Abstract
Flavonoids are a large group of plant secondary metabolites that have a high popularity as nutraceuticals. Further, they contribute to food quality, acting as preservatives, pigments and strong antioxidants. Flavonoids also play an important role in plant stress tolerance, with consequent contribution to crop productivity. The enhancement of flavonoid content is an alluring goal that meets the food requirements of an increasing and more demanding world population. After illustrating the relevance of flavonoids for human nutrition, food technology and plant protection, this review covers breeding and molecular strategies used to exploit flavonoid biodiversity present among plant species. Highlighted here are also recent advances in genome sequencing and -omics tools that facilitate the identification of genetic regions influencing flavonoid production in relevant agricultural species. Finally, the review outlines established and new biotechnological techniques which can help to functionalize and use flavonoid genes to improve both the quality and the quantity of these outstanding compounds. The final message of this review is that flavonoids can be an interesting target for molecular plant breeding that can greatly impact both primary agricultural products and food technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population growth is expected to increase dramatically in the near future. According to the United Nation predictions, world population will raise to 9.7 billion in 2050. Consequently, agriculture will deal with both new and existing challenges, including higher yield and sustainability in the use of agricultural resources; plant protection against biotic and abiotic stresses; food bio-fortification to alleviate malnutrition or to face the modern need of nutrition and food technology processes. Among strategies that can be exploited to accomplish these goals, those based on plant biodiversity are very promising given that phenotypic, genotypic and metabolic variability exist in both spontaneous and cultivated species. Secondary plant metabolites strongly affect this biodiversity and, due to their biological activity, they have been already used to improve crops and food products (Kliebenstein 2009; Moore et al. 2014).
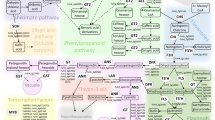
Among plant secondary metabolites, the class of phenylpropanoids is intensely studied for its antioxidant and free radical scavenging properties (Halliwell 2006; Korkina 2007; Sharma et al. 2012; Moore et al. 2014). They are widely widespread in raw foods, contributing to their quality even after some types of food transformation (Ioannou et al. 2012). Phenylpropanoids are not only important nutraceuticals. They have a role against environmental stresses as well (D’Amelia et al. 2017). This function, which is often underestimated, is directly connected with food production, because both biotic and abiotic stresses (e.g. pests, diseases, drought, cold, salinity) can seriously compromise food security and quality. Phenylpropanoids originate from deamination of phenylalanine and their metabolic pathway proceed them with a series of biochemical modifications to yield a wide array of different molecules (Fraser and Chapple 2011). Among them, flavonoids are particularly attractive. They are widespread through all plants and in algae (Goiris et al. 2014) and possess more than 6000 chemical structures (Hichri et al. 2011). The first step of flavonoid biosynthesis is directed by the activity of the enzyme chalcone synthase (CHS). Subsequent reactions catalysed by several enzymes, mostly conserved among plants, allow the production of different flavonoid subgroups such as flavones, flavonols and anthocyanins (Fig. 1a). The basic structure of flavonoids consists of two aromatic C6 rings (A and B) connected by a C3 unit benzene ring (C) (Fig. 1b). The position of catechol ring (ring B) on benzene (ring C) divides flavonoid (2-phenylchromans) from isoflavonoid (3-phenylchromans) (Kumar and Pandey 2013) and influences the antioxidant characteristics. This latter property is also determined by the number and position of hydroxyl substituents on the catechol group (ring B) (Racchi 2013) (Fig. 1c). Flavonoids may undergo to different decorations (i.e. hydroxylations, methylations, glycosylations and acylations). This extends the plant species-specific type of flavonoids and, consequently, their potential utilization (Winkel-Shirley 2001; Rinaldi et al. 2017).
Antioxidant flavonoids. a Scheme presenting the general pathway of flavonoid biosynthesis with the main responsible structural enzymes and some examples of transcription factors (circled) regulating some branches of flavonoid pathway in S. tuberosum (StAN1), S. lycopersicum (SlMYB12), Vitis vinifera (VvMYPA1) and Zea mays (ZmP1) (Grotewold et al. 1994; Bogs et al. 2007). For each class of flavonoids some examples of specific compounds are reported. In italic the structural genes regulating each steps: PAL, phenylalanine ammonia lyase; C4H, Cinnamate 4-hydroxylase; 4CL, 4-coumarate-CoA ligase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; FNS, flavone synthase; FNR, flavanone 4-reductase; FLS, flavonol synthase; F3′H, flavonoid-3′-hydroxylase; F3′5′H, flavonoid-3′5′-hydroxylase; DFR, dihydroflavonol reductase; ANS, anthocyanidin synthase; UFGT, UDP glucose: flavonoid-3-O-glucosyltransferase. b The basic structure of flavonoids consists of two aromatic rings (A and B) connected by a C3 unit benzene ring (C). The different type of residues (Rx) specifies for different subclasses of flavonoids. c The number and position of hydroxyl substituents on B catechol ring influences the antioxidant proprieties of flavonoids against reactive oxygen species (R·)
The objective of this paper is not to review recent findings on flavonoid biosynthesis and their health benefits, but rather to focus on the exploitation of their properties (antioxidant above all) for primary production and food applications. After introducing their beneficial attributes, we will discuss breeding strategies to exploit genetic resources and will outline biotechnological methods which can help to use genes enhancing flavonoid content and antioxidant properties.
Flavonoids as antioxidants: role for human nutrition and food technology
It is well known that an unbalanced diet can cause several chronic diseases (Martin et al. 2013). There is a vast literature testing the chemopreventive function of plant flavonoids in reducing the onset of these disorders (Yao et al. 2004; Martin et al. 2013; Raffa et al. 2017). Further, there is growing awareness that flavonoids underpin the beneficial health effects promoted by consumption of fruits and vegetable. Considering this popularity, plant researchers are becoming more and more addressed into both the identification of health-promoting flavonoids in major crops and the validation of their functional activity against human diseases. For example, flavonoids extracted from coloured potatoes have been reported to have biomedical activity against breast and haematological cancers (Bontempo et al. 2015), whereas those from red maize showed antiproliferative activity against prostate cancer (Herrera-Sotero et al. 2017). Additional examples are reported in Table 1. New functional foods containing flavonoids are being proposed. This is the case of blue maize tortillas made with flour enriched with flavonoids extracted from black beans or ready-to-eat snacks made with flour extracted from purple potatoes (Nems et al. 2015; Chavez-Santoscoy et al. 2016). The beneficial activity of flavonoids in human health promotion is mainly due to their antioxidant property. This is particularly strong compared to that of other phenolics due to the presence of multiple hydroxyl groups (Yashin et al. 2017). Flavonoids hold a strong scavenger activity towards reactive oxygen and nitrogen species (RONS; Martin et al. 2011; Martin and Li 2017). These latter compounds oxidize cellular proteins, nucleic acids and lipids, underpinning many degenerative and chronic pathologies (Heim et al. 2002). Flavonoids are able to transfer hydrogens and electrons to RONS, stabilizing them and giving rise to relatively stable flavonoid radicals. Furthermore, flavonoids can chelate metal catalysts involved in free radical generation (quercetin, for example, is known for its iron-chelating and iron-stabilizing properties). They can also activate antioxidant enzymes and inhibit those involved in RONS formation, such as the NADPH oxidase complex (Heim et al. 2002; Mladenka et al. 2010; Kumar and Pandey 2013).
Flavonoids are also valuable compounds for food storage and industry (Table 1). The Royal Society of London coined the term “sustainable intensification”. It indicates modern agricultural activities aimed at increasing food supplies while protecting biodiversity and ecosystem processes (Petersen and Snapp 2015). One of the most important aspects of “sustainable intensification” is the reduction of food waste. Consequently, there is an increasing need for continuing research in postharvest storage technologies and food preservatives. Since modern consumers are worried about the use of preservatives made by chemical synthesis, focus is being placed on natural products. At the same time industries are paying more emphasis on the use of plant compounds with antioxidant and antimicrobial properties to extend shelf-life. In this context, the antioxidant activity of flavonoids may not only ensure stabilization over the life of a finished food but can also provide action against necrotrophic fungi and food-borne pathogens. The antioxidant mechanism of flavonoids in food systems is mainly related to their ability to control lipid autoxidation, which is a major cause for food quality deterioration and shelf-life decreasing (Shahidi and Ambigaipalan 2015). Flavonoids can donate hydrogen atoms to lipid radicals and produce antioxidant radicals which are more stable and less subjected to autoxidation. The antioxidant action of flavonoid to extend shelf-life can be also due to their ability to reduce the susceptibility of fresh fruits and vegetables to specific postharvest pathogens. Zhang et al. (2013, 2015b) provided evidence that flavonoids, and in particular high hydroxylated anthocyanins, are able to block the development of grey mold (caused by Botrytis cinerea) perturbing the dynamics of the ROS burst during infection. Flavonoids can also provide antimicrobial activity against several microorganisms in that they can bind and inactivate proteins and may complex with bacterial cell walls (Hintz et al. 2015). For example, those extracted from almond skin, chilli seeds, bergamot and pomegranate fruits possess antibacterial and bacteriostatic actions against pathogenic species such as Salmonella enterica, Escherichia coli, Pseudomonas putida, Bacillus subtilis and Staphylococcus aureus (Mandalari et al. 2007, 2010; Mahboubi et al. 2015; Gurnani et al. 2016). Considering their beneficial properties, flavonoids have been used in various food applications. For example, they have been added as active antioxidant materials for the packaging of oxygen-sensitive foods to increase both product’s shelf-life and bioactive compound content (Lopez-de-Dicastillo et al. 2011). They have been also added to cooked pork patties or to raw mackerel fillets to reduce lipid oxidation (Rey et al. 2005; Pazos et al. 2005; Viji et al. 2015). Additional examples of similar applications are reported in Table 1.
Plant antioxidant flavonoids can be also used as food colorants. In this regard, the most important example is given by anthocyanins, the largest group of water soluble pigments in the plant kingdom (Mateus and de Freitas 2008). Common commercial preparations usually include anthocyanins such as 3-glucosides and 3,5-diglucosides of cyanidin, delphinidin and malvidin, often extracted from grapes and their by-products (Davies 2004; Ali et al. 2010). Unfortunately, the application of this class of flavonoids as natural colorant can be hampered by low stability, weak tinctorial strength, interactions with food ingredients, and inability to match desired hues. Therefore, there is a need for research aimed at identifying additional sources and/or types of anthocyanins as well as new stabilization methods (for a review see Cortez et al. 2017). Since high levels of glycosylation and acylation may enhance the stability and antioxidant properties, sources of more complex forms of flavonoids are being identified (Plaza et al. 2014). In this regard, genetic studies and innovative biotechnological applications are expected to play a key role to both identify and enhance flavonoid chemical variability. Such aspects will be discussed later in this review.
Flavonoids as antioxidants: role in plant protection
The antioxidant role of flavonoids is also important to protect plants from environmental stressors (Table 2). This indirectly impact food quality and security since it has been estimated that about half of the crop worldwide production is lost due to environmental stresses (Atkinson and Urwin 2012). Biotic and abiotic stresses often cause damages and have lethal effects on plants due to the production of unstable free radicals, among which the most reactive are those containing partial reduction of oxygen such as ROSs (Sharma et al. 2012). The accumulation of these molecules may result in biological damages to cell membranes and relevant macromolecules such as DNA, proteins, lipids and carbohydrates (Racchi 2013). In the absence of stress conditions, plants normally produce ROS as consequence of an aerobic metabolism (Halliwell 2006). However, these are physiologically maintained at a not toxic level (Gill and Tuteja 2010). During stress conditions, antioxidant enzymes (e.g. superoxide dismutase, catalase, ascorbate peroxidase, glutathione peroxidase, glutathione reductase etc.) are either inactivated or may have an activity that is not sufficient to face ROS. In these situations, the antioxidant functions of flavonoids become particularly important to complement the action of antioxidant enzymes (Hatier and Gould 2008; Fini et al. 2011; Di Ferdinando et al. 2012). Several abiotic stresses induce highly hydroxylated flavonoids. In these conditions the presence of an extra free hydroxyl (–OH) on the C-3′ of the B-ring contributes to a stronger scavenger ability (Tattini et al. 2004; Agati et al. 2012). For example, the dihydroxy B-ring-substituted flavonoid glycosides, such as quercetin 3-O or luteolin 7-O-glycosides are produced more than the monohydroxy B-ring kaempferol 3-O- or apigenin 7-O-glucoside during either UV-B or drought stress (Di Ferdinando et al. 2012). The vacuolar presence of flavonoids also suggests their role in reducing H2O2 molecules that usually escape from chloroplasts during stress (Bienert et al. 2007; Di Ferdinando et al. 2012). Glycosylation and phenylacilation of flavonoids can be considered another clue of their antioxidant role in plant cell protection (Di Ferdinando et al. 2012; Tohge et al. 2017a). Though the antioxidant activity of these molecules is reduced with glycosylation, the glycosylated forms are more soluble in cellular milieu and much more preserved from autoxidation (Pearse et al. 2005; Agati and Tattini 2010).
Flavonoids are very important also to protect plants from pathogenic bacteria and fungi. During plant/pathogen reactions, the earliest defence mechanism employed by infected plants is often the hypersensitive response, characterized by programmed cell death. In this situation, flavonoids may accumulate into the cell wall of necrotic and adjacent cells, chelating metals required by pathogen enzymes to attack plant cell structures (Treutter 2005; Mierziak et al. 2014). Pathogen attacks may disturb the delicate balance between ROS production and scavenging (Das and Roychoudhury 2014), leading to ROS accumulation (the so-called oxidative burst). In the case of pathogens that take advantage of dead cells (i.e. necrotrophic fungi or some bacteria), the property of flavonoids to reduce ROS burst is an important mechanism to limit their colonization (Venisse et al. 2001; Zhang et al. 2015b). Specific flavonols (such as myricetin) and anthocyanins (such as delphinidin), all having a high B-ring hydroxylation degree, are the strongest defence against pathogen attacks. Worth to mention is also the fact that leaf flavonoids participate in defence mechanisms against insects. Indeed, these stressors show preferences for green leaves for food or for oviposition and do not possess red colour receptors (Karageorgou and Manetas 2006; Schaefer and Rolshausen 2006; Chittka and Doring 2007).
Molecular breeding and biotechnological approaches to study and exploit plant flavonoids
Mendel can be considered the first geneticist who studied the genetics of flavonoids. Indeed, in his crossing experiments he examined the inheritance of the trait “pea flower colour” that is caused by anthocyanin accumulation (Harker et al. 1990). Since then, flavonoid genetics and biochemistry have been extensively investigated in a number of species. Nowadays we have a broad comprehension of the pathway and the genes that play decisive roles in flavonoid synthesis in different tissues, at different times, and under different conditions. A combination of conventional and non-conventional approaches has been used for such studies including genetic mapping, application of various -omics technologies and the exploitation of plant germplasm through genetic engineering. In this section, we provide an overview of main strategies used to study and exploit plant flavonoids.
Classical breeding methods
About 150 years later Mendel’s crossing experiments, conventional approaches based on intra- and inter-specific hybridization still represent a valid opportunity to develop flavonoid-rich plants. Wild species have often been used as donor parents to produce the genetic diversity necessary for breeding efforts (Rigano et al. 2016). Good examples of this kind of approach are reported in tomato. Willits et al. (2005) enhanced quercetin production in both fruit flesh and peel through crosses between cultivated Solanum lycopersicum and its wild relative S. pennellii. In this case, the use of the wild species restored the flavonol pathway, which was probably blocked during tomato domestication. Similarly, the untapped genetic diversity available in wild S. chilense (carrying the dominant allele Anthocyanin fruit, AFT), S. cheesmanie (carrying the recessive allele Atroviolacium, atv) and S. lycopersicoides (carrying the dominant allele Aubergine, ABG) was used to improve anthocyanin production in tomato fruits through interspecific crossing. Fruits of either AFT-/atvatv and ABG-/atvatv hybrids showed high production of anthocyanins in the peel (Jones et al. 2003; Mes et al. 2008; Gonzali et al. 2009). Exploitation of wild relatives in breeding programmes for increased flavonoid content are reported also in Allium. Since wild A. ursinum and A. victorialis contain novel flavonoids (Andersen and Fossen 1995; Wu et al. 2009), breeding strategies have been developed to introgress their relevant genes into cultivated A. sativum. Sources of genetic diversity for breeding are also represented by cultivated ecotypes, hairlooms and landraces. In South and Central America, open-pollinated varieties of maize named “morado”, for example, are an important source of anthocyanins and other flavonoids. They have been used as parents to introgress “strong” alleles of anthocyanin regulatory genes, such as Booster1 (B1) and Purple Plant1 (Pl1) in European/North American varieties (Petroni et al. 2014). Similarly, South American native germplasm represents a rich source of flavonoids for potato breeding. Brown et al. (2007) reported that cultivars “Negra” and “Challina” from Peru displayed particularly high levels of anthocyanins and suggested their use in varietal development through introgressive hybridization. An advantage in using these potato genetic resources is that their anthocyanin accumulation is regulated by dominant alleles (Jung et al. 2009; Zhang et al. 2009). Flavonoid variability is also present in important monocot crops. In the case of rice, for example, the biodiversity present in subspecies indica and japonica represented sources of different kinds of flavonoids (Dong et al. 2014).
There is no doubt that improvements of plant flavonoid composition would continue through traditional breeding methods. However, the ubiquitous number of genes to reshuffle, the abundance of genes and alleles and the myriad of their possible combinations mixed with environmental interactions make inter- and intra-specific sexual hybridization a challenging strategy to be pursued. Therefore, breeders are continuously seeking for new tools and strategies to improve the efficiency of plant improvement.
From molecular markers to -omics tools
Most contemporary plant breeders are using DNA-based molecular markers as aids to produce new cultivars. Molecular markers are DNA sequence variants that unequivocally characterize the genomic region of an individual and usually follow the same rules of inheritance of genes. They detect point mutations, insertions, deletions or inversions in DNA fragments and, as such, can be used to easily differentiate individuals of the same species, providing a powerful, quick and cheap fingerprint of plant germ plasm. They can be located very close to genes involved in the variation of a specific trait. In this case, they can mark traits that are not observable at early stages of plant life cycle or that are masked by environmental factors. These characteristics make molecular makers a quick tool that allows breeders to accelerate and reduce the costs of selection programs. Molecular markers such as EST-SSR (expressed microsatellite), DArT (different array technology) and AFLP (amplified fragments length polymorphisms) have been exploited to characterize flavonoids genetic variability in species where these information are limited (chinese peanut, white clover and orchids) (Ballizany et al. 2016; Bhattacharyya et al. 2017; Hou et al. 2017). Fingerprinting profiles of plants with different flavonoid content led to the identification of potential markers to apply in breeding work. These markers for flavonoids often tag structural genes related to the biochemical pathway. In onion, for example, Kim et al. (2005) developed a co-dominant PCR-based marker linked to the DFR-A gene, known to be involved in the last steps of anthocyanin biosynthesis. They proposed the use of this marker to expedite the screening of heterozygous red onions in segregating populations, thereby eliminating the need for time-consuming progeny tests. Similarly, Guo and Qiu (2013) found molecular markers tightly linked to two important flavonoid hydroxylase genes, F3′H and F3′5′H, involved in soybean pubescence colour. Since flavonoid accumulation is a quantitative trait (i.e. controlled by several genes), molecular markers can be used in association studies to mine physical links between genes and phenotypes. This may lead to the detection of the chromosomic regions affecting the quantitative trait, also known as QTL (quantitative trait loci). In apple, Chagné et al. (2012) identified specific QTLs for flavanols, flavonols and anthocyanins analysing a F1 population segregating for antioxidant content. As an alternative to the use of segregating population, landraces, ecotypes, varieties and introgression lines (ILs) can be exploited to identify regions connected to flavonoid biosynthesis. This material is particularly appropriate for QTL identification, especially when the traits are contrasting and variable inside the population used. For example, a very large collection of rice accessions, showing contrasting colour phenotypes, has been analysed by Shao et al. (2011) to identify specific markers and QTLs underlining grain colour and nutritional antioxidant quality caused by flavonoids and polyphenols. ILs represent an even more suitable plant material for QTL mapping since the phenotypic differences between lines are caused by a single genomic region introgressed from a wild donor into the cultivated genetic background. Tomato represents an excellent example on the use of ILs to identify QTLs controlling flavonoid content and, in general, secondary metabolites (Lippman et al. 2007; Tohge et al. 2017b). ILs largely used in breeding and genetic studies are those developed from S. pennelli × S. lycopersicum (Eshed and Zamir 1995). Using these materials, Rousseaux et al. (2005) analysed the fruit phenolic content in three seasons, identifying few QTLs whose contribution was highly influenced by environmental conditions. Di Matteo et al. (2013) performed the transcriptional profiling of one of these QTLs, revealing the presence in the wild genome of genes regulating part of the flavonoid biochemical pathway (in particular related to their transport). A recent work, based on more updated technologies, characterized 69 flavonoid QTLs of S. pennelli × S. lycopersicum ILs (Alseekh et al. 2015). The authors found that each chromosome holds a different number of QTLs that probably contribute differently to flavonoid biosynthesis. Using similar approaches, tomato ILs deriving from S. lycopersicum × S. chmielewskii have been recently studied. They allowed the identification of a single QTL on chromosome 5 with major effects on the accumulation of flavonol glycosides in ripe tomatoes (Ballester et al. 2016).
Studies aimed at mapping QTLs by examining the marker–flavonoid content associations have been particularly supported by the availability of plant genome sequences. Genome wide association studies (GWAS) used to enhance flavonoids content have in parallel highly increased. Rice, soybean and maize are among the major crops whose genome has been scanned to identify loci and markers (in particular single nucleotide polymorphisms, SNPs) influencing flavonoid content (Chen et al. 2014; Wen et al. 2014, 2015; Jin et al. 2017). Lin et al. (2014), for example, using GWAS studies analysed 231 tomato accessions and identified mutations which mark pink-fruited tomatoes owing to the absence of flavonoids. These mutations were placed in the promoter of SlMYB12, a transcription factor gene which is known to control flavonol accumulation. These mutations caused the loss of expression of this gene. This example suggests how nowadays whole genome analyses may lead to an easy identification of genetic determinants affecting specific traits of interest. The real revolution in this kind of analysis is the combination of GWAS with other high-throughput technologies grouped with name of -omics (in particular metabolomics and transcriptomics). Example of such comprehensive studies are provided by Wen et al. (2014), Chen et al. (2014), Rhodes et al. (2014) and Matsuda et al. (2015), who characterized the metabolic diversity and the genes necessary for flavonoid biochemical synthesis, transport and modification in maize kernel, rice leaves, sorghum and rice grains, respectively. The identification of flavonoid candidate genes through these integrative -omics approaches represents today a powerful tool for subsequent biotechnological applications. The -omics revolution also provides a major opportunity to decipher specific gene complements associated with individuals possessing a different capacity to accumulate flavonoids. For examples, using a comparative approach we recently investigated the variability in flavonoid gene copy number between the cultivated potato S. tuberosum and its wild relative S. commersonii. The genomes of both species have been sequenced (The Potato Genome Consortium 2011; Aversano et al. 2015), providing an unprecedented opportunity to explore whether genes involved in the biosynthesis of flavonoids in the cultivated potato were affected by structural changes when compared to its wild counterparts. We observed that the wild species possesses a higher number of copies of genes (Phenylalanine ammonia lyase, Cytochrome P450 and Glucosyltransferase) related to the phenylpropanoid pathway (Fig. 2; Aversano et al. 2017). Importantly, in S. commersonii a higher number of genes correlated to a higher amount of phenylpropanoids (Aversano et al. 2017) confirming that transcript accumulation and, consequently, the metabolic content may be affected by gene dosage (Kliebenstein 2008). The identified members of Cytochrome P450 are particularly appealing for future investigations. Their involvements on hydroxylation reactions may be used to enhance both the antioxidant ability and the molecule decoration of flavonoids (e.g. glycosylation and acylation degree; Plaza et al. 2014). The combination of several -omics approaches with the use of mutants, ILs and transgenic materials have been successfully employed to understand flavonoid biosynthesis, glycosylation and acylation in model and crop plants. This topic has been recently reviewed by Tohge et al. (2017b).
Results of a genome comparative study reporting the number of genes for six flavonoid enzymes in the cultivated potato Solanum tuberosum and its wild relative S. commersonii (readapted from Aversano et al. 2017). The comparison of the two phylogenetic trees (built using neighbor-joining method) shows that a higher number of genes for the different classes of enzymes is present in wild S. commersonii vis-a-vis the cultivated potato. Coloured lines represent the different flavonoid gene families and the sizes of the wedges reflect their numerical consistency
Established techniques of genetic modification
While genomic applications have been highly useful in characterizing existing genetic variation within species and, consequently, in complementing classical breeding, new genetic diversity and also new promising applications can be created through genetic engineering. The extended knowledge on flavonoid biosynthesis and regulation makes this approach very suitable. Indeed, specific biochemical pathways can be modified through the activation or repression of specific genes. Here, we discuss two potential approaches for flavonoid biosynthesis improvement. The first is based on the identification of transcription factors (TFs) as important alternative to multiple steps engineering; the second is founded on the use of inducible promoters to avoid the deleterious effects of a constitutive production.
The use of genes codifying for TFs is particularly advantageous in trans- and cis-genic transformation (Itkin and Aharoni 2009). Indeed, since TFs control the expression of multiple genes, just altering the expression of a single TF it is possible to obtain a multiple step control with the specific production of one or more types of metabolites. An example is reported in Fig. 3. Using a single MYB TF gene isolated from a purple potato cultivar, it was possible to activate in tobacco all the steps of flavonoid pathway, leading to anthocyanins production (D’Amelia et al. 2014). TF identification and functionalization represents an important prerequisite for determining the suitability of this kind of biotechnological applications. For this reason, in the last decades many papers appeared where specific TFs (activators or repressors) have been shown to control branches of the flavonoids and phenylpropanoid biosynthesis (Fig. 1a). Among TFs, the MYB class has a major role in controlling flavonoids (reviewed in Liu et al. 2015). Other classes of TFs can either directly or indirectly affect flavonoids biosynthesis, especially under specific stimuli or physiological processes. Examples of these classes of TFs are the grapevine VvibZIPC22 and WRKY26 (Malacarne et al. 2016; Amato et al. 2017), the Arabidopsis LNK1 and LNK2 (Zhou et al. 2017) and the peach NAC named BLOOD (Zhou et al. 2015a). A quick and easy technology used to functionalize TF genes is virus induced gene silencing (VIGS). It has been successful used in several crops, including tomato, pear, crabapple, chili pepper and soybean (Nagamatsu et al. 2007; Zhai et al. 2016; Tian et al. 2017) to discover flavonoid gene regulators through silencing them directly on the interested plant organ (De Luca et al. 2012).
Enhanced overproduction of anthocyanins in transgenic tobacco plants. Stable integration of StAN1 transcription factor in the genome of tobacco plants was mediated by Agrobacterium tumefaciens. Overexpression of StAN1 in tobacco cells regulates the biosynthesis of a class of flavonoids (mainly anthocyanins) that confer a strike purple-reddish pigmentation (a) with respect to the wild type plant (b). The production of anthocyanin compounds is evident in all plant tissues (c, e, g) compared to the respective wild type control (d, g, h). Even the anthers in the flowers resulted red coloured in transgenic StAN1 plants (arrowed in g) with respect to wild type control (arrowed in h)
Genetic engineering based on the use of a constitutive promoter leads to a strong metabolite biosynthesis activation in all tissues of a genetic modified plant. In some situations, the use of inducible rather than constitutive promoters is preferable. This is particularly true when flavonoids are exploited to defend plants against stresses. In fact, the biosynthesis of these complicated molecules is costly due to the shift of carbon flux from the growth to the defensive trait. The expression of a target gene in specific conditions or tissues offers the advantage to allow the production of a target metabolite only when/where necessary. An explicative example comes from the model plant Arabidopsis thaliana, where antioxidant flavonoids have been induced by Feng et al. (2011) only during stress condition. In details, two TFs named PAP1 (Production of Anthocyanin Pigment 1) and CBF1 (C-repeat/DRE Binding Factor 1) were put under the osmotic inducible promoter RD29a (Responsive to Dehydration 29) to induce anthocyanin accumulation only after cold stress. An additional example is provided by the transgenic tomato lines “Del/Ros” and “MYB12” or their hybrid named “Indigo” (Butelli et al. 2008; Luo et al. 2008; Zhang et al. 2015a), where the quality and quantity of tomato fruits has been enhanced through an increase in flavonoid content. These lines accumulate high amount of anthocyanins and flavonols specifically in the fruits through the use of an ethylene responsive promoter (E8). Worth to mention in this context is the potential of inducible promoters in tune with UV–visible flavonoids, such as anthocyanins. In fact, they can be used to produce biosensors to monitor conditions of abiotic and biotic stress and facilitate remedial actions in a short time. This represents an alternative to electron devices.
New plant breeding techniques
In the last few years, genome editing has emerged as new technology that overcomes the limits of homologous integration in plants. Zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats/Cas9 (CRISPR/Cas9) have been developed as biotechnological tools to perform in a precise and directed manner the manipulation of genomes (Lee et al. 2016). Today, genome editing by CRISPR/Cas9 system is considered more affordable compared with the other methods (Cardi and Neal Stewart 2016; Demirci et al. 2017). Through these technologies it is now possible to “re-design” the nutritional propriety of foods to better respond to the needs of consumers and producers. The perspective of editing flavonoids is particularly attractive due to the enormous knowledge gained so far on the genes controlling their biosynthesis and chemical modifications. Such detailed knowledge allows to reprogram the pathway of phenylpropanoids. In Populus, for example, CRISPR/Cas9-based editing was used to knock-out a specific isoform of 4-coumarate: CoA ligase (4CL) which promotes flavonoids biosynthesis rather than the biosynthesis of condensed tannins or proanthocyanidins (Zhou et al. 2015b). Though the previous example is representative of the great potential of CRISPR/Cas9 based genome editing on flavonoids, reports on their improvement through genome editing are still limited. We believe it may be particularly interesting to modify the quality rather than the quantity of flavonoids. For example, changing the type of decorative enzymes or the enzymes responsible for molecule hydroxylation (e.g. those belonging to cytochrome P450 superfamily), the stability and the beneficial proprieties of flavonoids can be increased (Ayabe and Akashi 2006; Plaza et al. 2014).
Conclusions
Flavonoids hold several beneficial proprieties not only for human health and food processes, but also for plant protection. In this review we emphasized the antioxidant function of flavonoids as common denominator for various applications in food production. We believe that enhancing the quantity as well as the antioxidant capacity of flavonoids may have success in both primary agricultural products and in food quality. In a modern agriculture, which has to face an increased population growth rate and more and more demanding consumers, flavonoids can be an interesting target for breeding programs and biotechnological applications.
Abbreviations
- bHLH:
-
Basic helix-loop-helix
- bZIP:
-
Basic leucine zipper
- 4CL:
-
4-Coumarate:CoaLigase
- CBF1:
-
C-repeat/DRE binding factor 1
- CRISPR/Cas9:
-
Clustered regularly interspaced short palindromic repeats-associated protein-9 nuclease
- DFR:
-
Dihydroflavonol 4-reductase
- F3′H:
-
Flavonoid 3′ hydroxylase
- F3′5′H:
-
Flavonoid 3′,5′-hydroxylase
- GWAS:
-
Genome wide association studies
- LNK:
-
Night light-inducible and clock-regulated genes
- MAPK:
-
Mitogen-activated protein kinase
- NAC:
-
Non apical meristem
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- PAP1:
-
Production of anthocyanin pigment 1
- QTL:
-
Quantitative trait loci
- RD29a:
-
Responsive to dehydration 29a
- RONS:
-
Reactive oxygen and nitrogen species
- ROS:
-
Reactive oxygen species
- SNP:
-
Single nucleotide polymorphisms
- TALENs:
-
Transcription activator-like effector nucleases
- TF:
-
Transcription factor
- VIGS:
-
Virus induced gene silencing
- ZFNs:
-
Zinc finger nucleases
References
Agati G, Tattini M (2010) Multiple functional roles of flavonoids in photoprotection. New Phytol 186:786–793
Agati G, Azzarello E, Pollastri S et al (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76
Ahmed NU, Park JI, Jung HJ et al (2015) Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in Brassica rapa. Funct Integr Genomic 15:383–394
Ali K, Maltese F, Choi YH et al (2010) Metabolic constituents of grapevine and grape-derived products. Phytochem Rev 9:357–378
Alseekh S, Tohge T, Wendenberg R et al (2015) Identification and mode of inheritance of quantitative trait loci for secondary metabolite abundance in tomato. Plant Cell 27:485–512
Amato A, Cavallini E, Zenoni S et al (2017) A grapevine TTG2-like WRKY transcription factor is involved in regulating vacuolar transport and flavonoid biosynthesis. Front Plant Sci 7:1979
Andersen OM, Fossen T (1995) Anthocyanins with an unusual acylation pattern from stem of Allium victorialis. Phytochemistry 40:1809–1812
Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 63:3523–3543
Aversano R, Contaldi F, Ercolano MR et al (2015) The Solanum commersonii genome sequence provides insights into adaptation to stress conditions and genome evolution of wild potato relatives. Plant Cell 27:954–968
Aversano R, Contaldi F, Adelfi MG et al (2017) Comparative metabolite and genome analysis of tuber-bearing potato species. Phytochemistry 137:42–51
Ayabe S, Akashi T (2006) Cytochrome P450s in flavonoid metabolism. Phytochem Rev 5:271–282
Ballester AR, Tikunov Y, Molthoff J et al (2016) Identification of loci affecting accumulation of secondary metabolites in tomato fruit of a Solanum lycopersicum × Solanum chmielewskii introgression line population. Front Plant Sci 7:1428
Ballizany WL, Griffiths AG, Franzmayr BK et al (2016) Marker-trait associations for flavonoids and biomass in white clover (Trifolium Repens L.). In: Roldán-Ruiz I, Baert J, Reheul D (eds) Breeding in a world of scarcity. Springer, Cham, p 225
Bhattacharyya P, Ghosh S, Sen Mandi S et al (2017) Genetic variability and association of AFLP markers with some important biochemical traits in Dendrobium thyrsiflorum, a threatened medicinal orchid. S Afr J Bot 109:214–222
Bienert GP, Moller ALB, Kristiansen KA et al (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282:1183–1192
Bogs J, Jaffe FW, Takos AM et al (2007) The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol 143:1347–1361
Bontempo P, De Masi L, Carafa V et al (2015) Anticancer activities of anthocyanin extract from genotyped Solanum tuberosum L. “Vitelotte”. J Funct Foods 19:584–593
Brown CR, Culley D, Bonierbale M et al (2007) Anthocyanin, carotenoid content, and antioxidant values in native South American potato cultivars. HortScience 42:1733–1736
Buchweitz M, Brauch J, Carle R et al (2013) Colour and stability assessment of blue ferric anthocyanin chelates in liquid pectin-stabilised model systems. Food Chem 138:2026–2035
Butelli E, Titta L, Giorgio M et al (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26:1301–1308
Cardi T, Neal Stewart C Jr (2016) Progress of targeted genome modification approaches in higher plants. Plant Cell Rep 35:1401–1416
Chagné D, Krieger C, Rassam M et al (2012) QTL and candidate gene mapping for polyphenolic composition in apple fruit. BMC Plant Biol 12:12
Chavez-Santoscoy RA, Gutierrez-Uribe JA, Serna-Saldivar SO et al (2016) Production of maize tortillas and cookies from nixtamalized flour enriched with anthocyanins, flavonoids and saponins extracted from black bean (Phaseolus vulgaris) seed coats. Food Chem 192:90–97
Chen W, Gao YQ, Xie WB et al (2014) Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat Genet 46:714–721
Chittka L, Doring TF (2007) Are autumn foliage colors red signals to aphids? PLoS Biol 5:1640–1644
Cortez R, Luna-Vital DA, Margulis D et al (2017) Natural pigments: stabilization methods of anthocyanins for food applications. Compr Rev Food Sci F 16:180–198
D’Amelia V, Aversano R, Batelli G et al (2014) High AN1 variability and interaction with basic helix-loop-helix co-factors related to anthocyanin biosynthesis in potato leaves. Plant J 80:527–540
D’Amelia V, Aversano R, Ruggiero A et al (2017) Subfunctionalization of duplicate MYB genes in Solanum commersonii generated the cold-induced ScAN2 and the anthocyanin regulator ScAN1. Plant Cell Environ. https://doi.org/10.1111/pce.12966
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53
Davies KM (2004) An introduction to plant pigments in biology and in commerce. In: Davies KM (ed) Plant pigments and their manipulation, vol 14. CRC Press, Oxford, pp 1–22
De Luca V, Salim V, Atsumi SM et al (2012) Mining the biodiversity of plants: a revolution in the making. Science 336:1658–1661
Demirci Y, Zhang B, Unver T (2017) CRISPR/Cas9: an RNA-guided highly precise synthetic tool for plant genome editing. J Cell Physiol 21:21
Di Ferdinando M, Brunetti C, Fini A et al (2012) Flavonoids as antioxidants in plants under abiotic stress. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants. Springer, New York, p 159
Di Matteo A, Ruggieri V, Sacco A et al (2013) Identification of candidate genes for phenolics accumulation in tomato fruit. Plant Sci 205:87–96
Dong XK, Chen W, Wang WS et al (2014) Comprehensive profiling and natural variation of flavonoids in rice. J Integr Plant Biol 56:876–886
Du YG, Chu H, Wang MF et al (2010) Identification of flavone phytoalexins and a pathogen-inducible flavone synthase II gene (SbFNSII) in Sorghum. J Exp Bot 61:983–994
Eshed Y, Zamir D (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141:1147–1162
Espinoza-Moreno RJ, Reyes-Moreno C, Milan-Carrillo J et al (2016) Healthy ready-to-eat expanded snack with high nutritional and antioxidant value produced from whole amarantin transgenic maize and black common bean. Plant Food Hum Nutr 71:218–224
Esposito D, Chen A, Grace MH et al (2014) Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J Agric Food Chem 62:7022–7028
Feng Y, Cao CM, Vikram M et al (2011) A three-component gene expression system and its application for inducible flavonoid overproduction in transgenic Arabidopsis thaliana. PLoS ONE 6:e17603
Fini A, Brunetti C, Di Ferdinando M et al (2011) Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal Behav 6:709–711
Fraser CM, Chapple C (2011) The phenylpropanoid pathway in Arabidopsis. Arabidopsis Book 9:e0152
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch 48:909–930
Goiris K, Muylaert K, Voorspoels S et al (2014) Detection of flavonoids in microalgae from different evolutionary lineages. J Phycol 50:483–492
Gonzali S, Mazzucato A, Perata P (2009) Purple as a tomato: towards high anthocyanin tomatoes. Trends Plant Sci 14:237–241
Grotewold E, Drummond BJ, Bowen B et al (1994) The MYB-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell 76:543–553
Guo Y, Qiu LJ (2013) Allele-specific marker development and selection efficiencies for both flavonoid 3’-hydroxylase and flavonoid 3’,5’-hydroxylase genes in soybean subgenus soja. Theor Appl Genet 126:1445–1455
Gurnani N, Gupta M, Mehta D et al (2016) Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.). J Taibah Univ Sci 10:462–470
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Harker CL, Ellis THN, Coen ES (1990) Identification and genetic-regulation of the chalcone synthase multigene family in pea. Plant Cell 2:185–194
Hasegawa M, Mitsuhara I, Seo S et al (2014) Analysis on blast fungus-responsive characters of a flavonoid phytoalexin sakuranetin; accumulation in infected rice leaves, antifungal activity and detoxification by fungus. Molecules 19:11404–11418
Hatier JHB, Gould KS (2008) Foliar anthocyanins as modulators of stress signals. J Theor Biol 253:625–627
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584
Herrera-Sotero MY, Cruz-Hernández CD, Trujillo-Carretero C et al (2017) Antioxidant and antiproliferative activity of blue corn and tortilla from native maize. Chem Cent J 11:110
Hichri I, Barrieu F, Bogs J et al (2011) Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J Exp Bot 62:2465–2483
Hintz T, Matthews KK, Di R (2015) The use of plant antimicrobial compounds for food preservation. Biomed Res Int 2015:246264
Hou MY, Mu GJ, Zhang YJ et al (2017) Evaluation of total flavonoid content and analysis of related EST-SSR in chinese peanut germ plasm. Crop Breed Appl Biot 17:221–227
Hwang SL, Shih PH, Yen GC (2012) Neuroprotective effects of citrus flavonoids. J Agric Food Chem 60:877–885
Ioannou I, Hafsa I, Hamdi S et al (2012) Review of the effects of food processing and formulation on flavonol and anthocyanin behaviour. J Food Eng 111:208–217
Ismail H, Maksimovic JD, Maksimovic V et al (2016) Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct Plant Biol 43:75–86
Itkin M, Aharoni A (2009) Bioengineering. In: Osbourn A, Lanzotti V (eds) Plant-derived natural products, p 435
Izbiańska K, Arasimowicz-Jelonek M, Deckert J (2014) Phenylpropanoid pathway metabolites promote tolerance response of lupine roots to lead stress. Ecotox Environ Safe 110:61–67
Jin M, Zang X, Zhao M et al (2017) Integrated genomics-based mapping reveals the genetics underlying maize flavonoid biosynthesis. BMC Plant Biol 17:17
Jones CM, Mes P, Myers JR (2003) Characterization and inheritance of the anthocyanin fruit (Aft) tomato. J Hered 94:449–456
Jung CS, Griffiths HM, De Jong DM et al (2009) The potato developer (D) locus encodes an R2R3 MYB transcription factor that regulates expression of multiple anthocyanin structural genes in tuber skin. Theor Appl Genet 120:45–57
Kang J, Li Z, Wu T et al (2010) Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.). Food Chem 122:610–617
Karaaslan M, Ozden M, Vardin H et al (2011) Phenolic fortification of yogurt using grape and callus extracts. Lwt-Food Sci Technol 44:1065–1072
Karageorgou P, Manetas Y (2006) The importance of being red when young: anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiol 26:613–621
Kim S, Yoo KS, Pike LM (2005) Development of a co-dominant, PCR-based marker for allelic selection of the pink trait in onions (Allium cepa), based on the insertion mutation in the promoter of the anthocyanidin synthase gene. Theor Appl Genet 110:1167
Kliebenstein DJ (2008) A role for gene duplication and natural variation of gene expression in the evolution of metabolism. PLoS ONE 3:e1838
Kliebenstein DJ (2009) Use of secondary metabolite variation in crop improvement. In: Osbourn A, Lanzotti V (eds) Plant-derived natural products. Springer, New York, p 83
Korkina LG (2007) Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol 53:15–25
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 2013:162750
Lee J, Chung JH, Kim HM et al (2016) Designed nucleases for targeted genome editing. Plant Biotechnol J 14:448–462
Li PM, Cheng LL (2009) The elevated anthocyanin level in the shaded peel of ‘Anjou’ pear enhances its tolerance to high temperature under high light. Plant Sci 177:418–426
Lim S, Xu JT, Kim J et al (2013) Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol Nutr Food Res 57:1908–1917
Lin T, Zhu GT, Zhang JH et al (2014) Genomic analyses provide insights into the history of tomato breeding. Nat Genet 46:1220–1226
Lippman ZB, Semel Y, Zamir D (2007) An integrated view of quantitative trait variation using tomato interspecific introgression lines. Curr Opin Genet Dev 17:545–552
Liu JY, Osbourn A, Ma PD (2015) MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol Plant 8:689–708
Lopez-de-Dicastillo C, Catala R, Gavara R et al (2011) Development of new antioxidant active packaging films based on ethylene vinyl alcohol copolymer (EVOH) and green tea extract. J Agric Food Chem 59:7832–7840
Lopez-Gresa MP, Torres C, Campos L et al (2011) Identification of defence metabolites in tomato plants infected by the bacterial pathogen Pseudomonas syringae. Environ Exp Bot 74:216–228
Luo J, Butelli E, Hill L et al (2008) AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: expression in fruit results in very high levels of both types of polyphenol. Plant J 56:316–326
Ma DY, Sun DX, Wang CY et al (2014) Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol Biochem 80:60–66
Mahboubi A, Asgarpanah J, Sadaghiyani PN et al (2015) Total phenolic and flavonoid content and antibacterial activity of Punica granatum L. Var. Pleniflora flowers (golnar) against bacterial strains causing foodborne diseases. BMC Compl Altern Med 15:366
Malacarne G, Coller E, Czemmel S et al (2016) The grapevine VvibZIPC22 transcription factor is involved in the regulation of flavonoid biosynthesis. J Exp Bot 67:3509–3522
Mandalari G, Bennett RN, Bisignano G et al (2007) Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J Appl Microbiol 103:2056–2064
Mandalari G, Bisignano C, D’Arrigo M et al (2010) Antimicrobial potential of polyphenols extracted from almond skins. Lett Appl Microbiol 51:83–89
Martin C, Li J (2017) Medicine is not health care, food is health care: plant metabolic engineering, diet and human health. New Phytol 216:699–719
Martin C, Butelli E, Petroni K et al (2011) How can research on plants contribute to promoting human health? Plant Cell 23:1685–1699
Martin C, Zhang Y, Tonelli C et al (2013) Plants, diet, and health. Annu Rev Plant Biol 64:19–46
Mateus N, de Freitas V (2008) Anthocyanin as food colorants. In: Winefield C, Davies K, Gould K (eds) Anthocyanins. Springer, New York, p 283
Matsuda F, Nakabayashi R, Yang ZG et al (2015) Metabolome-genome-wide association study dissects genetic architecture for generating natural variation in rice secondary metabolism. Plant J 81:13–23
Mes PJ, Boches P, Myers JR et al (2008) Characterization of tomatoes expressing anthocyanin in the fruit. J Am Soc Hortic Sci 133:262–269
Mierziak J, Kostyn K, Kulma A (2014) Flavonoids as important molecules of plant interactions with the environment. Molecules 19:16240–16265
Mladenka P, Zatloukalova L, Filipsky T et al (2010) Cardiovascular effects of flavonoids are not caused only by direct antioxidant activity. Free Radical Biol Med 49:963–975
Moore B, Andrew RL, Kulheim C et al (2014) Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol 201:733–750
Morand C, Dubray C, Milenkovic D et al (2011) Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr 93:73–80
Morkunas I, Wozniak A, Formela M et al (2016) Pea aphid infestation induces changes in flavonoids, antioxidative defence, soluble sugars and sugar transporter expression in leaves of pea seedlings. Protoplasma 253:1063–1079
Nagamatsu A, Masuta C, Senda M et al (2007) Functional analysis of soybean genes involved in flavonoid biosynthesis by virus-induced gene silencing. Plant Biotechnol J 5:778–790
Nems A, Peksa A, Kucharska AZ et al (2015) Anthocyanin and antioxidant activity of snacks with coloured potato. Food Chem 172:175–182
Pazos M, Gallardo JM, Torres JL et al (2005) Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem 3:547–557
Pearse IS, Heath KD, Cheeseman JM (2005) Biochemical and ecological characterization of two peroxidase isoenzymes from the mangrove, Rhizophora mangle. Plant Cell Environ 28:612–622
Peng XF, Ma JY, Cheng KW et al (2010) The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem 119:49–53
Petersen B, Snapp S (2015) What is sustainable intensification? Views from experts. Land Use Policy 46:1–10
Petroni K, Pilu R, Tonelli C (2014) Anthocyanins in corn: a wealth of genes for human health. Planta 240:901–911
Plaza M, Pozzo T, Liu JY et al (2014) Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J Agric Food Chem 62:3321–3333
Racchi ML (2013) Antioxidant defenses in plants with attention to prunus and Citrus spp. Antioxidants 2:340–369
Raffa D, Maggio B, Raimondi MV et al (2017) Recent discoveries of anticancer flavonoids. Euro J Med Chem 142:213–228
Rey AI, Hopia A, Kivikari R et al (2005) Use of natural food/plant extracts: cloudberry (Rubus Chamaemorus), beetroot (Beta Vulgaris “Vulgaris”) or willow herb (Epilobium angustifolium) to reduce lipid oxidation of cooked pork patties. LWT- Food Sci Technol 38:363–370
Rhodes DH, Hoffmann L, Rooney WL et al (2014) Genome-wide association study of grain polyphenol concentrations in global sorghum [Sorghum bicolor (L.) Moench] germplasm. J Agric Food Chem 62:10916–10927
Rigano MM, Raiola A, Docimo T et al (2016) Metabolic and molecular changes of the phenylpropanoid pathway in tomato (Solanum lycopersicum) lines carrying different Solanum pennellii wild chromosomal regions. Front Plant Sci 7:1484
Rinaldi A, Villano C, Lanzillo C et al (2017) Metabolic and RNA profiling elucidates proanthocyanidins accumulation in Aglianico grape. Food Chem 233:52–59
Rousseaux MC, Jones CM, Adams D et al (2005) QTL analysis of fruit antioxidants in tomato using Lycopersicon pennellii introgression lines. Theor Appl Genet 111:1396–1408
Schaefer HM, Rolshausen G (2006) Plants on red alert: Do insects pay attention? BioEssays 28:65–71
Shahidi F, Ambigaipalan P (2015) Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects—a review. J Funct Foods 18:820–897
Shao YF, Jin L, Zhang G et al (2011) Association mapping of grain color, phenolic content, flavonoid content and antioxidant capacity in dehulled rice. Theor Appl Genet 122:1005–1016
Sharma P, Jha AB, Dubey RS et al (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26
Synowiec A, Gniewosz M, Krasniewska K et al (2014) Antimicrobial and antioxidant properties of pullulan film containing sweet basil extract and an evaluation of coating effectiveness in the prolongation of the shelf life of apples stored in refrigeration conditions. Innov Food Sci Emerg 23:171–181
Tai HH, Goyer C, Murphy AM (2013) Potato MYB and bHLH transcription factors associated with anthocyanin intensity and common scab resistance. Botany 91:722–730
Tattini M, Galardi C, Pinelli P et al (2004) Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol 163:547–561
The Potato Genome Consortium (2011) Genome sequence and analysis of the tuber crop potato. Nature 475:189–194
Tian J, Zhang J, Han ZY et al (2017) McMYB12 transcription factors co-regulate proanthocyanidin and anthocyanin biosynthesis in Malus crabapple. Sci Rep 7:43715
Toda K, Takahashi R, Iwashina T et al (2011) Difference in chilling-induced flavonoid profiles, antioxidant activity and chilling tolerance between soybean near-isogenic lines for the pubescence color gene. J Plant Res 124:173–182
Tohge T, Perez de Souza L, Fernie AR (2017a) On the natural diversity of phenylacylated-flavonoid and their in planta function under conditions of stress. Phytochem Rev 1–12
Tohge T, Perez de Souza L, Fernie AR (2017b) Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J Exp Bot 68:4013–4028
Treutter D (2005) Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol 7:581–591
Venisse JS, Gullner G, Brisset MN (2001) Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol 125:2164–2172
Viji P, Binsi PK, Visnuvinayagam S et al (2015) Efficacy of mint (Mentha arvensis) leaf and citrus (Citrus aurantium) peel extracts as natural preservatives for shelf life extension of chill stored indian mackerel. J Food Sci Technol Mys 52:6278–6289
Wahid A, Ghazanfar A (2006) Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J Plant Physiol 163:723–730
Wang B, Zhang X (2012) Inhibitory effects of broccolini leaf flavonoids on human cancer cells. Scanning 34:1–5
Wen WW, Li D, Li X et al (2014) Metabolome-based genome-wide association study of maize kernel leads to novel biochemical insights. Nat Commun 5:3438
Wen ZX, Boyse JF, Song QJ et al (2015) Genomic consequences of selection and genome-wide association mapping in soybean. BMC Genom 16:671
Willits MG, Kramer CM, Prata RTN et al (2005) Utilization of the genetic resources of wild species to create a nontransgenic high flavonoid tomato. J Agric Food Chem 53:1231–1236
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Wu H, Dushenkov S, Ho CT et al (2009) Novel acetylated flavonoid glycosides from the leaves of Allium ursinum. Food Chem 115:592–595
Yan JH, Wang BA, Jiang YN et al (2014) GmFNSII-controlled soybean flavone metabolism responds to abiotic stresses and regulates plant salt tolerance. Plant Cell Physiol 55:74–86
Yao LH, Jiang YM, Shi J et al (2004) Flavonoids in food and their health benefits. Plant Food Hum Nutr 59:113–122
Yashin A, Yashin Y, Xia X et al (2017) Antioxidant activity of spices and their impact on human health: a review. Antioxidants 6:70
Zhai R, Wang ZM, Zhang SW et al (2016) Two MYB transcription factors regulate flavonoid biosynthesis in pear fruit (Pyrus bretschneideri Rehd.). J Exp Bot 67:1275–1284
Zhang YF, Jung CS, De Jong WS (2009) Genetic analysis of pigmented tuber flesh in potato. Theor Appl Genet 119:143–150
Zhang Y, Butelli E, De Stefano R et al (2013) Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr Biol 23:1094–1100
Zhang Y, Butelli E, Alseekh S et al (2015a) Multi-level engineering facilitates the production of phenylpropanoid compounds in tomato. Nat Commun 6:8635
Zhang Y, De Stefano R, Robine M et al (2015b) Different reactive oxygen species scavenging properties of flavonoids determine their abilities to extend the shelf life of tomato. Plant Physiol 169:1568–1583
Zhou H, Wang-Li K, Wang H et al (2015a) Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors. Plant J 82:105–121
Zhou X, Jacobs TB, Xue LJ et al (2015b) Exploiting SNPs for biallelic CRISPR mutations in the outcrossing woody perennial populus reveals 4-coumarate: CoA ligase specificity and redundancy. New Phytol 208:298–301
Zhou M, Zhang K, Sun Z et al (2017) LNK1 and LNK2 corepressors interact with the MYB3 transcription factor in phenylpropanoid biosynthesis. Plant Physiol 174:1348–1358
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Amelia, V., Aversano, R., Chiaiese, P. et al. The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding. Phytochem Rev 17, 611–625 (2018). https://doi.org/10.1007/s11101-018-9568-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-018-9568-y