Abstract
Background
Transsphenoidal selective adenomectomy is the first-line treatment for Cushing’s disease. At experienced centers, early remission rates after transsphenoidal surgery range from 65 to 98 %, however disease relapse frequently occurs with rates ranging from 2 to 35 % at long-term follow up.
Methods
This article discusses recently reported studies on the surgical outcomes from transsphenoidal surgery for Cushing's disease.
Conclusions
One of the keys to a successful long-term surgical outcome is meticulous dissection using the adenoma’s pseudocapsule as a surgical plane for complete resection. MRI-negative and invasive ACTH-secreting adenomas pose particular challenges for pituitary surgeons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cushing’s disease remission and relapse after transsphenoidal surgery
Consistent diagnosis and successful treatment of ACTH-secreting pituitary adenomas associated with Cushing’s disease remains a challenge for neuro-endocrinologists and pituitary surgeons. Unsuccessful treatment leads to increased morbidity, a higher mortality rate, and poor quality of life among patients with Cushing’s disease [1–3].
Once the diagnosis has been firmly established, selective adenomectomy via a transsphenoidal approach is the treatment of choice for a vast majority of patients with Cushing’s disease. Early surgical remission rates after transsphenoidal surgery range from 65 to 98 % at experienced centers, however as many as 20–35 % of patients with Cushing’s disease are not in remission after surgery, leaving these patients at risk to the detrimental effects of persistent hypercortisolemia [4–10].
Failure to achieve remission after selective adenomectomy is caused by residual adenoma. This can occur after an incomplete resection of an invasive or large tumor, incorrect removal of a secondary, incidental, lesion instead of the ACTH-secreting adenoma, extrapituitary or parasellar adenomas, or inability to find the adenoma during careful exploration of the pituitary gland [11]. In these instances, early repeat surgery may be performed to re-explore previously unexplored portions of the pituitary gland in an attempt to identify and remove the ACTH-producing adenoma [12–16]. If none is found, the surgeon may elect to perform a partial or total hypophesectomy, often with favorable outcomes.
Although transsphenoidal surgery is effective in attaining and sustaining disease remission in a majority of cases, disease relapse is reported in 2–35 % of patients [6–10, 17, 18]. The variation of reported remission and relapse rates is clearly a result of the varying definitions used to define remission and length of follow up. For instance, Patil et al. [18] describe recurrences in 26 % of patients with 2 year follow up, 33 % at 3 years, and 46 % at 5 years. Over the past two years several large series have been published with long durations of follow up, some of which have reported much higher rates of disease relapse than prior studies (see Table 1) [19–29].
In the largest of recent case series, Lambert et al. [26] studied the long-term outcomes in 257 patients with Cushing’s disease treated by transsphenoidal surgery. Follow up times ranged from 1 month to 30 years with an average time of 6.3 years. Immediate remission with hypocortisolism was noted in 76 % of patients, delayed remission (which included remission after Gamma Knife, adrenalectomy, etc.) in 13.6 %, and persistent disease in 10.5 %. Overall disease relapse occurred in 21 % of the patients, with relapses occurring in 10.8 and 57.1 % of patients with postoperative hypocortisolism and delayed remission, respectively [26]. Alexandraki et al. [20] studied the long-term outcomes of 124 patients with Cushing’s disease treated with transsphenoidal surgery with a minimum of 6 years follow up (range 6–29 years). They report an overall early remission in 68 % of patients, and there was a 24 % rate of disease relapse; this included recurrence in 15 % of the patients with hypocortisolim immediately after surgery using stringent criteria (serum cortisol <50 nmol/l; 1.8 µg/dl) [20].
Dimopoulou et al. [24] studied the outcomes of 120 patients who had first-time surgery and 36 patients who had revision surgery at two institutions in Munich, Germany with a mean follow up time of 79 months. The remission rates for patients having first time surgery and repeat surgery were 71 and 42 %, respectively. Among patients with remission after initial surgery, 76 % (65/85 patients) had early postoperative hypocortisolism (serum cortisol <5 µg/dl). These patients were 0.7 times less likely to have disease recurrence compared to patients with early postoperative eucortisolism. Relapse rates were 34 and 58 % for first-time and repeat surgery, respectively with an average time to relapse of 54 months (range 5–205 months) [24]. Aranda et al. [21] reported rate of relapse at 65.6 % in a 41 patient study with an average follow up of 14 years, which is much higher than previous reports. The average time to relapse was 2.4 years (range 0.5–5 years).
Among the aforementioned studies, the authors and others have attempted to identify early postoperative predictors of sustained remission and disease relapse. The best early predictor of successful surgery is hypocortisolemia in the first few days following surgery, with serum cortisol values <5 μg/dl predicting remission and profound hypocortisolism (<1–2 μg/dl) likely being highly predictive for sustained remission, although Lindsay et al. [30] reported no statistical difference in the rate of recurrence in patients with postoperative AM cortisol nadir of <1 μg/dl, <2 ng/ml, or <5 ng/ml). Note that the summary material in Table 1 suggests that the higher relapse rates tend to occur in the series with a greater fraction of patients with eucortisolism in the early postoperative interval, as well as with longer follow up. Other studies have found that prolonged hypocortisolism without recovery of the HPA axis as a good indicator of remission, although many of these patients may also have panhypopituitarism. Moreover, although identifying early predictors of surgical success has been the focus of many studies, disease relapse also occurs in patients with undetectable plasma cortisol levels after surgery and detection and treatment of it is a significant factor in postoperative patient care. All patients with Cushing’s disease need prolonged postoperative assessment by an endocrinologist.
Most disease relapses occur within the first 5 years, although relapse has been reported up to 30 years after initial surgery [21, 24, 31]. Most relapse can be attributable to microscopic residual left at the adenoma margin or unrecognized microscopic dural invasion along the wall of the cavernous sinus [11]. In a study that compared the location of adenomas in patients with relapsed Cushing’s disease, all recurrent tumors were found at the same site of the adenoma at the initial operation or within the adjacent dura, usually in the wall of the cavernous sinus [11]. Furthermore, a high percentage of these recurrent adenomas had surgical evidence of dural invasion that was unrecognized during the initial surgery. Repeat transsphenoidal surgery is effective in obtaining remission in 61–73 % of patients with disease relapse, which is slightly lower than remission rates after initial surgery. Friedman et al. [32] studied the outcomes of 31 patients who had recurrent Cushing’s disease after transsphenoidal surgery and found that 73 % had remission after repeat transsphenoidal surgery. Patil et al. [33] conducted a similar study in 36 patients with recurrent Cushing’s disease and obtained remission in 22 (61 %) with repeat surgery. In these cases, careful patient selection is required for optimal outcomes.
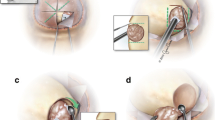
The histological pseudocapsule for adenoma resection
Careful pituitary exploration, adenoma dissection and complete removal are essential for optimal short and long-term outcomes. Over the last 20 years there has been an emphasis on the method for surgical approach to the sella in the pituitary surgery literature with the development of endoscopic techniques. However, few studies describe and report outcomes of pituitary surgery that focus on the method for adenoma removal, the critical portion of the operation [34–36].
The anterior pituitary gland is covered by a thin, durable capsule, which separates the gland from the adjacent dura of the sella and cavernous sinuses. The parenchyma of the anterior gland is composed of cells arranged in cords or acini. The intervening stromal tissue contains a rich network of blood vessels and supporting structural elements including collagen and reticulin. This structural arrangement gives the anterior pituitary gland a firm texture, compared to the adjacent posterior pituitary gland, which does not contain a dense collagen matrix for support.
Pituitary adenomas originate from the proliferation of a single abnormal cell without the formation of normal intervening structural support. Thus, pituitary adenomas tend to have a soft consistency. During adenoma growth, the normal pituitary tissue is compressed and displaced, leading to an interface between the adenoma and normal gland that is comprised of compressed normal gland that contains collagen and reticulin [36]. This results in the formation of an envelope around the adenoma termed the histological pseudocapsule.
An important surgical advance for Cushing’s disease came with the recognition that the adenoma’s pseudocapsule could be identified during careful dissection and used as a surgical plane to remove adenomas [36]. This surgical method has several advantages. First, it allows complete adenoma resection and avoids possible incomplete resection using a piecemeal approach. Second it allows the surgeon to carefully inspect the adenoma borders and appreciate areas of invasion into the pituitary capsule and adjacent dura. Finally, this method affords maximal preservation of the remaining normal pituitary gland. The use of the pseudocapsule as a surgical plane can be applied to adenomas of all sizes except for very small tumors. In adenomas <1 mm in size, there appears to be little compression of the surrounding gland, however with adenomas as small as 2–3 mm in size there is sufficient compression of adjacent normal pituitary gland to form a histological pseudocapsule [36].
One of us (EHO) has focused on the use of the histological pseudocapsule as a surgical capsule for selective adenomectomy since the early 1980s, and has previously reported the long-term results using the histological pseudocapsule as a surgical plane to remove intrasellar adenomas in Cushing’s disease. In this study, all 261 patients with adenomas identified at surgery and in whom the adenoma was contained within the pituitary gland had reversal of hypercortisolism [256 (98 %) with hypocortisolism, 5 with eucortisolism] at hospital discharge, which included a large number of patients with MRI negative imaging (48 %) [8]. During the hospitalization 9 of these patients underwent early re-operation for persistent hyercortisolemia. There were only 6 patients (2 %) with disease relapse at an average interval of 56 months during a mean overall follow up of 84 months.
Following complete resection of an adenoma using this method, patients become hypocortisolemic 19.4 h after surgery on average. This rate of decline in serum cortisol level is significantly faster than the rate occurring in patients who received incomplete resections or resections using a piecemeal method [37]. This supports the idea that dissection and adenoma removal using the pseudocapsular method enables complete adenoma removal, including microscopic disease in the tumor envelope that could otherwise be potentially left behind and contribute to disease relapse.
Challenges in pituitary surgery for Cushing’s disease
Negative preoperative MRI
Among patients with microadenomas that are detected on preoperative imaging and are contained within the pituitary gland, the remission rate following surgery is high [8, 10]. However, Cushing’s disease can be caused by very small adenomas that can be undetectable on even the most sensitive preoperative MRIs [38, 39]. Reported remission rates are lower for MRI-negative microadenomas [27, 28, 40]. One step that can be taken to enhance the likelihood of identifying the site of the adenoma before surgery is the use of high resolution, thin cut MRI, which demonstrates the adenoma in some patients despite negative conventional MRI [39, 41]. These cases present a unique challenge for pituitary surgeons, since there is no clear surgical target to guide dissection [35]. Inferior petrosal sinus sampling for ACTH gradients are commonly used in patients with suspected Cushing’s disease and negative pituitary MR imaging to establish the diagnosis [42], although this technique is not reliable for predicting the location of the adenoma within the sella [38, 43]. This circumstance requires a careful exploration of the pituitary gland until an adenoma is found, the entire gland if necessary. Furthermore, some small ACTH-secreting pituitary adenomas can present in unusual locations outside the sella, including the sphenoid sinus mucosa, within the cavernous sinus, or within the posterior pituitary gland [44–46].
In cases where an adenoma is not found after careful exploration of the entire anterior pituitary gland, surgeons must decide whether to proceed with total or partial hypophysecotomy. Disease remission occurs in 60–75 % of patients who receive total or partial hypophysectomies, although essentially all these patients have panhypopituitarism after surgery. Since hemihypophysectomy (favoring the size of highest IPSS gradient) or subtotal hypophysectomy (where 30 % of the gland is removed from either side and 20 % is removed from the inferior aspect, leaving 20–30 % attached to the pituitary stalk), sparing a remnant of normal pituitary gland attached to the stalk, the rate of postoperative pituitary insufficiency is only 15–20 % (personal experience), which is much lower than total hypophesectomy.
Invasion
Invasive macradenomas that cause Cushing’s disease are also a challenge for pituitary surgeons [47]. Several studies have reported adenoma size as a factor in achieving surgical remission, and remission rates are lower invasive macroadenomas [9, 48]. Dural invasion is higher with larger tumors; the challenge with larger tumors may be a product of the invasion, rather than tumor size. Dural invasion of ACTH-secreting adenomas is likely underappreciated. In a prospective study, Lonser et al. [49] studied a consecutive series of eighty-seven patients with Cushing’s disease who underwent surgery. Among this group, 34 % of patients had histologically confirmed dural invasion [49]. In this study, there was a correlation between adenoma size and dural invasion with larger tumors more frequently invading adjacent dura [49].
Among adenomas whose invasion is limited to the dura of the medial wall of the cavernous sinus, a complete resection of all microscopic adenoma is feasible by excision of the involved dura, and there is a high rate of remission [29, 47, 49]. However, if an adenoma invades beyond the medial wall to involve structures within the cavernous sinus, a complete resection of all microscopic disease with cure is unlikely [47]. A thorough debulking of the adenoma within the cavernous sinus may lead to early disease remission, but long-term surgical remission seems unlikely without further treatment with radiosurgery.
Conclusions
Transsphenoidal surgery is an effective, definitive treatment for Cushing’s disease. However, not all patients are in remission after surgery and others experience disease relapse after some time of remission. Careful pituitary exploration in a bloodless operative field, identification of the tumor, and complete removal, often by taking advantage of the tissue envelope surrounding the tumor provided by the histological pseudocapsule, are essential for optimal surgical outcomes. Patients with negative MRI and invasive adenomas remain challenging patients for surgical success.
References
Clayton RN, Raskauskiene D, Reulen RC, Jones PW (2011) Mortality and morbidity in Cushing’s disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab 96(3):632–642
Dekkers OM, Biermasz NR, Pereira AM et al (2007) Mortality in patients treated for Cushing’s disease is increased, compared with patients treated for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab 92(3):976–981
Swearingen B, Biller BM, Barker FG II et al (1999) Long-term mortality after transsphenoidal surgery for Cushing disease. Ann Intern Med 130(10):821–824
Chen JC, Amar AP, Choi S, Singer P, Couldwell WT, Weiss MH (2003) Transsphenoidal microsurgical treatment of Cushing disease: postoperative assessment of surgical efficacy by application of an overnight low-dose dexamethasone suppression test. J Neurosurg 98(5):967–973
Ciric I, Zhao JC, Du H et al (2012) Transsphenoidal surgery for Cushing disease: experience with 136 patients. Neurosurgery 70(1):70–80 (discussion 80–71)
Hammer GD, Tyrrell JB, Lamborn KR et al (2004) Transsphenoidal microsurgery for Cushing’s disease: initial outcome and long-term results. J Clin Endocrinol Metab 89(12):6348–6357
Hofmann BM, Hlavac M, Martinez R, Buchfelder M, Muller OA, Fahlbusch R (2008) Long-term results after microsurgery for Cushing disease: experience with 426 primary operations over 35 years. J Neurosurg 108(1):9–18
Jagannathan J, Smith R, DeVroom HL et al (2009) Outcome of using the histological pseudocapsule as a surgical capsule in Cushing disease. J Neurosurg 111(3):531–539
Pouratian N, Prevedello DM, Jagannathan J, Lopes MB, Vance ML, Laws ER Jr (2007) Outcomes and management of patients with Cushing’s disease without pathological confirmation of tumor resection after transsphenoidal surgery. J Clin Endocrinol Metab 92(9):3383–3388
Prevedello DM, Pouratian N, Sherman J et al (2008) Management of Cushing’s disease: outcome in patients with microadenoma detected on pituitary magnetic resonance imaging. J Neurosurg 109(4):751–759
Dickerman RD, Oldfield EH (2002) Basis of persistent and recurrent Cushing disease: an analysis of findings at repeated pituitary surgery. J Neurosurg 97(6):1343–1349
Bertagna X, Guignat L (2013) Approach to the Cushing’s disease patient with persistent/recurrent hypercortisolism after pituitary surgery. J Clin Endocrinol Metab 98(4):1307–1318
Friedman RB, Oldfield EH, Nieman LK et al (1989) Repeat transsphenoidal surgery for Cushing’s disease. J Neurosurg 71(4):520–527
McLaughlin N, Kassam AB, Prevedello DM, Kelly DF (2011) Management of Cushing’s disease after failed surgery—a review. Can J Neurol Sci 38(1):12–21
Ram Z, Nieman LK, Cutler GB Jr, Chrousos GP, Doppman JL, Oldfield EH (1994) Early repeat surgery for persistent Cushing’s disease. J Neurosurg 80(1):37–45
Valderrabano P, Aller J, Garcia-Valdecasas L et al (2014) Results of repeated transsphenoidal surgery in Cushing’s disease. Long-term follow-up. Endocrinol Nutr 61(4):176–183
Shimon I, Ram Z, Cohen ZR, Hadani M (2002) Transsphenoidal surgery for Cushing’s disease: endocrinological follow-up monitoring of 82 patients. Neurosurgery 51(1):57–61 (discussion 61–52)
Patil CG, Prevedello DM, Lad SP et al (2008) Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab 93(2):358–362
Alahmadi H, Cusimano MD, Woo K et al (2013) Impact of technique on cushing disease outcome using strict remission criteria. Can J Neurol Sci 40(3):334–341
Alexandraki KI, Kaltsas GA, Isidori AM et al (2013) Long-term remission and recurrence rates in Cushing’s disease: predictive factors in a single-centre study. Eur J Endocrinol 68(4):639–648
Aranda G, Ensenat J, Mora M et al (2015) Long-term remission and recurrence rate in a cohort of Cushing’s disease: the need for long-term follow-up. Pituitary 18(1):142–149
Berker M, Isikay I, Berker D, Bayraktar M, Gurlek A (2013) Early promising results for the endoscopic surgical treatment of Cushing’s disease. Neurosurg Rev (Epub ahead of print; PMID: 24233258)
Costenaro F, Rodrigues TC, Rollin GA, Ferreira NP, Czepielewski MA (2014) Evaluation of Cushing’s disease remission after transsphenoidal surgery based on early serum cortisol dynamics. Clin Endocrinol 80(3):411–418
Dimopoulou C, Schopohl J, Rachinger W, Buchfelder M, Honegger J, Reincke M, Stalla GK (2013) Long-term remission and recurrence rates after first and second transsphenoidal surgery for Cushing’s disease: care reality in the Munich Metropolitan Region. Eur J Endocrinol 170(2):283–292
Hameed N, Yedinak CG, Brzana J et al (2013) Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: a large single center experience. Pituitary 16(4):452–458
Lambert JK, Goldberg L, Fayngold S, Kostadinov J, Post KD, Geer EB (2013) Predictors of mortality and long-term outcomes in treated Cushing’s disease: a study of 346 patients. J Clin Endocrinol Metab 98(3):1022–1030
Starke RM, Reames DL, Chen CJ, Laws ER, Jane JA Jr (2013) Endoscopic transsphenoidal surgery for cushing disease: techniques, outcomes, and predictors of remission. Neurosurgery 72(2):240–247 (discussion 247)
Wagenmakers MA, Boogaarts HD, Roerink SH et al (2013) Endoscopic transsphenoidal pituitary surgery: a good and safe primary treatment option for Cushing’s disease, even in case of macroadenomas or invasive adenomas. Eur J Endocrinol 169(3):329–337
Lonser RR, Wind JJ, Nieman LK, Weil RJ, DeVroom HL, Oldfield EH (2013) Outcome of surgical treatment of 200 children with Cushing’s disease. J Clin Endocrinol Metabol 98(3):892–901
Lindsay JR, Oldfield EH, Stratakis CA, Nieman LK (2011) The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing’s disease after transsphenoidal surgery. J Clin Endocrinol Metab 96(7):2057–2064
Nakane T, Kuwayama A, Watanabe M et al (1987) Long term results of transsphenoidal adenomectomy in patients with Cushing’s disease. Neurosurgery 21(2):218–222
Friedman RB, Oldfield EH, Nieman LK, Chrousos GP, Doppman JL, Cutler GB Jr, Loriaux DL (1989) Repeat transsphenoidal surgery for Cushing’s disease. J Neurosurg 71(4):520–527
Patil CG, Veeravagu A, Prevedello DM, Katznelson L, Vance ML, Laws ER Jr (2008) Outcomes after repeat transsphenoidal surgery for recurrent Cushing’s disease. Neurosurgery 63(2):266–270 (discussion 270–271)
Kawamata T, Kubo O, Hori T (2005) Surgical removal of growth hormone-secreting pituitary adenomas with intensive microsurgical pseudocapsule resection results in complete remission of acromegaly. Neurosurg Rev 28(3):201–208
Oldfield EH (2011) Surgical management of Cushing’s disease: a personal perspective. Clin Neurosurg 58:13–26
Oldfield EH, Vortmeyer AO (2006) Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg 104(1):7–19
Monteith SJ, Starke RM, Jane JA Jr, Oldfield EH (2012) Use of the histological pseudocapsule in surgery for Cushing disease: rapid postoperative cortisol decline predicting complete tumor resection. J Neurosurg 116(4):721–727
Kasaliwal R, Sankhe SS, Lila AR et al (2013) Volume interpolated 3D-spoiled gradient echo sequence is better than dynamic contrast spin echo sequence for MRI detection of corticotropin secreting pituitary microadenomas. Clin Endocrinol 78(6):825–830
Patronas N, Bulakbasi N, Stratakis CA et al (2003) Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab 88(4):1565–1569
Yamada S, Fukuhara N, Nishioka H et al (2012) Surgical management and outcomes in patients with Cushing disease with negative pituitary magnetic resonance imaging. World Neurosurg 77(3–4):525–532
Batista D, Courkoutsakis NA, Oldfield EH et al (2005) Detection of adrenocorticotropin-secreting pituitary adenomas by magnetic resonance imaging in children and adolescents with cushing disease. J Clin Endocrinol Metab 90(9):5134–5140
Oldfield EH, Doppman JL, Nieman LK et al (1991) Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med 325(13):897–905
Wind JJ, Lonser RR, Nieman LK, DeVroom HL, Chang R, Oldfield EH (2013) The lateralization accuracy of inferior petrosal sinus sampling in 501 patients with Cushing’s disease. J Clin Endocrinol Metab 98(6):2285–2293
Oldfield EH, Vance ML (2013) A cryptic cause of Cushing’s disease. J Clin Endocrinol Metab 98(12):4593–4594
Pluta RM, Nieman L, Doppman JL et al (1999) Extrapituitary parasellar microadenoma in Cushing’s disease. J Clin Endocrinol Metab 84(8):2912–2923
Weil RJ, Vortmeyer AO, Nieman LK, Devroom HL, Wanebo J, Oldfield EH (2006) Surgical remission of pituitary adenomas confined to the neurohypophysis in Cushing’s disease. J Clin Endocrinol Metab 91(7):2656–2664
Oldfield EH (2014) Editorial: Management of invasion by pituitary adenomas. J Neurosurg 121(3):501–503
Kakade HR, Kasaliwal R, Khadilkar KS et al (2014) Clinical, biochemical and imaging characteristics of Cushing’s macroadenomas and their long-term treatment outcome. Clin Endocrinol 81(3):336–342
Lonser RR, Ksendzovsky A, Wind JJ, Vortmeyer AO, Oldfield EH (2012) Prospective evaluation of the characteristics and incidence of adenoma-associated dural invasion in Cushing disease. J Neurosurg 116(2):272–279
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dallapiazza, R.F., Oldfield, E.H. & Jane, J.A. Surgical management of Cushing’s disease. Pituitary 18, 211–216 (2015). https://doi.org/10.1007/s11102-015-0646-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-015-0646-5