Abstract
Postoperative serum cortisol is used as an indicator of Cushing’s disease (CD) remission following transsphenoidal surgery (TSS) and guides (controversially) the need for immediate adjuvant treatment for CD. We investigated postoperative cortisol and adrenocorticotropic hormone (ACTH) levels as predictors of remission/recurrence in CD in a large retrospective cohort of patients with pathologically confirmed CD, over 6 years at a single institution. Midnight and morning cortisol, and ACTH at 24–48 h postoperatively (>24 h after last hydrocortisone dose) were measured. Remission was defined as normal 24-h urine free cortisol, normal midnight salivary cortisol, a normal dexamethasone-corticotropin releasing hormone (CRH) test or continued need for hydrocortisone, assessed periodically. Statistical analysis was performed using PASW 18. Follow up data was available for 52 patients (38 females and 14 males), median follow up was 16.5 month (range 2–143 months), median age was 45 years (range 21–72 years), 28 tumors were microadenomas and 16 were macroadenomas, and in eight cases no tumor was observed on magnetic resonance imaging. No patient with postoperative cortisol levels >10 mcg/dl were found to be in remission. Ten of the 52 patients with cortisol >10 mcg/dl by postoperative day 1–2 underwent a second TSS within 7 days. Forty-three patients (82.7 %) achieved CD remission (36 after one TSS and 7 after a second early TSS) and six patients suffered disease recurrence (mean 39.2 ± 52.4 months). An immediate second TSS induced additional hormonal deficiencies (diabetes insipidus) in three patients with no surgical complications. Persistent disease was noted in nine patients despite three patients having an immediate second TSS. Positive predictive value for remission of cortisol <2 mcg/dl and ACTH <5 pg/ml was 100 %. Cortisol and ACTH levels (at all postoperative time points and at 2 months) were correlated (r = 0.37, P < 0.001). Nadir serum cortisol of ≤2 mcg/dl and ACTH <5 pg/ml predicted remission (P < 0.005), but no level predicted lack of recurrence. Immediate postoperative ACTH/cortisol did not predict length of remission. No patients with postoperative cortisol >10 mcg/dl were observed to have delayed remission; all required additional treatment. There was no significant difference in age, body mass index, tumor size and length of follow-up between postoperative cortisol groups: cortisol ≤2 mcg/dl, cortisol >5 mcg/dl and cortisol >10 mcg/dl. Immediate postoperative cortisol levels should routinely be obtained in CD patients post TSS, until better tools to identify early remission are available. Immediate repeat TSS could be beneficial in patients with cortisol >10 mcg/dl and positive CD pathology: our combined (micro- and macroadenomas) remission rate with this approach was 82.7 %. ACTH measurements correlate well with cortisol. However, because no single cortisol or ACTH cutoff value excludes all recurrences, patients require long-term clinical and biochemical follow-up. Further research is needed in this area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cushing’s disease (CD) represents hypercortisolism caused by an adrenocorticotropic hormone (ACTH)-secreting pituitary adenoma [1, 2]. Prevalence is estimated at 2.4 cases per million inhabitants with an incidence of 1.2–2.4 cases per million per year [3]. The primary modality for definitive treatment is transsphenoidal surgery (TSS) [1, 3–7]. If surgery is unsuccessful or hypercortisolism recurs, second line treatment options include repeat surgery, medical therapy, radiation, bilateral adrenalectomy or a combination of these options [4, 8, 9].
The consequences of persistent hypercortisolism are severe and include immunosuppression, poor wound healing, diabetes, high blood pressure, cardiac insufficiency, severe osteoporosis, and increased mortality [3, 10]. Hence, early identification of patients at risk of treatment failure is exigent.
Diagnosing persistent or recurrent CD is perhaps even more challenging than making the initial CD diagnosis. Several investigators have recommended serial measurement of serum cortisol in the days after pituitary surgery to identify patients with persistent CD [5, 7, 11]. Subnormal or undetectable levels of serum cortisol in the immediate postoperative period have been suggested as predictive of long-term remission [1, 12], however, this has not been confirmed in subsequent studies [13]. Other investigators have suggested that normalization of 24 h urine free cortisol (UFC), normalization of circadian rhythm, suppressibility to dexamethasone [14] corticotropin releasing hormone (CRH) tests [5] or ACTH stimulation tests [15, 16] could be used to determine remission. To date, no consensus has been established. Remission after an initial TSS has been reported to occur in anywhere from 50 to 94 % of subjects [1, 3, 4, 6, 17] with long term relapse rates of up to 46 % [5]. Variability relates to neurosurgical expertise, size and location of tumor, methodology and the biochemical criteria used in postoperative assessments, as well as remission definition and duration of follow up [1, 4].
If a first surgery fails, the choice of second-line therapy, particularly repeat TSS, remains controversial. Repeat surgery can be successful when residual tumor is detectable on magnetic resonance (MR) imaging and is supported by low morbidity (1.8 %) and mortality (<1 %) rates [1]. Recommendations regarding the ideal timing of repeat surgery vary significantly, in the literature [1, 18, 19]. Re-exploration of the sella within 15 days after the primary procedure has been advocated by a number of pituitary centers [1, 18–21]. Early surgery is reported to allow the surgeon to re-explore the sella with minimal additional trauma and no major concerns about altered surgical anatomy related to scar tissue formation [1, 18–20]. Conversely, it has been suggested that hormonal assessment in the immediate TSS postoperative period for CD may be misleading because delayed remission can occur in a subset of patients [6]. However, because there is no consensus regarding the optimal strategy for determination of immediate postoperative remission nor a definition of remission, direct comparisons between centers remains challenging [5].
In this study we aimed to assess clinical outcomes using repeat surgery in the immediate postoperative period (within 1 week post initial TSS) in patients with pathologically confirmed CD. We also assessed the value of early postoperative cortisol and ACTH levels as predictors of remission versus recurrence.
Patients and methods
Patients
We conducted a retrospective cross-sectional chart review of all patients with pathologically confirmed CD who underwent TSS from 2006 to 2011 at Oregon Health & Science University (OHSU). The study was approved by the OHSU Institutional Review Board. Reticulin staining confirmed positive adenoma pathology (acinar architecture was disrupted with reticulin). Positive pathology was defined as the presence of ACTH staining, basophilic hyperplasia and Crooke’s hyaline changes in the tumor. Immunohistochemical staining included ACTH, human growth hormone, prolactin, and cytokeratin CAM 5.2. Immunohistochemistry was performed on formalin-fixed, paraffin embedded tissue, using a biotin-free protocol that included appropriate positive and negative controls. All surgeries were performed by the same neurosurgeon.
Study design and assessments
During the time period described the postoperative protocol (43 patients) included administration of 50 mg of hydrocortisone intraoperatively and on postoperative day (POD) 0. No further glucocorticoid was administered until POD 2. Serum ACTH and cortisol levels were collected every 6 h starting at 06:00 a.m. (repeated at 06:30 a.m.) on POD 1. Hence, the POD 2 midnight, 06:00 a.m. and 06:30 a.m. ACTH/cortisol levels were collected at least 24 h after the last steroid dose. For the additional nine patients, administration of 50 mg of hydrocortisone was every 8 h × 3 (POD 0–1) and cortisol and ACTH levels were drawn on POD 2 at midnight, and POD 3 at 06:00 a.m. and 06:30 a.m.
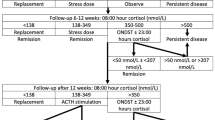
Following discharge, patients were consistently evaluated by a combined neurosurgical and endocrine team at 4 and 12 weeks postoperatively, at 6 months, and then annually thereafter. Remission was defined as a continued need for hydrocortisone or a normalized 24 h UFC, and midnight salivary cortisol or dexamethasone suppression-CRH stimulation test (serum cortisol of >2.5 mcg/dl) [22]. Aside from the evaluation of hypocortisolemia, with/without signs of adrenal insufficiency, in the immediate postoperative setting, all other tests were completed at outpatient visits.
Plasma cortisol was measured by using a 1-step competitive binding immunoenzymatic assay run on a Beckman Coulter UniCel DxI 800 with use of chemiluminescence technology. The detection level was 0.4 mcg, with intra assay and inter assay coefficient variations of 4–7 %. Plasma ACTH was measured using a commercial immunoluminescent kit (Immulite 2000; the intra assay variability was 6.7–9.5 %, and inter assay variability was 6.1–10 %).
Statistical analysis was performed using PASW18 and included descriptive analysis. Statistical significance was accepted for P < 0.05. Comparison of patients groups defined as in remission and not in remission was performed using independent samples t test, both with and without equal variance (Levene’s test) as necessary and Man Whitney U tests when variables were not normally distributed. Analysis of variance of the means between groups was performed for all variables and followed by Tamhane post hoc testing for data rejecting the null hypothesis. Bivariate correlational analysis was performed using Spearmans Rho for data not normally distributed.
Results
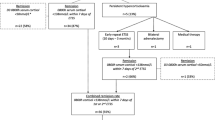
Fifty-eight patients were identified and cortisol and ACTH draws were aborted in six patients who developed severe hypotension and symptoms of adrenal insufficiency requiring stress dose steroids. Fifty-two (38 females and 14 males) patients were included in final analyses.
There were 28 microadenomas, 16 macroadenomas and eight patients in whom no tumor was identified on MR imaging. Forty-three of 52 patients (82.7 %) achieved remission: 36 (69.3 %) patients achieved remission after one TSS. Remission rate for patients with microadenomas or when no tumor was observed on MR imaging was 86.1 %. Whereas, remission rate for patients with macroadenomas was 75 %. As expected, the larger the tumor, the lower the remission rate (r = 0.73, P = 0.007).
Thirteen of 43 (30.2 %) patients who achieved remission had a postoperative cortisol level of <2 mcg/dl during 48 h postoperatively. Sixteen patients (30.7 %) did not achieve remission after one TSS. Of these patients, 10 had a repeat TSS within 1 week and seven of the 10 patients achieved remission (Fig. 1).
There was no statistically significant difference noted between remission and non-remission groups in terms of age (45.5 vs. 41.2 years), body mass index (BMI; 34 vs. 38.2) and duration of follow up (23.3 vs. 21.8 months), respectively (Table 1).
Positive predictive value for remission with postoperative nadir cortisol <2 mcg/dl and ACTH <5 pg/ml was 100 % (P < 0.005). Positive predictive value for non-remission of ACTH >15 pg/ml was 87.5 %. Postoperative ACTH >5 pg/ml and/or cortisol >5 mcg/dl correlated with lower likelihood of remission (rs = −0.394, P = 0.028 and rs = −0.604, P < 0.001), respectively. Of the 9 patients with persistent disease, 6 had postoperative nadir cortisol levels >5 mcg/dl and 5/6 had cortisol levels >10 mcg/dl. Morning ACTH at 2 months after surgery correlated with immediate postoperative midnight and 06:00 a.m. ACTH levels (rs = 0.628, P = 0.029 and rs = 0.619, P = 0.032), respectively (Figs. 2, 3, 4, 5).
Total hypophysectomy, either for the 1st or second TSS was not performed in any patient. With the exception of transient diabetes insipidus in three patients after the second TSS, no additional pituitary dysfunction was found postoperatively after the 2nd TSS.
Discussion
Short term remission rates in our subjects, are higher than those reported overall in CD, particularly for macroadenomas [3, 12, 23]. In cases of surgical failure, repeat exploration of the sella represents a controversial treatment option [1, 6, 7, 18, 20, 24, 25]. Limited data is available in the literature on the long-term effect of immediate repeat TSS versus delaying repeat surgery for persistent CD [1, 6, 7, 18, 20, 24, 25]. Locatelli et al. [1], analyzed the strategy of immediate re-operation in cases of surgical failure judged by plasma cortisol levels that did not fall to 2 mcg/dl or less within 72 h of surgery. Of 12 patients who underwent repeat TSS within 2 weeks of the first, based on persistently elevated postoperative cortisol levels, eight patients achieved remission, three required subsequent treatments (radiotherapy, gamma knife radiosurgery, bilateral adrenalectomy) and one patient died of heart failure. The advantages of early re-operation are absence of scar tissue and altered anatomy that may be encountered with delayed surgery with the possible downside of higher rates of postoperative complications including cerebrospinal fluid (CSF) leak, diabetes insipidus and hypopituitarism [11]. Indeed, eight of 12 patients in Locatelli’s study developed pan-hypopituitarism; however, 10 of 12 patients underwent total hypophysectomy. In our study seven of 10 (70 %) patients who underwent a second TSS, achieved remission with no further persistent pituitary dysfunction suggesting that immediate repeat TSS in selected cases in the hands of an experienced surgeon, could be beneficial. However, more long term studies are required for substantiation.
Complete restoration of normal hypothalamic–pituitary–adrenal axis function has been considered by some authors as defining remission of CD [11]. Lack of ACTH production postoperatively may result in a temporary or long-standing adrenal insufficiency from suppression of normal corticotrophs by the excess cortisol of the disease state. Unfortunately, regrowth or progression of residual tumor may restore normal ACTH production and subsequently produce excess ACTH despite apparent normalization of cortisol levels immediately postoperatively [11, 23]. These patients may experience a temporary phase of normal cortisol levels followed by development of recurrent hypercortisolism. Notably, this phase of normocortisolemia is often characterized by lack of a normal diurnal variation in cortisol secretion [11]. In some cases, marked adrenal hyperplasia from long standing ACTH excess has been hypothesized to result in autonomous adrenal cortisol hypersecretion with persistent elevated postoperative cortisol [11]. Likewise, vascular injury at the time of surgery has been hypothesized to lead to ischemia of residual tumor with decreasing ACTH secretion and progressive tumor necrosis with subsequent delayed remission [6, 23]. However, we did not observe delayed remission in any of the patients in our study population.
Reasons for surgical failure after initial TSS have been previously described in detail; the presence of residual tumor either missed or hidden in the gland, intrasellar or perisellar region ectopic ACTH secretion, residual invasive tumor within the dura mater of the sella turcica or tumor within the cavernous sinus [1, 5, 11]. The identification of a tumor radiographically has been associated with higher remission rates [3]. In some series, remission rates were higher when corticotroph tumor was confirmed on pathology [26, 27]. Conversely, other studies have not shown any correlation between corticotroph tumor confirmation and remission [28–30].
Much discussion has revolved around the determination of a cutoff value of cortisol below which disease remission can be assumed. In a study involving 331 patients with histologically proven ACTH pituitary adenomas, and at least 1 year of disease free follow up, postoperative nadir serum cortisol <2 mcg/dl was reported to have a positive predictive value (PPV) for remission of 90.5 % [5]. Other studies have reported postoperative cortisol levels <2 mcg/dl with PPV rates between 87 and 93 % [11]. However, in a National Institutes of Health study, 9.5 % of patients suffered a later recurrence with a 7 % recurrence rate reported among patients with a nadir cortisol below 1 mcg/dl [5]. Conversely after following 44 patients with CD for 49 months Acebes et al. [3], concluded that 08:00 a.m. cortisol levels of 2 mcg/dl were 100 % sensitive and 90 % specific for surgical failure. In our study population, postoperative cortisol <2 mcg/dl within 48 h postoperatively, after glucocorticoids were held for at least 24 h, revealed a 100 % PPV for remission. Our results were in agreement with these studies in that no single cortisol cutoff value was shown to be an absolute predictor of recurrence [4, 5, 11].
More recently, the phenomenon of “delayed remission” has been described as more frequent than previously thought. Valassi et al. [6], characterized patients without immediate postoperative remission, who have a delayed decrease to normal or low cortisol levels without further therapy. In a cohort of 620 patients who underwent TSS for CD at three tertiary care centers 23.9 % had persistent hypercortisolism. A delayed decline in the cortisol levels at a mean of 38 ± 50 days (median 9 days, range 4–180 days) postoperatively was found in 5.6 % of these patients. However, in our study, no patient with a nadir postoperative serum cortisol level >10 mcg/dl, after one or two surgeries, achieved delayed remission (over a mean follow up period of 23.1 ± 26.3 months). All of these patients required some form of additional treatment (repeat TSS, medical therapy, radiotherapy, bilateral adrenalectomy). We cannot exclude the possibility of a missed delayed remission in any of the 10 patients with cortisol >10 mcg/dl who had immediate TSS.
Undetectable cortisol levels immediately (within 24 h) or within 2 weeks after adenomectomy are considered by some investigators as the defining criteria of remission [3, 5, 6, 11]. This relies on the theory that hypercortisolism suppresses the normal corticotroph activity that may remain suppressed or could be restored to normal function once the tumor is removed [5]. This theory has led to the practice in some centers of withholding glucocorticoids until signs and symptoms of hypoadrenalism appear to determine remission status. This practice is opposed by others as an unnecessary risk [11, 13]. It has been hypothesized that glucocorticoids perioperatively may inhibit the secretion of ACTH and cortisol complicating the interpretation of remission [3, 5]. Our approach includes administration of glucocorticoids immediately postoperatively but to withhold them POD 1–2. All blood for analysis in our study was drawn at least 24 h after the last dose of hydrocortisone was administered.
In a recent meta-analysis [11] patients with sub-normal early postoperative cortisol levels showed a cumulative rate of recurrence of 9 % compared to 24 % in the patients with early postoperative cortisol levels in the normal range. In an analysis of their own patient population, Sughrue et al., found a recurrence rate of 4 % amongst patients with subnormal POD 1 (08:00 a.m.) cortisol levels compared to recurrence rate of 22 % in patients with levels in normal range (8–25 mcg/dl). They concluded that sub-normal postoperative cortisol levels do not guarantee long term remission and a normal cortisol level, although associated with a higher risk of recurrence, does not always imply persistent disease. In this meta-analysis there was a reduced rate of recurrence in patients with undetectable immediate postoperative cortisol level of <2 mcg/dl (follow up period 32–115 months), which concurs with findings in our study. However, no cutoff for ACTH or cortisol predicted disease recurrence.
Both the timing of cortisol draws and timing of repetitions, plus the subject’s cortisol clearance may influence determination of disease remission. Roelfsema et al. [31], recently reported that both basal and pulsatile cortisol levels, which are elevated in active CD, return to the level of healthy controls when the disease is in remission. Comparison among studies is complicated by differences in the exact timing of cortisol measurement, which varies between studies. This ranges from POD 1 to POD 28, with the majority of measurements done within 2 weeks after surgery [3, 5, 6, 11]. The time of day is most often described as between 06:00 a.m. and 08:00 a.m. and the number of measurements is often unclear. Additionally, the impact of individual cortisol clearance parameters may play a role in postoperative serum levels [32] and may impact determination of remission. Consistent with other studies measurements in our study were obtained at 06:00 a.m. but were repeated at 06:30 a.m. with findings at both time intervals included in the analysis.
The predictive value of postoperative ACTH levels has not been widely documented in the literature and remains controversial [3, 4, 23]. Due to a short plasma half-life (8 min) an early decline in ACTH levels has been proposed by some to differentiate postoperative remission versus persistent disease [4, 33]. Other groups have not reported an absolute ACTH level threshold shown to ensure remission [4]. In our study, an ACTH level of <5 pg/ml within 48 h postoperatively was 100 % predictive of remission. Conversely, we found that no immediate postoperative cortisol or ACTH level was a negative predictor of recurrence or predicted the length of remission. Srinivasan et al. [4], found that plasma ACTH decreased more in subjects with hypocortisolemia versus those with persistent disease within the first 48 h of surgery. Plasma ACTH fell and remained below 20 pg/ml in all subjects in their postoperative hypocortisolemia group and preceded attainment of hypocortisolemia by a mean of 11 h. Graham et al. [34], also found that at least 40 % ACTH reduction predicted postsurgical remission. Conversely, ACTH >15 pg/ml was highly indicative of non-remission [4]. However, Srinivansan et al., hypothesized that the immunoreactivity of ACTH may be substantially longer than the plasma half-life creating higher levels in the immediate postoperative period. Roelfsema et al. [31], found that pulsatile and basal secretions of ACTH secretion levels remained elevated post-surgery compared to those patients in remission and healthy controls. However, patients in remission also had non-significant ACTH elevations when compared to healthy controls [31]. Further evaluation of the predictive role of immediate postoperative ACTH levels is warranted.
Conclusion
The search for reliable treatment outcome predictors of remission in CD is somewhat hindered by the rarity of this disease and the small number of patients at any single center.
In contrast to other studies, we found a lower cutoff value for ACTH and cortisol a sensitive predictor of remission, with early postoperative cortisol <2 mcg/dl and ACTH <5 pg/ml being able to distinguish between patients in remission and treatment failure. The utility of plasma cortisol and ACTH measurements, including delayed normalization in the postoperative management of CD needs further study. These patients will continue to need lifelong follow up as no value predicts absence of recurrence.
Our results also show that a second surgery in the immediate postoperative period in patients with persistent hypercortisolemia can increase the likelihood of CD remission (especially for macroadenomas) in selected patients, with no significant disadvantages. The treatment paradigm of a second surgery and optimal timing should be analyzed further in the context of newly available medical therapies for CD.
References
Locatelli M, Vance ML, Laws ER (2005) Clinical review: the strategy of immediate reoperation for transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 90:5478–5482
Ben-Shlomo A, Liu N-A, Melmed S (2011) Pathogenesis of corticotropic tumors. In: Bronstein MD (ed) Cushing’s syndrome. Contemporary endocrinology. Humana Press, Totowa, pp 31–40
Acebes JJ, Martino J, Masuet C, Montanya E, Soler J (2007) Early post-operative ACTH and cortisol as predictors of remission in Cushing’s disease. Acta Neurochir (Wien) 149:471–477
Srinivasan L, Laws ER, Dodd RL, Monita MM, Tannenbaum CE, Kirkeby KM, Chu OS, Harsh GR, Katznelson L (2011) The dynamics of post-operative plasma ACTH values following transsphenoidal surgery for Cushing’s disease. Pituitary 14:312–317
Lindsay JR, Oldfield EH, Stratakis CA, Nieman LK (2011) The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing’s disease after transsphenoidal surgery. J Clin Endocrinol Metab 96:2057–2064
Valassi E, Biller BM, Swearingen B, Giraldi FP, Losa M, Mortini P, Hayden D, Cavagnini F, Klibanski A (2010) Delayed remission after transsphenoidal surgery in patients with Cushing’s disease. J Clin Endocrinol Metab 95:601–610
Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93:2454–2462
Fleseriu M, Loriaux DL, Ludlam WH (2007) Second-line treatment for Cushing’s disease when initial pituitary surgery is unsuccessful. Curr Opin Endocrinol Diabetes Obes 14:323–328
Fleseriu M, Petersenn S (2012) Medical management of Cushing’s disease: what is the future? Pituitary 15:330–341
Clayton RN, Raskauskiene D, Reulen RC, Jones PW (2011) Mortality and morbidity in Cushing’s disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab 96:632–642
Sughrue ME, Shah JK, Devin JK, Kunwar S, Blevins LS Jr (2010) Utility of the immediate postoperative cortisol concentrations in patients with Cushing’s disease. Neurosurgery 67:688–695
McCance DR, Besser M, Atkinson AB (1996) Assessment of cure after transsphenoidal surgery for Cushing’s disease. Clin Endocrinol (Oxf) 44:1–6
Blevins LS Jr, Christy JH, Khajavi M, Tindall GT (1998) Outcomes of therapy for Cushing’s disease due to adrenocorticotropin-secreting pituitary macroadenomas. J Clin Endocrinol Metab 83:63–67
Bochicchio D, Losa M, Buchfelder M (1995) Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s Disease Survey Group. J Clin Endocrinol Metab 80:3114–3120
Alwani RA, de Herder WW, de Jong FH, Lamberts SW, van der Lely AJ, Feelders RA (2011) Rapid decrease in adrenal responsiveness to ACTH stimulation after successful pituitary surgery in patients with Cushing’s disease. Clin Endocrinol (Oxf) 75:602–607
Alwani RA, de Herder WW, van Aken MO, van den Berge JH, Delwel EJ, Dallenga AH, De Jong FH, Lamberts SW, van der Lely AJ, Feelders RA (2010) Biochemical predictors of outcome of pituitary surgery for Cushing’s disease. Neuroendocrinology 91:169–178
Pouratian N, Prevedello DM, Jagannathan J, Lopes MB, Vance ML, Laws ER Jr (2007) Outcomes and management of patients with Cushing’s disease without pathological confirmation of tumor resection after transsphenoidal surgery. J Clin Endocrinol Metab 92:3383–3388
Ram Z, Nieman LK, Cutler GB Jr, Chrousos GP, Doppman JL, Oldfield EH (1994) Early repeat surgery for persistent Cushing’s disease. J Neurosurg 80:37–45
Trainer PJ, Lawrie HS, Verhelst J, Howlett TA, Lowe DG, Grossman AB, Savage MO, Afshar F, Besser GM (1993) Transsphenoidal resection in Cushing’s disease: undetectable serum cortisol as the definition of successful treatment. Clin Endocrinol (Oxf) 38:73–78
Friedman RB, Oldfield EH, Nieman LK, Chrousos GP, Doppman JL, Cutler GB Jr, Loriaux DL (1989) Repeat transsphenoidal surgery for Cushing’s disease. J Neurosurg 71:520–527
Patil CG, Veeravagu A, Prevedello DM, Katznelson L, Vance ML, Laws ER Jr (2008) Outcomes after repeat transsphenoidal surgery for recurrent Cushing’s disease. Neurosurgery 63:266–270
Erickson D, Natt N, Nippoldt T, Young WF Jr, Carpenter PC, Petterson T, Christianson T (2007) Dexamethasone-suppressed corticotropin-releasing hormone stimulation test for diagnosis of mild hypercortisolism. J Clin Endocrinol Metab 92:2972–2976
Pereira AM, van Aken MO, van Dulken H, Schutte PJ, Biermasz NR, Smit JW, Roelfsema F, Romijn JA (2003) Long-term predictive value of postsurgical cortisol concentrations for cure and risk of recurrence in Cushing’s disease. J Clin Endocrinol Metab 88:5858–5864
Utz AL, Swearingen B, Biller BM (2005) Pituitary surgery and postoperative management in Cushing’s disease. Endocrinol Metab Clin North Am 34:459–478
Hofmann BM, Hlavac M, Kreutzer J, Grabenbauer G, Fahlbusch R (2006) Surgical treatment of recurrent Cushing’s disease. Neurosurgery 58:1108–1118; discussion 1108–1118
Guilhaume B, Bertagna X, Thomsen M, Bricaire C, Vila-Porcile E, Olivier L, Racadot J, Derome P, Laudat MH, Girard F et al (1988) Transsphenoidal pituitary surgery for the treatment of Cushing’s disease: results in 64 patients and long term follow-up studies. J Clin Endocrinol Metab 66:1056–1064
Invitti C, Pecori Giraldi F, de Martin M, Cavagnini F (1999) Diagnosis and management of Cushing’s syndrome: results of an Italian multicentre study. Study group of the Italian society of endocrinology on the pathophysiology of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab 84:440–448
Estrada J, Garcia-Uria J, Lamas C, Alfaro J, Lucas T, Diez S, Salto L, Barcelo B (2001) The complete normalization of the adrenocortical function as the criterion of cure after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 86:5695–5699
Burke CW, Adams CB, Esiri MM, Morris C, Bevan JS (1990) Transsphenoidal surgery for Cushing’s disease: does what is removed determine the endocrine outcome? Clin Endocrinol (Oxf) 33:525–537
Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF (2002) Long-term follow-up results of transsphenoidal surgery for Cushing’s disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf) 56:541–551
Roelfsema F, Keenan DM, Veldhuis JD (2011) Endogenous ACTH concentration-cortisol secretion dose analysis unmasks decreased ACTH potency in Cushing’s disease with restoration after successful pituitary adenomectomy. J Clin Endocrinol Metab 96:3768–3774
Veldhuis JD, Iranmanesh A, Lizarralde G, Johnson ML (1989) Amplitude modulation of a burstlike mode of cortisol secretion subserves the circadian glucocorticoid rhythm. Am J Physiol 257:E6–E14
Czirjak S, Bezzegh A, Gal A, Racz K (2002) Intra- and postoperative plasma ACTH concentrations in patients with Cushing’s disease cured by transsphenoidal pituitary surgery. Acta Neurochir (Wien) 144:971–977
Graham KE, Samuels MH, Raff H, Barnwell SL, Cook DM (1997) Intraoperative adrenocorticotropin levels during transsphenoidal surgery for Cushing’s disease do not predict cure. J Clin Endocrinol Metab 82:1776–1779
Acknowledgments
The authors thank Shirley McCartney, PhD for editorial assistance and Andy Rekito, MS for graphic assistance. This data was summarized and presented at ENDO 2012 in Houston, Texas, June 23 –26.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nadia Hameed and Chris G. Yedinak contributed equally and should be considered joint first authors.
Rights and permissions
About this article
Cite this article
Hameed, N., Yedinak, C.G., Brzana, J. et al. Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: a large single center experience. Pituitary 16, 452–458 (2013). https://doi.org/10.1007/s11102-012-0455-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-012-0455-z