-tolerant genotypes with contrasting behaviours in current flag leaf photosynthesis and remobilization of stored assimilates under rain-fed conditions were selected for further study based on a preliminary screening. To better understand the response of vegetative tissues to drought stress at the grain filling period, the transcription profiles of genes encoding fructan and sucrose metabolism were studied by quantitative real-time PCR (qRT-PCR) in the stem, penultimate internode (pn) and root of the two selected genotypes with the aim of (1) comparing the behaviours of stem and root tissues in the remobilization of assimilates to the grain and (2) determining a suitable strategy for the development of wheat genotypes under drought stress during grain filling.
Materials and Methods
Two experiments were carried out at the research farm and greenhouse of Gorgan University of Agricultural Sciences and Natural Resources (36°54′N, 54°24′E and 13 m) from 2012 to 2015.
Field Experiment
The Tabasi mutated lines were produced by the Institute of Agricultural, Medical and Industrial Research, Tehran, Iran. These mutant lines were the result of a mutation breeding program by Co-60 gamma radiation, 300 Gray at 55 rad min−1 (Daei et al. 2009). Tabasi is a spring cultivar with an acceptable level of drought tolerance (Kordenaeej et al. 2008). Fifteen mutant lines (M6) of bread wheat along with their wild type (Tabasi cultivar) were grown under rain-fed conditions for 2 years (2012–2014) in the field (listed in Supplemental Table 1). In this area, the grain filling period occurs during June and July, and the average daily temperatures and precipitation in June 2013/2014 were 26.4/26.6 °C and 12.2/7.8 mm, respectively. In July 2013/2014, the average daily temperature was 27.5/28.1 °C and there was no precipitation. The experiment was conducted in a randomized complete block design (RCBD), with three replications. There were five rows in each plot, the distance between rows was 20 cm, and the length of each row was 2 m. The measurements were randomly taken from the tagged main stems with the same length in four stages (each experimental unit consisting of eight plants) after anthesis at intervals of 10 days (at 10, 20, 20 and 40 days after anthesis) from the stem and the flag leaf. To determine the dry weight, stem samples were first dried in an oven at 80 °C for 48 h and then weighed. Remobilization based on specific weight was calculated as the difference between the maximum and minimum of specific weight of stems after anthesis (Ehdaie et al. 2006a, b). The specific weight was calculated by dividing the stem weights by their lengths. The remobilization efficiency was calculated as the ratio of remobilization to the maximum specific weight (Ehdaie et al. 2006a, b). Furthermore, to determine the photosynthetic capacity of the flag leaf, stomatal conductance was measured using a porometer (Delta T AP4, UK), and the chlorophyll content was obtained using a chlorophyll meter (SPAD CCM-200, USA), reporting the average of the four stages. Genotypes were plotted against stomatal conductance and chlorophyll content of the flag leaf, remobilization and remobilization efficiency of the main stem. Consequently, the T-65-7-1 mutant line (with a high remobilization and a low photosynthetic capacity) and the Tabasi wild type (with a low remobilization and a high photosynthetic capacity) were selected for further study.
Greenhouse Experiment
The two selected genotypes (Tabasi, the wild type, and T-65-7-1, the mutant line) were grown in a greenhouse in a completely randomized design (2 × 2 factorial experiment) with five replications during the growing season of 2014–2015. Each pot was filled with 10 kg of soil containing a combination of clay, loamy sand and animal fertilizer at a 1:2:1 ratio. The diameter and depth of the pots were 28 and 40 cm, respectively, and there were 10 plants in each experimental unit (pot). The plants were grown in the controlled greenhouse environment with 16 h of light (28 °C) and 8 h of dark (18 °C). The relative humidity was 55–60% and the photon flux density was approximately 250 µmol m−2 s−1. The plants were grown in the same conditions under well-watered conditions until stress was imposed. Plants were irrigated three times with 1/2 Hoagland solution (Supplemental Table 2) until flag leaf emergence (Zadoks 37).
Imposing Stress and Sampling
Imposing stress by terminating irrigation was initiated at the Zadoks 60 (full heading emergence) stage (Zadoks et al. 1974). The main stems of the plants in each pot were tagged as the first spikes emerged from the flag leaf sheaths. The soil moisture of plots under well-watered conditions was kept at 90% field capacity (FC) through regular irrigation, whereas under drought stress conditions, the soil moisture was held at 40% of the field capacity using the procedure explained by Bazargani et al. (2011) through regular weighing. Random sampling of flag leaf, pn internode (from the main stem) and roots occurred at five stages (S1–S5) from anthesis to ripening at 7-day intervals (0, 7, 14, 21, and 28 days), with each plot consisting of five plants. According to previous studies, among the stem internodes, the pn internode has the highest level of stored carbohydrates and the highest remobilization efficiency; therefore, in this study, the pn internodes (with leaf sheath) were used (Blum 1998; Wardlaw and Willenbrink 2000; Scofield et al. 2007; Sharbatkhari et al. 2016). Roots with soil sticking to them were rapidly put on a wire mesh and washed with water until the soil was completely eliminated (Ehdaie et al. 2012). Tissue samples needed for gene expression evaluation were kept in − 80 °C after being frozen in liquid nitrogen.
Fructan Remobilization and Remobilization Efficiency of the pn Internode
Fructan remobilization of the pn internode was calculated as the difference between the maximum and minimum fructan concentrations after anthesis. Remobilization efficiency was the ratio of remobilization to the maximum fructan concentration (Ehdaie et al. 2006).
Chlorophyll Content and Relative Water Content (RWC) of the Flag Leaf
The flag leaf chlorophyll content was measured using the spectrophotometric method and by employing pure acetone (Lichtenthaler 1987). The leaf RWC was measured using the method described by Dhanda and Sethi (1998).
Water Soluble Carbohydrate (WSC), Sucrose and Fructan Contents
The total WSCs of stems (pn) and roots was extracted using water at 70 °C and measuring the concentration of WSCs with the phenol–sulphuric acid approach (Dubois et al. 1990). Glucose, fructose and sucrose were measured in accordance with the method described by Hajirezaei et al. (2000). Glucose and fructose were measured by determining the d-glucose and d-fructose contents due to the activity of hexokinase, phosphoglucose isomerase and glucose 6-phosphate dehydrogenase. The NADPH content formed in this reaction is stoichiometric with the content of d-glucose d-fructose. It is the NADPH which is determined by the increase in absorbance at wavelengths of 340 nm. Sucrose is hydrolyzed to d-fructose and d-glucose using β-fructosidas. By subtracting the amounts of d-fructose and d-glucose obtained from the previous reaction, the amount of sucrose was calculated. Fructans and sucrose were hydrolyzed to glucose and fructose by perchloric acid (HClO4). Increasing the concentration of fructose indicated the concentration of sucrose and fructans, and consequently the concentration of fructans was obtained by subtracting the amount of the primary proportion of sucrose and fructose in the sample.
Quantitative Real-Time PCR Analysis
To evaluate the expression of genes, total RNA was extracted from 100 mg of the samples prepared using the P-Biozol kit (Bio Flux-Bioer, Tokyo, Japan). After DNaseI treatment, the quantity and quality of extracted RNA were determined by spectrophotometry and agarose gel electrophoresis. The cDNA strand was synthesized with an RT reagent kit (Fermentas company, Vilnius, Lithuania) according to the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was done using SYBR Green I in a 20 µl reaction volume that contained 1× of SYBR Bio Pars (GUASNR). Reactions were carried out in an iCycler iQ5 thermocycler (Bio Rad Company). Specific primers used to assess the transcript profiles of fructan (1-SST, 6-SFT, 1-FFT, 1-FEHw3, 6-FEH), sucrose (SPS, SPP, SuS, INV, SUT1, SUT2) and photosynthetic genes involved in the Calvin cycle (RBCL: Ribulose 1,5bisphosphate carboxylase/oxygenase large subunit, RBCS: Ribulose 1,5-bisphosphate carboxylase/oxygenase small subunit, RCA: Ribulose bisphosphate carboxylase activase) are shown in Table 1. Some of these primers were used in other studies (Table 1), and others were designed using the software AllelID7 (PREMIER Biosoft, Palo Alto, CA, USA). Genes involved in the metabolism of sucrose and fructan were studied in the stem (pn internode along with leaf sheath) and root, and photosynthesis genes were studied only in the flag leaf. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control (Gonçalves et al. 2005; Zhang et al. 2009). Gene expression was assessed by 2−ΔΔCT (Pfaffl 2001) in relation to the control plants at the same stage with three biological replicates (each replicate consisted of four pooled plants).
Statistical Analysis
Gene expression data were analyzed using GenEx 6 (MultiDAnalyses, Goteborg, Sweden). The analysis of morphophysiological data was conducted using SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA) with proc GLM, and the comparison of means was performed using LSD at a 5% probability level.
Results
Current Photosynthesis and Remobilization of Assimilates
Based on the results of the field experiment, the genotypes differed in their parameters associated with photosynthesis (Supplemental Table 1) and stem remobilization under rain-fed conditions. For instance, the flag leaf SPAD value in genotypes varied between 26.95 and 42.88. The flag leaf stomatal conductance of genotypes ranged from 25.87 to 63.34 m mol H2O m−2 s−1 (Fig. 1a). Furthermore, the SPAD value and the stomatal conductance of the flag leaf as photosynthetic parameters had a strong relationship (r = 0.87**) (Fig. 1a). The stem remobilization of genotypes varied from 3.66 to 6.92 mg cm−1, and the remobilization efficiency varied between 19.61 and 34.13% (Fig. 1b). The correlation between remobilization and remobilization efficiency was also strong (r = 0.80**) (Fig. 1b). The behaviours of Tabasi and T-65-7-1 were completely opposite with respect to these parameters. Tabasi depicted a high current photosynthesis and a low remobilization efficiency whereas T-65-7-1 showed lower current photosynthesis and higher remobilization efficiency (Fig. 1). However, the two genotypes did not differ in terms of flowering time and fertile tillers (data not shown). Based on the results of the field experiment, the T-65-7-1 mutant line and the Tabasi wild type were selected for further study in the greenhouse environment.
Scatter plots: a stomatal conductance and SPAD value, b remobilization and remobilization efficiency in the main stem of 16 wheat genotypes over two growing seasons (2012–2014) under natural rain-fed conditions. Each point represents a mean computed from 192 (a) and 48 (b) independent main stems. Arrows show genotypes, 1 (Tabasi; wild type) and 8 (T-65-7-1; mutant line). Remobilization = maximum specific weight − specific minimum weight. Remobilization efficiency = (remobilization/maximum specific weight) × 100
Grain Yield, Flag Leaf Chlorophyll Content, and RWC in T-65-7-1 and Tabasi
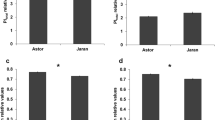
Under both moisture conditions, the grain yield of T-65-7-1 was significantly higher than Tabasi (Fig. 2a). Drought stress reduced grain yield per plant in the both genotypes, and this yield reduction was lower in T-65-7-1 (17.43%) than Tabasi (25.34%) (Fig. 2a). Two genotypes under well-watered conditions had no significant differences in chlorophyll content during the grain filling period excluding S5 (Fig. 2b). Drought stress conditions significantly reduced chlorophyll content in T-65-7-1 at all stages, but the decline in Tabasi was not significant at S1, S2 and S3 (Fig. 2b). Similarly, under well-watered conditions, these two genotypes were not significantly different in terms of RWC (Fig. 2c). Flag leaf RWC decreased in in T-65-7-1 at all stages under drought stress conditions (Fig. 2c). Flag leaf RWC of Tabasi was significantly higher than T-65-7-1 during the grain filling period under drought stress conditions (Fig. 2c).
Grain yield (a), chlorophyll content (b) and relative water content (c) in Tabasi and T-65-7-1 in well-watered (WW) and drought stress (DS) conditions in the controlled greenhouse environment. The vertical lines represent ± SE of the mean of three replicates. Stages represent days after anthesis 0 (S1), 7 (S2), 14 (S3), 21 (S4) and 28 (S5), respectively. Means followed by same letters are not significantly different (P < 0.05). Same letters in parentheses indicate non-significant difference between each genotype under WW compared to DS (P < 0.05)
Fructan and Sucrose Contents of the Stem (pn Internode) During the Grain Filling Period
Generally, under well-watered conditions, the trend of fructan changes in the stem (pn) was similar for both genotypes, in which after anthesis and during grain filling, the stem (pn) fructan started to increase and peaked at S3, and then, after decreasing, reached a minimum at S5 (Fig. 3). Under both moisture conditions, the peak of fructan concentration in T-65-7-1 was greater than Tabasi. The fructan concentration under drought stress conditions reached the peak earlier at S2 in T-65-7-1. Additionally, the fructan concentration in T-65-7-1 decreased more sharply after attaining the peak under drought stress conditions (Fig. 3a). Under well-watered conditions, the differences in fructan remobilization and remobilization efficiency of stem tissues (pn) in the two genotypes were not significant, but under drought stress conditions, remobilization was significantly greater in T-65-7-1 than Tabasi (Fig. 5a).
The sucrose concentration of the stem (pn) reached the peak earlier in T-65-7-1. After anthesis, T-65-7-1 (at S3) and Tabasi (at S4) peaked and then reached a minimum at S5 (Fig. 3b). The peak of sucrose concentration in both conditions was greater in T-65-7-1 than Tabasi. Drought increased the concentration of sucrose at the S2 and S3 stages in both genotypes. Compared to Tabasi, the sucrose concentration of T-65-7-1 decreased more intensively after peaking (Fig. 3b).
Fructan and Sucrose Contents in the Root During the Grain Filling Period
Under both moisture conditions, trends in fructan changes of roots were similar for both genotypes. The peak of fructan concentration was observed at S2 (Fig. 4a). Drought stress conditions caused a significant increase in the fructan concentration of T-65-7-1, so that the peak of fructan concentration in roots of T-65-7-1 was significantly greater than Tabasi (Fig. 4a). Drought stress conditions caused a significant increase in sucrose concentrations in T-65-7-1 at S2 and S3. Compared to Tabasi, T-65-7-1 sucrose concentrations decreased more sharply after peaking under drought stress conditions (Fig. 4b). Additionally, similar to the stem (pn) under well-watered conditions, the two genotypes did not significantly differ in fructan remobilization and remobilization efficiency of the root, but under drought stress conditions, remobilization was significantly higher in T-65-7-1 than Tabasi (Fig. 5b).
Fructan remobilization and remobilization efficiency, a in the stem (pn) and b root in Tabasi and T-65-7-1 in well-watered (WW) and drought stress (DS) conditions. The vertical lines represent ± SE of the mean of three replicates. Means followed by same letters are not significantly different (P < 0.05). Same letters in parentheses indicate non-significant difference between each genotype under WW compared to DS (P < 0.05)
Expression of Photosynthetic Genes Under Drought Stress in the Flag Leaf
The expression of photosynthetic genes showed that the photosynthetic apparatus of T-65-7-1 was affected more quickly than Tabasi under drought stress (Fig. 6). For T-65-7-1, drought stress caused a significant reduction in the expression of photosynthetic genes after S2, while Tabasi exhibited a significant reduction at S4 and S5 (Fig. 6). The reduction in expression of RBCL, RBCS and RCA at S2 to S5 was significantly higher in T-65-7-1 than Tabasi (except at S2 for RBCL). As the plants reached the final stages of the grain filling period, the differences in expression of photosynthetic genes became greater between T-65-7-1 and Tabasi (Fig. 6).
Relative expression of photosynthetic genes aRBCS, bRBCL and cRCA in the flag leaf of Tabasi and T-65-7-1, under drought stress during the grain filling period. Stages represent days after anthesis 0 (S1), 7 (S2), 14 (S3), 21 (S4) and 28 (S5), respectively. The vertical lines represent ± SE of the mean of three replicates
Expression of Genes Involved in the Metabolism of Sucrose and Fructan Under Drought Stress in the Stem (pn Internode) and Root
The expression of key genes involved in fructan biosynthesis (1-SST, 6-SFT and 1-FFT) in the stem (pn) and root showed that drought stress conditions caused a significant increase in the 1-SST and 6-SFT genes (especially at S2, S3 and S4), whereas the expression of the 1-FFT gene was not significantly changed due to drought stress (Fig. 7). The expression of 1-SST and 6-SFT in the stem (pn) and root of T-65-7-1 peaked earlier (S2), whereas the peak of expression was observed at S3 in Tabasi. In general, the expression of genes involved in fructan biosynthesis under drought stress conditions in the stem (pn) and root of T-65-7-1 was equal to or greater than Tabasi at all of the stages (Fig. 7). Under drought stress conditions, expression of 1-FEHw3 in T-65-7-1 was significantly greater than Tabasi in both tissues at S3, S4 and S5 (Fig. 8). Also expression of 6-FEH in the stem (pn) of T-65-7-1 was significantly higher than Tabasi at S1, S3, S4 and S5, and in the root at S3, S4 and S5 (Fig. 8).
Relative expression of fructan biosynthesis genes a1-SST, b6-SFT and c1-FFT in the stem (pn) and root of Tabasi and T-65-7-1, under drought stress during the grain filling period. Stages represent days after anthesis 0 (S1), 7 (S2), 14 (S3), 21 (S4) and 28 (S5), respectively. The vertical lines represent ± SE of the mean of three replicates
Relative expression of fructan hydrolysis genes a1-FEH and b6-FEH in the stem (pn) and root of Tabasi and T-65-7-1, under drought stress during the grain filling period. Stages represent days after anthesis 0 (S1), 7 (S2), 14 (S3), 21 (S4) and 28 (S5), respectively. The vertical lines represent ± SE of the mean of three replicates
Under drought stress conditions, the gene expression changes of SPS and SPP were similar in both stem (pn) and root tissues, peaking at S3 and then declining. SPS gene expression was significantly higher than Tabasi in the stem (pn) of T-65-7-1 at stages S3 to S5, and in the root at all stages excluding S5 (Fig. 9). Under drought stress conditions, SPP gene expression in the stem (pn) and root was significantly higher in T-65-7-1 than Tabasi (Fig. 9) at all stages excluding S5 for the root. Significant changes in SuS gene expression were not observed in the stem (pn) or root of both genotypes under drought stress conditions (Fig. 9). Expression of INV increased under drought stress conditions at all of the stages in the stem (pn) and root of T-65-7-1. In T-65-7-1, INV gene expression was higher than Tabasi in both tissues since the expression of Tabasi remained almost constant during the grain filling period (Fig. 9).
Relative expression of sucrose biosynthesis/hydrolysis genes aSPS, bSPP, cSUS and dINV in the stem (pn) and root of Tabasi and T-65-7-1, under drought stress during the grain filling period. Stages represent days after anthesis 0 (S1), 7 (S2), 14 (S3), 21 (S4) and 28 (S5), respectively. The vertical lines represent ± SE of the mean of three replicates
The expression patterns of sucrose transporter genes (SUT1 and SUT2) were almost identical in both genotypes (Fig. 10). After anthesis in T-65-7-1, the sucrose transporter gene expression increased as a result of drought stress, peaked at S3, and then decreased (Fig. 10). Changes in SUT1 gene expression in the stem (pn) and root of the wild type under drought stress conditions were not significant at any of the stages but were significantly higher in the stem (pn) (S3 and S4) and root (S2, S3 and S4) of T-65-7-1 and were higher than Tabasi (Fig. 10). Under drought stress conditions, SUT2 gene expression in the stem (pn) and root of Tabasi increased significantly at only one stage (stem S2, root S3). However, SUT2 increased significantly in T-65-7-1 at three stages (S2, S3, S4). In the stem (pn), SUT2 increased in two stages (S3 and S4), and in the root, SUT2 increased in three stages (S2, S3 and S4), and this increase was significantly greater than Tabasi (Fig. 10).
Relative expression of sucrose transporter genes aSUT1, bSUT2 in the stem (pn) and root of Tabasi and T-65-7-1 under drought stress during the grain filling period. Stages represent days after anthesis 0 (S1), 7 (S2), 14 (S3), 21 (S4) and 28 (S5), respectively. The vertical lines represent ± SE of the mean of three replicates
Correlation of the Expression of Genes Involved in Carbohydrate Metabolism with Contents of Fructan, Sucrose and WSCs
There was a positive and significant correlation between the expression of genes involved in fructan biosynthesis (1-FFT, 6-SFT, 1-SST), and the fructan and WSC contents in both tissues (Table 2). A positive and significant correlation between the expression of SPS and SPP and sucrose was observed in the stem (pn) and root (Table 2). In both tissues, there was a positive and significant correlation between sucrose transporter gene expression (SUT1 and SUT2) and sucrose (Table 2). In contrast, a negative significant correlation was observed for 1-FEHw3 and 6-FEH with fructan and WSC contents in the stem (pn) and root. Moreover, the correlation between INV gene expression and fructan, sucrose and WSC contents was negative in both tissues (Table 2).
Correlation of the Expression of Genes Involved in Carbohydrate Metabolism Between Stem (pn Internode) and Root
Among all of the evaluated genes involved in the metabolism of fructan and sucrose, the correlation between stem (pn) and root was not significant for only 1-FFT. In the expression of the other genes in the stem (pn) and root, correlations were positive and significant (Supplemental Fig. 1).
Discussion
In plants that are not drought-escaping, some genotypes mostly react to drought stress by resisting the reduction of photosynthesis and some by remobilizing assimilates stored in vegetative tissues (Farooq et al. 2014). In the screening of bread wheat mutant lines and their wild type, a huge difference was observed with respect to the parameters associated with photosynthesis and stem remobilization under rain-fed conditions (Fig. 1). In other studies, genetic variation is frequently mentioned for remobilization and remobilization efficiency (Ehdaie et al. 2006a, b; Ruuska et al. 2006; Xue et al. 2009; Gupta et al. 2011; Joudi et al. 2012).
The results showed that under both moisture conditions, grain yield per plant in T-65-7-1 was significantly greater than Tabasi. Drought stress conditions caused lower reductions in the yield of T-65-7-1 (17.43%) compared to Tabasi (25.34%) (Fig. 2a). It seems that the remobilization of assimilates to grain during the grain filling period is a more effective strategy than maintenance of photosynthesis under drought stress conditions because drought stress is imposed during the grain filling period. Jagadish et al. (2015) reported that both staying green and senescence are physiological reactions of plants against abiotic stresses. When the plant is faced with stress before anthesis or fertility, the ability to stay green causes more fertility and, as a result, increases the grain number whereas under stress after anthesis and during grain filling, senescence leads to increased remobilization of stored material to grains and changes in grain weight (Jagadish et al. 2015).
In this study, based on the results of the expression of photosynthetic genes, flag leaf chlorophyll and RWC, it seems that Tabasi maintains and continues photosynthesis and uses the current assimilates during grain filling under drought stress (Figs. 2b, c, 6). The obvious signs of leaf senescence are the controlled changes of physiological reactions including photosynthesis decline, chloroplast degradation, a significant reduction in chlorophyll content and RWC (Chandlee 2001; Hörtensteiner and Feller 2002). The expression of photosynthetic genes (RBCL, RBCS and RCA) during the grain filling period under drought stress conditions showed that the photosynthetic system of T-65-7-1 was affected more quickly and severely by drought than Tabasi (Fig. 6). Rubisco is degraded during senescence, and its degradation products are re-utilized as a source for developing tissues (Suzuki et al. 2001). Senescence increases the remobilization of assimilates stored in vegetative tissue for grain filling in monocarpic plants such as wheat (Yang and Zhang 2006). The stem and root are capable of transporting the stored assimilates (Lawlor and Paul 2014). Our results showed that, under well-watered conditions, fructan remobilization and remobilization efficiency of the stem and root were not significantly different between the two cultivars, whereas under drought stress conditions, these were significantly higher in T-65-7-1 than Tabasi (Fig. 5), which is probably due to early senescence. Bazargani et al. (2011) studied the proteome of two wheat landraces (contrasting in stem remobilization) and reported that the expression of several related senescence proteins, and consequently the degradation of photosynthetic proteins, increased under drought stress conditions in the genotype with higher remobilization.
Several studies have been conducted on the remobilization from stem to grain under drought stress conditions in wheat (Wardlaw and Willenbrink 2000; Yang et al. 2000, 2004; Ehdaie et al. 2006a, b, 2008; Xue et al. 2009; Gupta et al. 2011; Zhang et al. 2015). There are few reports on the importance of remobilization of root assimilates during the grain filling period under drought stress conditions (Lopes and Reynolds 2010; Zhang et al. 2016), although there have been some studies of the role of root system architecture (Manschadi et al. 2006; Palta et al. 2011; Nakhforoosh et al. 2014). Drought stress conditions cause root development in deeper soil layers for moisture absorption (Wasson et al. 2012). In wheat breeding programs, varieties with deeper root systems were selected to create a drought-tolerant variety. However, in rain-fed Mediterranean regions, the evaporation rate is high due to the limited (or non-existent) rainfall and high temperatures at the end of the growing season. Therefore, any rainfall that occurs will only affect the uppermost soil layer and is not enough to help develop deeper roots (Zhang et al. 2016). Under these conditions, the importance of the remobilization of assimilates from root to grain is vital. Roots, as a partial fructan pool, can be involved in assimilate remobilization (Zhang et al. 2016). In this study, the amount of root fructan remobilization was one-fourth of the pn stem internode (Fig. 5). Zhang et al. (2016) reported that the WSCs and root fructan were approximately one-third of the stem. Further, compared to Tabasi, the peak of fructan and sucrose contents in T-65-7-1 was higher in vegetative tissues (Figs. 3, 4).
Fructan comprises most of the WSCs in wheat vegetative tissues, which contain both β-(2–1)-linked and β-(2–6)-linked (fructose Van den Ende et al. 2003; Chalmers et al. 2005; Van den Ende and El-Esawe 2014). The β-(2–6)-linked fructans are formed by the sequential action of 1-SST and 6-SFT, while β-(2–1)-linked fructans are formed by the action of 1-FFT; the latter reaction is least important in fructan synthesis (Verspreet et al. 2013). The present results showed that the expression of 1-SST and 6-SFT in the stem (pn) and root significantly increased under drought stress (especially at S2, S3, and 4S), whereas 1-FFT gene expression was not affected by drought stress (Fig. 7). The reason that 1-FFT is not affected may be because of a less important role in the synthesis of fructan. These results suggested that β-(2–6) linkages, the predominant form of fructan linkages, were affected by the drought. In this study, under drought stress conditions, the expression of 1-SST and 6-SFT in the stem (pn) and root of T-65-7-1 were equal to or greater than Tabasi at all stages (Fig. 7). A significant positive correlation was observed between the expression of genes involved in fructan biosynthesis and contents of fructan and WSCs in both the stem (pn) and the root (Table 2). These results clearly indicate that the contents of fructan and WSCs in the root and stem are a function of the activity of enzymes encoded by 1-SST and 6-SFT. Moreover, the genetic variation in fructan accumulation among genotypes is caused by 1-SST and 6-SFT (Xue et al. 2008).
The hydrolysis of fructan and its conversion to sucrose and fructose are conducted by 1-FEHs and 6-FEHs, which preferentially cleave the ß-(2–1)- and ß-(2–6)-links, respectively (Van den Ende et al. 2003; Van Riet et al. 2006). There are three forms of 1-FEH: 1-FEHw1 (1-FEH-6A), 1-FEHw2 (1-FEH-6D) and 1-FEHw3 (1-FEH-6B). However, 1-FEHw3 is the key gene in the remobilization of fructan (Zhang et al. 2009). The expression of 1-FEHw3 and 6- FEH in the stem (pn) and root of T-65-7-1 were significantly higher at the final stages (S3, S4 and S5) than Tabasi (Fig. 8). Khoshro et al. (2014) studied the effect of terminal draught stress on two wheat cultivars and reported that the 1-FEHw3 and 6-FEH is up-regulated during remobilization of assimilates. In this study, negative and significant correlations were observed between the FEHs genes (1-FEHw3 and 6-FEH) and fructan and WSC contents in the stem (pn) and root (Table 2), which represents a further reduction of fructan content under drought stress conditions at the final stages of grain filling, especially in T-65-7-1. The negative and significant correlations between the FEHs genes expression and WSCs under terminal drought stress has been reported by the other researchers (Zhang et al. 2009; Khoshro et al. 2014). When the demand for grain filling is high, sucrose is limited, and fructan is converted to sucrose and fructose by FEHs (Zhang et al. 2009). It seems that the higher sink strength (grain yield) of T-65-7-1 limits sucrose and increases FEHs genes expression to supply sucrose (Figs. 2a, 8).
After fructan hydrolysis, fructose is used as the substrate for resynthesis of sucrose (Joudi et al. 2012). In plant tissues, sucrose is synthesized by the activity of the enzymes SPS, SPP, and SuS (Xue et al. 2008a, b). At most of the stages, SPS and SPP gene expression were significantly higher in the stem (pn) and the root of T-65-7-1 than Tabasi (Fig. 9a, b). However, no significant change was observed in the SuS gene expression in the stem (pn) and root of both genotypes under drought stress (Fig. 9c). These results reflect the higher synthesis of sucrose in the stem (pn) and root of T-65-7-1 compared to Tabasi, especially at intermediate stages (S2, S3, and S4). A significant positive correlation between the expression of SPS and SPP and sucrose in the stem (pn) and root indicates the role of these genes in determining the sucrose content of these tissues (Table 2).
Hydrolysis of sucrose and its conversion into ɑ-d-glucose and fructose in the vacuole is done by vacuolar invertase (INV) (Ruan 2014). The results showed that INV expression for T-65-7-1 under drought stress conditions was higher than Tabasi in both shoot and root tissues for all stages (Fig. 9d). Therefore, more hexose sugars are produced under drought stress conditions in T-65-7-1, and the required substrate for producing sucrose and fructan are provided. The effect of the increase in INV gene expression in the varieties with high remobilization under drought and salt stress has been reported by the other researchers (Khoshro et al. 2014; Sharbatkhari et al. 2016). In this study, a negative correlation was observed between INV gene expression and fructan and WSC contents in the stem (pn) and root, though this was not statistically significant (Table 2). This was not unexpected because the INVs are mostly regulated at the post-translational level by invertase inhibitors (Tauzin et al. 2014).
The metabolism and transport of sucrose are very important for growth and senescence (Wang et al. 2016). The transportation of sugars from source to sink is one of the main necessities of plant growth (Lemoine et al. 2013). Transport of sucrose from the vacuole to the cytosol and the cytosol to the apoplast are due to the action of SUT2 and SUT1, respectively (Aoki et al. 2004; Deol et al. 2013). The expression of sucrose transporter genes (SUT1 and SUT2) was increased in T-65-7-1 at most of the evaluated stages, while Tabasi was not affected by drought and had a constant trend at most stages (Fig. 10). The over-expression of sucrose transporter genes (SUT1 and SUT2) in T-65-7-1 correlates an increase in sucrose transport in the remobilization from vegetative tissues to grains. In both tissues, a positive significant correlation was observed between the expression of sucrose transporter genes (SUT1 and SUT2) and sucrose (Table 2), which represents the fast transport of sucrose from vegetative tissues to the sink during terminal drought, especially in T-65-7-1.
Our results showed that both stem (pn) and root tissues display similar behaviours in the remobilization of stored assimilates to the grain during grain filling under drought stress conditions. In most of the examined genes in the fructan and sucrose metabolism pathway, a positive and significant correlation was observed between gene expression in the stem (pn) and root (Supplemental Fig. 1). Drought-induced leaf senescence increases the remobilization of assimilates from vegetative tissues to the grains (Yang and Zhang 2006; Distelfeld et al. 2014). It seems that the wheat stem (pn) and root exhibit similar behaviours for responding to senescence.
The majority of the investigated genes in the current study, including 1-SST, 6-SFT,1-FEHw3, 6-FEH, SPS, SPP, INV, SUT1 and SUT2, provide valuable molecular indicators for achieving wheat genotypes with high fructan content and more remobilization under terminal drought stress and will aid in the development of new drought-tolerant genotypes in wheat breeding programmes.
Conclusions
Our results showed that senescence was induced in T-65-7-1 but not in Tabasi as a result of drought. Additionally, the expression of photosynthetic genes, chlorophyll content, and RWC of the flag leaf were sharply reduced in T-65-7-1. These changes may have contributed to the damage of the photosynthetic apparatus under drought stress conditions. Higher fructan remobilization through vegetative tissues to the grains in T-65-7-1 compared to Tabasi under drought stress was caused by the regulation of genes involved in the synthesis and hydrolysis of fructan (1-SST, 6-SFT, 1-FEHw3, 6-FEH) as well as the synthesis, hydrolysis, and transport of sucrose (SPS, SPP, INV, SUT1, SUT2) (Fig. 11). WSCs stored in the root as a partial pool participated in the remobilization of assimilates during the grain filling period, while root fructan remobilization was one-fourth of the stem. Stem (pn) and root tissues exhibited similar behaviour in the remobilization of assimilates to grain during grain filling under drought stress. Our results showed that the remobilization of assimilates to the grains during the grain filling period was a more effective strategy than maintenance of photosynthesis under terminal drought stress conditions, so that the grain yield reduction in T-65-7-1 was lower than Tabasi. This research provides molecular indicators for selecting drought-tolerant wheat genotypes with high fructan content and greater remobilization under drought stress during the grain filling period in wheat breeding programmes.
The effect of flag leaf senescence on the increase of remobilization of WSCs from the stem (pn) and root to grain, and changes in the expression of genes involved in fructan and sucrose metabolism in the stem (pn) and root of T-65-7-1 compared to Tabasi. Overall expression changes of the genes are indicated by colour, with red for drought up-regulated genes, blue for drought down-regulated genes and black for genes with no significant change in T-65-7-1 compared to Tabasi. Sucrose transport from the vacuole to the cytosol and the cytosol to the apoplast occurs by SUT2 and SUT1, respectively. The tonoplast monosaccharide transporters (TMTs) reversibly transfer glucose and fructose between the vacuole and cytosol, which is still poorly understood in wheat. (Color figure online)
References
Aoki N, Scofield GN, Wang XD, Patrick JW, Offler CE, Furbank RT (2004) Expression and localisation analysis of the wheat sucrose transporter TaSUT1 in vegetative tissues. Planta 219(1):176–184. https://doi.org/10.1007/s00425-004-1232-7
Bazargani MM, Sarhadi E, Bushehri A-AS, Matros A, Mock H-P, Naghavi M-R, Hajihoseini V, Mardi M, Hajirezaei M-R, Moradi F (2011) A proteomics view on the role of drought-induced senescence and oxidative stress defense in enhanced stem reserves remobilization in wheat. J Proteomics 74(10):1959–1973
Bazargani MM, Hajirezaei M-R, Salekdeh GH, Bushehri A-AS, Falahati-Anbaran M, Moradi F, Naghavi M-R, Ehdaie B (2012) A view on the role of metabolites in enhanced stem reserves remobilization in wheat under drought during grain filling. Aust J Crop Sci 6(12):1613–1623
Blum A (1998) Improving wheat grain filling under stress by stem reserve mobilisation. Euphytica 100(1–3):77–83
Chalmers J, Lidgett A, Cummings N, Cao Y, Forster J, Spangenberg G (2005) Molecular genetics of fructan metabolism in perennial ryegrass. Plant Biotechnol J 3(5):459–474
Chandlee JM (2001) Current molecular understanding of the genetically programmed process of leaf senescence. Physiol Plant 113(1):1–8
Daei G, Ardekani M, Rejali F, Teimuri S, Miransari M (2009) Alleviation of salinity stress on wheat yield, yield components, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J Plant Physiol 166(6):617–625
Deol KK, Mukherjee S, Gao F, Brûlé-Babel A, Stasolla C, Ayele BT (2013) Identification and characterization of the three homeologues of a new sucrose transporter in hexaploid wheat (Triticum aestivum L.). BMC Plant Biol 13(1):181
Dhanda S, Sethi G (1998) Inheritance of excised-leaf water loss and relative water content in bread wheat (Triticum aestivum). Euphytica 104(1):39–47
Distelfeld A, Avni R, Fischer AM (2014) Senescence, nutrient remobilization, and yield in wheat and barley. J Exp Bot 65(14):3783–3798
Dubois D, Winzeler M, Nösberger J (1990) Fructan accumulation and sucrose: sucrose fructosyltransferase activity in stems of spring wheat genotypes. Crop Sci 30(2):315–319
Ehdaie B, Alloush G, Madore M, Waines J (2006a) Genotypic variation for stem reserves and mobilization in wheat: I. Postanthesis changes in internode dry matter. Crop Sci 46(2):735–747
Ehdaie B, Alloush G, Madore M, Waines J (2006b) Genotypic variation for stem reserves and mobilization in wheat: II. Postanthesis changes in internode water-soluble carbohydrates. Crop Sci 46(5):2093–2103
Ehdaie B, Alloush G, Waines J (2008) Genotypic variation in linear rate of grain growth and contribution of stem reserves to grain yield in wheat. Field Crops Res 106(1):34–43
Ehdaie B, Layne AP, Waines JG (2012) Root system plasticity to drought influences grain yield in bread wheat. Euphytica 186(1):219–232
Farooq M, Hussain M, Siddique KH (2014) Drought stress in wheat during flowering and grain-filling periods. Crit Rev Plant Sci 33(4):331–349
Gebbing T (2003) The enclosed and exposed part of the peduncle of wheat (Triticum aestivum)–spatial separation of fructan storage. New Phytol 159(1):245–252
Gonçalves S, Cairney J, Maroco J, Oliveira MM, Miguel C (2005) Evaluation of control transcripts in real-time RT-PCR expression analysis during maritime pine embryogenesis. Planta 222(3):556–563
Gupta AK, Kaur K, Kaur N (2011) Stem reserve mobilization and sink activity in wheat under drought conditions. Am J Plant Sci 2(01):70–77
Hajirezaei MR, Takahata Y, Trethewey RN, Willmitzer L, Sonnewald U (2000) Impact of elevated cytosolic and apoplastic invertase activity on carbon metabolism during potato tuber development. J Exp Bot 51(suppl 1):439–445
Hörtensteiner S, Feller U (2002) Nitrogen metabolism and remobilization during senescence. J Exp Bot 53(370):927–937
Jagadish KS, Kavi Kishor PB, Bahuguna RN, von Wiren N, Sreenivasulu N (2015) Staying alive or going to die during terminal senescence-an enigma surrounding yield stability. Front Plant Sci 6:1070. https://doi.org/10.3389/fpls.2015.01070
Joudi M, Ahmadi A, Mohamadi V, Abbasi A, Vergauwen R, Mohammadi H, Van den Ende W (2012) Comparison of fructan dynamics in two wheat cultivars with different capacities of accumulation and remobilization under drought stress. Physiol Plant 144(1):1–12
Khoshro HH, Taleei A, Bihamta MR, Shahbazi M, Abbasi A, Ramezanpour SS (2014) Expression analysis of the genes involved in accumulation and remobilization of assimilates in wheat stem under terminal drought stress. Plant Growth Regul 74(2):165–176. https://doi.org/10.1007/s10725-014-9908-x
Königshofer H, Löppert H-G (2015) Regulation of invertase activity in different root zones of wheat (Triticum aestivum L.) seedlings in the course of osmotic adjustment under water deficit conditions. J Plant Physiol 183:130–137
Kordenaeej A, Nasrollah-Nejad A, Shojaeian A, Lelley T (2008) Mapping QTLs related to yield and yield components under drought in bread wheat. In: Appels R, Eastwood R, Lagudah E, Langridge P, Lynne MM (eds) The 11th international wheat genetics symposium proceedings. Sydney University Press, Sydney
Kumar RR, Goswami S, Singh K, Dubey K, Singh S, Sharma R, Verma N, Kala YK, Rai GK, Grover M, Mishra DC, Singh B, Pathak H, Chinnusamy V, Rai A, Praveen S (2016) Identification of putative RuBisCo activase (TaRca1)—the catalytic chaperone regulating carbon assimilatory pathway in wheat (Triticum aestivum) under the heat stress. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00986
Lawlor DW, Paul MJ (2014) Source/sink interactions underpin crop yield: the case for trehalose 6-phosphate/SnRK1 in improvement of wheat. Front Plant Sci 5:418
Lemoine R, La Camera S, Atanassova R, Dédaldéchamp F, Allario T, Pourtau N, Bonnemain J-L, Laloi M, Coutos-Thévenot P, Maurousset L (2013) Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci 4:272
Lichtenthaler HK (1987) Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J Plant Physiol 131(1–2):101–110
Lopes MS, Reynolds MP (2010) Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Funct Plant Biol 37(2):147–156
Manschadi AM, Christopher J, deVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Biol 33(9):823–837. https://doi.org/10.1071/FP06055
Nakhforoosh A, Grausgruber H, Kaul H-P, Bodner G (2014) Wheat root diversity and root functional characterization. Plant Soil 380(1–2):211–229
Palta JA, Chen X, Milroy SP, Rebetzke GJ, Dreccer MF, Watt M (2011) Large root systems: are they useful in adapting wheat to dry environments? Funct Plant Biol 38(5):347–354
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29(9):e45–e45
Ritsema T, Smeekens SC (2003) Engineering fructan metabolism in plants. J Plant Physiol 160(7):811–820
Ruan Y-L (2014) Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol 65:33–67
Ruuska SA, Rebetzke GJ, van Herwaarden AF, Richards RA, Fettell NA, Tabe L, Jenkins CL (2006) Genotypic variation in water-soluble carbohydrate accumulation in wheat. Funct Plant Biol 33(9):799–809
Scofield GN, Hirose T, Aoki N, Furbank RT (2007) Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J Exp Bot 58(12):3155–3169. https://doi.org/10.1093/jxb/erm153
Sharbatkhari M, Shobbar Z-S, Galeshi S, Nakhoda B (2016) Wheat stem reserves and salinity tolerance: molecular dissection of fructan biosynthesis and remobilization to grains. Planta 244(1):191–202
Suzuki Y, Makino A, Mae T (2001) Changes in the turnover of Rubisco and levels of mRNAs of rbcL and rbcS in rice leaves from emergence to senescence. Plant Cell Environ 24(12):1353–1360
Tauzin AS, Sulzenbacher G, Lafond M, Desseaux V, Reca IB, Perrier J, Bellincampi D, Fourquet P, Lévêque C, Giardina T (2014) Functional characterization of a vacuolar invertase from Solanum lycopersicum: post-translational regulation by N-glycosylation and a proteinaceous inhibitor. Biochimie 101:39–49
Valluru R, Van den Ende W (2008) Plant fructans in stress environments: emerging concepts and future prospects. J Exp Bot 59(11):2905–2916
Van den Ende W, El-Esawe SK (2014) Sucrose signaling pathways leading to fructan and anthocyanin accumulation: a dual function in abiotic and biotic stress responses? Environ Exper Bot 108:4–13
Van den Ende W, Clerens S, Vergauwen R, Van Riet L, Van Laere A, Yoshida M, Kawakami A (2003) Fructan 1-exohydrolases. β-(2,1)-trimmers during graminan biosynthesis in stems of wheat? Purification, characterization, mass mapping, and cloning of two fructan 1-exohydrolase isoforms. Plant Physiol 131(2):621–631
Van Riet L, Nagaraj V, Van den Ende W, Clerens S, Wiemken A, Van Laere A (2006) Purification, cloning and functional characterization of a fructan 6-exohydrolase from wheat (Triticum aestivum L.). J Exp Bot 57(1):213–223
Verspreet J, Cimini S, Vergauwen R, Dornez E, Locato V, Le Roy K, De Gara L, Van den Ende W, Delcour JA, Courtin CM (2013) Fructan metabolism in developing wheat (Triticum aestivum L.) kernels. Plant Cell Physiol 54(12):2047–2057
Wang W, Hao Q, Tian F, Li Q, Wang W (2016) Cytokinin-regulated sucrose metabolism in stay-green wheat phenotype. PLoS ONE 11(8):e0161351. https://doi.org/10.1371/journal.pone.0161351
Wardlaw I, Willenbrink J (2000) Mobilization of fructan reserves and changes in enzyme activities in wheat stems correlate with water stress during kernel filling. New Phytol 148(3):413–422
Wasson A, Richards R, Chatrath R, Misra S, Prasad SS, Rebetzke G, Kirkegaard J, Christopher J, Watt M (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63(9):3485–3498
Xue G-P, McIntyre CL, Glassop D, Shorter R (2008a) Use of expression analysis to dissect alterations in carbohydrate metabolism in wheat leaves during drought stress. Plant Mol Biol 67(3):197–214. https://doi.org/10.1007/s11103-008-9311-y
Xue GP, McIntyre CL, Jenkins CLD, Glassop D, van Herwaarden AF, Shorter R (2008b) Molecular dissection of variation in carbohydrate metabolism related to water-soluble carbohydrate accumulation in stems of wheat. Plant Physiol 146(2):441–454. https://doi.org/10.1104/pp.107.113076
Xue G-P, McIntyre CL, Rattey AR, van Herwaarden AF, Shorter R (2009) Use of dry matter content as a rapid and low-cost estimate for ranking genotypic differences in water-soluble carbohydrate concentrations in the stem and leaf sheath of Triticum aestivum. Crop Pasture Sci 60(1):51–59
Xue G-P, Drenth J, Glassop D, Kooiker M, McIntyre CL (2013) Dissecting the molecular basis of the contribution of source strength to high fructan accumulation in wheat. Plant Mol Biol 81(1–2):71–92. https://doi.org/10.1007/s11103-012-9983-1
Yang J, Zhang J (2006) Grain filling of cereals under soil drying. New Phytol 169(2):223–236
Yang J, Zhang J, Huang Z, Zhu Q, Wang L (2000) Remobilization of carbon reserves is improved by controlled soil-drying during grain filling of wheat. Crop Sci 40(6):1645–1655
Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2004) Activities of fructan- and sucrose-metabolizing enzymes in wheat stems subjected to water stress during grain filling. Planta 220(2):331–343. https://doi.org/10.1007/s00425-004-1338-y
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14(6):415–421
Zhang J, Huang S, Fosu-Nyarko J, Dell B, McNeil M, Waters I, Moolhuijzen P, Conocono E, Appels R (2008) The genome structure of the 1-FEH genes in wheat (Triticum aestivum L.): new markers to track stem carbohydrates and grain filling QTLs in breeding. Mol Breed 22(3):339–351
Zhang J, Dell B, Conocono E, Waters I, Setter T, Appels R (2009) Water deficits in wheat: fructan exohydrolase (1-FEH) mRNA expression and relationship to soluble carbohydrate concentrations in two varieties. New Phytol 181(4):843–850. https://doi.org/10.1111/j.1469-8137.2008.02713.x
Zhang J, Chen W, Dell B, Vergauwen R, Zhang X, Mayer JE, Van den Ende W (2015) Wheat genotypic variation in dynamic fluxes of WSC components in different stem segments under drought during grain filling. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00624
Zhang J, Dell B, Ma W, Vergauwen R, Zhang X, Oteri T, Foreman A, Laird D, Van den Ende W (2016) Contributions of root WSC during grain filling in wheat under drought. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00904
Acknowledgements
This study has been supported by Gorgan University of Agricultural Sciences and Natural Resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bagherikia, S., Pahlevani, M., Yamchi, A. et al. Transcript Profiling of Genes Encoding Fructan and Sucrose Metabolism in Wheat Under Terminal Drought Stress. J Plant Growth Regul 38, 148–163 (2019). https://doi.org/10.1007/s00344-018-9822-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9822-y