Abstract
The perception of aphid infestation induces highly coordinated and sequential defensive reactions in plants at the cellular and molecular levels. The aim of the study was to explore kinetics of induced antioxidative defence responses in leaf cells of Pisum sativum L.cv. Cysterski upon infestation of the pea aphid Acyrthosiphon pisum at varying population sizes, including accumulation of flavonoids, changes of carbon metabolism, and expression of nuclear genes involved in sugar transport. Within the first 96 h, after A. pisum infestation, flavonoid accumulation and increased peroxidase activity were observed in leaves. The level of pisatin increased after 48 h of infestation and reached a maximum at 96 h. At this time point, a higher concentration of flavonols was observed in the infested tissue than in the control. Additionally, strong post-infestation accumulation of chalcone synthase (CHS) and isoflavone synthase (IFS) transcription products was also found. The levels of sucrose and fructose in 24-h leaves infested by 10, 20, and 30 aphids were significantly lower than in the control. Moreover, in leaves infested by 30 aphids, the reduced sucrose level observed up to 48 h was accompanied by a considerable increase in the expression level of the PsSUT1 gene encoding the sucrose transporter. In conclusion, A. pisum infestation on pea leads to stimulation of metabolic pathways associated with defence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among secondary metabolites, flavonoids are responsible for many key functions, which are critical for plant survival (Gould and Lister 2006). Flavonoids, like other phenolics, play an important role in the defence against insect herbivores, such as aphids (Simmonds 2003). Studies on insect–plant interactions have revealed important contributions of isoflavonoids (Simmonds and Stevenson 2001), likewise flavones, flavonols, and anthocyanins are equally important (Gould and Lister 2006; Lev-Yadun and Gould 2009). The accumulation of these secondary metabolites observed in response to aphid attacks (Lattanzio et al. 2000) resembles cellular action against other stress factors of both biotic (Morkunas et al. 2007, 2011a, b, 2013) and abiotic origin (Harborne and Williams 2000; Pourcel et al. 2007). These metabolites are extremely important not only for plant defence reactions, including against oxidative stress, but also due to their function as intracellular signaling molecules (Peer and Murphy 2006). Therefore, flavonoids may affect transcription, translation, enzyme activity, signal transduction, and enhance cell–cell communication in plants. The present study highlights the important role of flavonoids in pea, particularly pisatin (an isoflavonoid derivative belonging to the pterocarpan family) as phytoalexin (Jeandet et al. 2013, 2014) involved in the defence strategy against the pea aphids, and the significance of the allocation of carbon in the stimulation of secondary metabolism, including the phenylpropanoid pathway. Simultaneously, we emphasize the essential role of sugar transporters for the distribution of carbohydrates inside plant cells during plant–aphids interaction. This is the first report revealing the kinetics of induced antioxidative defence, including post-infestation changes of soluble sugars, expression of nuclear genes engaged in sucrose transport and subcellular location of flavonoids, and total antioxidant capacity in plant response to aphid attack. Only very few studies have demonstrated a significant role of the free flavonoids aglycones in the mechanism of plant resistance to aphid feeding (Lattanzio et al. 2000; Ateyyat et al. 2012). Recent research on plant defense response to other biotic stress factors than insects revealed that flavonoids’ biosynthesis requires an effective flow of carbon to the phenylpropanoid pathway. A significantly stimulatory role of sugars in the secondary metabolism was mainly demonstrated in in vitro studies (Morkunas et al. 2005; Solfanelli et al. 2006; Formela et al. 2014). It has been reported that soluble sugars such as sucrose, glucose, and fructose can be donors of the carbon skeletons for secondary metabolism and signaling molecules regulating the expression of genes encoding flavonoid biosynthesis enzymes (Morkunas et al. 2007, 2011a). However, sucrose has a central position in plant metabolism as the first free sugar formed during photosynthesis (Koch 2004; Van den Ende 2013) and the major form of translocated sugars in the phloem (Shiratake 2007; Van Bel and Hess 2008). Besides, sucrose creates the driving force for long-distance translocation of all other compounds in the phloem (Chen et al. 2015). Sucrose partitioning from the site of synthesis to different sites of storage, conversion into other storage compounds or metabolic degradation, involves various steps of cell-to-cell movement and transport. Sucrose translocation and accumulation are regulated by the sucrose/proton symporter proteins (SUTs) (Sauer 2007; Kühn and Grof 2010; Kühn 2010). SUTs play an important role in apoplastic phloem loading in higher plants (Sauer 2007; Reinders et al. 2012). Sucrose enters the phloem for long-distance transport either symplastically via plasmodesmata or apoplastically via SWEET-mediated export from phloem parenchyma cells, followed by SUT-mediated uptake into phloem companion cells (Chen et al 2012; Berthier et al. 2009). As reported by Kühn and Grof (2010), the phloem-specific expression of high affinity group II plasma membrane SUTs is consistent with a role in apoplastic phloem loading and subsequent recovery of sucrose that escapes during phloem transport. Electron microscopic localisation of the SUT1 protein and mRNA revealed sieve element-specific localisation of the protein, while mRNA was found in both the companion cells and sieve elements and was most abundant in the vicinity of pore plasmodesmata units (PPU) connecting both cell types (Kühn et al. 2003). In pea, plants sucrose transporters have been identified that exhibit pH- and energy-independent sucrose transport in a bi-directional manner (Zhou et al. 2007). These transport properties, together with counter transport, are consistent with a sucrose facilitator (SUF) function. The cellular and subcellular localisation of PsSUT1, PsSUF1, and PsSUF4, cloned from pea, was carried out to determine their potential contribution to phloem loading and unloading of sucrose in planta (Dibley 2012). Immunolocalisation studies in seed coat tissues revealed that PsSUF1 and PsSUF4 are present in the inner layers of parenchyma, the putative site of sucrose release in developing seed coats. In pea, phloem loading of leaf minor veins follows an apoplastic pathway (Wimmers and Turgeon 1991). As such, two transport events—efflux from the symplasm and subsequent uptake by accumulation in the sieve elements phloem—occur in series. Thus, the minor veins of source leaves were investigated as another region of symplasmic discontinuity and hence as a site of sucrose efflux. SUFs were not located in putative efflux cells (bundle sheath or phloem parenchyma cells), but were present, along with PsSUT1, on the plasma membranes of elements (Dibley 2012). It has been also demonstrated that all three transporters of pea are detectable at the plasma membrane. The likely physiological role for PsSUT1 involves the uptake of sucrose into cells against the prevailing gradient, while for the facilitators a role appears likely in equilibrating sucrose between the cytoplasm and apoplasm. Furthermore, GFP-fusion studies have shown that sucrose transporters can localise in vivo to different cellular membranes—the plasma membrane (Sivitz et al. 2007; Chincinska et al. 2008) or the tonoplast (Weschke et al. 2000; Endler et al. 2006; Reinders et al. 2008. Payyavula et al. 2011).
The multiple roles of plant sucrose transporters and especially the central role of sucrose loading into the phloem suggest sucrose transport to be strongly regulated by many environmental factors, including biotic stresses (Sauer 2007; Wahl et al. 2010; Lemoine et al. 2013). For example, the distribution of sucrose in plant cells may be crucial for the induction of plant defence against biotic stresses such as fungal pathogens (Doidy et al. 2012). Recent studies demonstrated the crucial role of cell wall invertases, which after sucrose release into the apoplast by sucrose transporters cleave sucrose into the hexoses glucose and fructose which can subsequently be taken up via hexose transporters (Proels and Huckelhoven 2014). Thereby, cell wall invertases are not only involved in metabolic reprogramming during plant-pathogen interactions like recently shown in Nicotiana attenuata (Ferrieri et al. 2015) but also play a crucial role in symbiotic interactions between host plants and arbuscular mycorrhizal fungi (Schaarschmidt et al. 2006). Thus, the expression of sucrose transporters seems to be tightly regulated in accordance with cell wall invertases in response to many environmental factors including biotic stresses (Bitterlich et al. 2014; Ferrieri et al. 2015).
Sucrose taken by aphid is present in plant phloem sap at high concentrations, often in the range of 0.5–1 M and is responsible for high osmotic pressure, often up to five times that of the feeding Hemipteran body fluids (Douglas 2006). We focused on the sucrose and sucrose transporter and sucrose facilitator expression during the infestation of the pea aphid of Pisum sativum L. leaves because sucrose is the most important donor of carbon skeleton for the phenylpropanoid pathway via which pisatin is synthesized and a signaling molecule and the determination of expression of sucrose transporters may be essential in the context of pea defence response to feeding by aphid Acyrthosiphon pisum. Besides, aphids absorb sucrose from the phloem sap and excrete from body as honeydew. Aphids success likely results from their ability to simultaneously suppress plant defense responses and manipulate phloem flow and composition (Voelckel et al. 2004).
Plant defense response to aphid feeding is the result of injury and effectors in saliva (Smith and Chuang 2014). Aphids using fine stylets penetrate tissues intercellularly, making punctures, and wait a few seconds to analyze the physicochemical properties of the microenvironment around the style tip (Tjallingi 2006). Aphid effectors are most likely expressed in salivary glands and secreted into saliva, which is delivered inside the host during feeding and probing. Aphid effectors are able to promote aphid virulence upon transient and/or transgenic over expression. For example, the effector C002, which was first identified in A. pisum, is an abundant salivary protein that is essential for aphid feeding (Mutti et al. 2008). Little is known about the time-dependent aspect of induced defensive reactions at the cellular and molecular levels (Kuśnierczyk et al. 2008). Unique differences in responses associated with the generation of signaling molecules by P. sativum L. to A. pisum infestation were revealed previously (Mai et al. 2013, 2014).
We started our studies with the aim to verify synthesis and accumulation of secondary metabolites, such as flavonoids, in particularly pisatin, in response to the infestation of the pea aphid of P. sativum L. leaves. Apart from these investigations, transcript levels of genes encoding important enzymes engaged in the biosynthesis of flavonoids, such as chalcone synthase (CHS) and isoflavone synthase (IFS) were analyzed. The second goal was to determine the total antioxidant capacity (TAC), and peroxidase (POX) activity toward the phenolic substrate syringaldazine was examined. The third goal of the study was to determine the level of soluble sugars (sucrose, glucose, and fructose) and the expression of genes encoding the sucrose transporter (SUT1) and facilitators (SUF1 and SUF4).
Materials and methods
Material
Experiments were conducted on leaves of edible pea seedlings (P. sativum L. cv. Cysterski). Pea seeds were obtained from the Plant Breeding Company at Tulce near Poznan in Poland. Surface-sterilization seeds and seeds imbibition were performed exactly, as described by Mai et al. (2014). The imbibed seeds were sown in 20-cm-diameter pots containing sterilized perlite. Pea seedlings were kept in a growth chamber at 22–23 °C, 65 % relative humidity, and light intensity of 130–150 μM photons m−2 s−1 with the 14/10 h (light/dark) photoperiod.
Aphids and infestation experiment
A. pisum (Harris) originally cultured and supplied by the Department of Entomology Poznań University of Life Sciences, Poland was reared on P. sativum L. in a growth chamber under conditions as mentioned above. On the 10th day of culture, pea seedlings were infested with 10, 20, or 30 apterous adult females of A. pisum (Mai et al. 2013). The aphid populations were monitored throughout all experiments. The control plants were pea seedlings with no pea aphids.
Cytochemical localization and accumulation of flavonoids
Flavonoids were detected in cells by staining pea leaves with diphenylboric acid 2-aminoethyl ester (DPBA; Merck), as described by Morkunas et al. (2011a). Leaves were collected at 0, 24, 48, 72, and 96 hpi and analysed by confocal laser scanning microscopy. Fluorescence was visualized under Zeiss LSM 510 laser scanning confocal microscope (argon laser excitation 488 nm, emission band pass 505–550 nm, long distance-LD 63 objective lens). Fluorescence was observed in the epidermal and cortical cells of leaves without and after pea aphid infestation. Confocal microscopy experiments were repeated three times with similar results.
Analysis of pisatin and flavonols
Isolation of phenolic compounds
Plant material, which was frozen at −80 °C, was homogenized in 80 % methanol (20 ml g−1 FW) and sonicated for 3 min using VirTis VirSonic 60 sonicator (Morkunas et al. 2013). The suspension was filtered through a Büchner funnel and concentrated under vacuum at 40 °C. Samples of plant extracts for LC analyses were prepared from 0.5 g FW pea tissue. The samples were purified and concentrated by solid-phase extraction on cartridges containing a cation exchanger and RP C-18 silica gel (Alltech, Carnforth, England) used in tandem, according to the method of Stobiecki et al. (1997).
Liquid chromatography–mass spectrometry (LC/UV/ESI/MS/MS)
Analyses of plant extract samples were performed with a Waters UPLC Acquity system connected to a micrOToF-Q mass spectrometer produced by Bruker Daltonics (Bremen, Germany). An Agilent Poroshell RP-C18 column (100 × 2.1 mm; 2.7 um) was used. During LC analyses, elution was carried out with two solvent mixtures: A (95 % H2O, 4.5 % acetonitrile, 0.5 % acetic acid; v/v/v) and B (95 % acetonitrile, 4.5 % H2O, 0.5 % acetic acid; v/v/v). Elution steps were as follows: 0–5 min 10–30 % B, 5–12 min isocratic at 30 % B, 12–13 min linear gradient up to 95 % of B, and 13–15 min isocratic at 95 % of B. Pisatin and flavonols were identified by comparing their retention times and mass spectra with the data obtained from respective standards. The micrOToF-Q mass spectrometer consisted of an ESI source operating at a voltage of ±4.5 kV, nebulization with nitrogen at 1.2 bar, and dry gas flow of 8.0 l/min at a temperature of 220 °C. The instrument was operated using the micrOTOF Control program version 2.3, and data were analyzed using the Bruker Data Analysis ver. 4 package. Targeted MS/MS experiments were performed using a collision energy ranging from 10 to 25 eV, depending on the molecular masses of compounds. The instrument operated at a resolution higher than 15,000 full widths at half maximum.
For quantitative analysis, the LC-UV trace from a DAD detector (Waters) was used, with peaks plotted at 259 and 350 nm. p-hydroxybenzoic acid was added to each analyzed sample as an internal standard at a final concentration of 125 mM (LC retention time and UV spectral data did not interfere with those of the studied compounds).
Total RNA extraction and reverse transcription–polymerase chain reaction (RT-PCR) analysis
Pea seedling leaves (0.50 g) were frozen in liquid nitrogen and ground with a mortar and pestle in the presence of liquid nitrogen. For RT-PCR analyses of a target gene, total RNA was isolated from 45 mg tissue using the SV Total RNA Isolation System (Promega) according to the recommendations of the manufacturer (Morkunas et al. 2010; Mai et al. 2014). The PCR products were then analyzed on agarose gel. The sequences of the specific primers for CHS and IFS were used for PCR reactions, such as
-
the forward primer for CHS: TATAGCCTTGACTGCAGCC
-
the reverse primer for CHS: TCCACATAGTGATGGAGC
-
the forward primer for IFS: GGACCTTACTGGAAGTTCAT
-
the reverse primer for IFS: CACAACAAGACCCTTGATT
The following actin primers were used as positive controls:
-
the forward primer for actin: GTATTGTTGGCCGACCTCG
-
the reverse primer for actin: TGGGCTTCATCACCTACATAG
Carbohydrate analysis
Leaves of pea seedlings (150 mg) were ground in liquid nitrogen using a 30 Hz laboratory ball mill (1 min, two balls per 2 ml Eppendorf tube) and flooded with 1.4 ml 80 % cooled methanol (MeOH, HPLC). The samples were supplemented with 25 μl ribitol (1 mg/1 ml) and then derivatization was performed, as described by Formela et al. (2014). Endogenous carbohydrate levels were determined by gas chromatography coupled with mass spectrometry (GC-MS) (a 6890 N gas chromatograph by Agilent with a GCT Premier mass spectrometer by Waters) using a DB-5MS column (30 m × 0.25 mm × 0.25 μm J&W Scientific). Carbohydrate content was expressed in microgram per 1 mg fresh weight.
Quantification of mRNAs encoding sugar transporters
RNA extraction
Total RNA from pea seedling leaves was isolated with the Trizol RNA isolation kit (Invitrogen, CA, USA) according to the manufacturer’s protocol. Approximately 100 mg fresh weight of P. sativum seedlings with or without aphid infestation were sampled and processed as described. In addition to the manufacturer’s protocol, RNA pellets were washed three times with 1.5 ml 75 % (v/v) EtOH to reliably remove traces of phenol or chaotropic salts. The RNA pellet was resuspended in 30–50 μl ultrapure RNase free water (Milli-Q water, autoclaved) and incubated for 10 min at 55 °C to completely dissolve the pellet. For storage, RNA was kept at −80 °C, and all work was conducted on ice with sterile RNase- and DNase-free filter tips.
qPCR
Quantitative real time PCR analyses were performed with the C1000 Thermocycler coupled with the CFX96 Real time System (Bio-Rad). Reactions of 10 μl were set up with the SensiMix SYBR No-ROX One-Step Kit (Bioline) on flat 96-well PCR plates (VWR) covered with MicroAmp optical adhesive films (Applied Biosystems). The amount of cDNA template was based on the total RNA used for cDNA synthesis. Standard cycle conditions were used for all reactions with 40 cycles and 61 °C annealing temperature. The relative transcription level was calculated using the 2-∆∆C T method as described by Livak and Schmittgen (2001).
Primers used for qPCR
Relative quantification of transcript amounts was always calculated in relation to the respective standard transcript level and given in arbitrary units. Primers were designed to obtain a 50–100 bp amplicon using Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Primer sequences used for real time PCR analysis: ß-tubulin fw: GCT CCC AGC AGT ACA GGA CTC T; ß-tubulin rev: TGG CAT CCC ACA TTT GTT GA; PsSUT1 fw: GCT TGT CCA GCC TGT AGT CG; PsSUT1 rev: CGA TAG CTA CGG CGA TTG AG; PsSUF1 f. CGG TCG CAG TCT TTC TCA TC; PsSUF1 a rev AGC GAC GTC GAG TAT CCA GA; PsSUF4 a fw: CGG ATT TGG GAT GGA AGT TT; PsSUF4 a rev: CAA AAC CCG AAC ACG AAA AA; PsPPA2 a rev: GAG CCC AGA ACA GGA GCT AAC A; PsPPA2 a fw: CCA CAT TAC CTG TAT CGG ATG ACA. Real time PCR data were corrected individually by calculation of PCR efficiency using the LinReg PCR software.
Extraction and assay of peroxidase activity
Frozen pea seedling leaves (400 mg) were homogenized at 4 °C with a mortar and pestle in 1.8 mL of 100 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 10 % (w/v) polyvinylopyrrolidone (PVP). The slurry was centrifuged at 15,000 g for 30 min at 4 °C, and the supernatants were used for enzyme assays. The protein concentration in the samples was estimated according to Bradford (1976) using bovine serum albumin as a standard. Peroxidase (EC 1.11.1.7) activity was measured spectrophotometrically using the phenolic substrate syringaldazine, as described by Morkunas and Gmerek (2007). The activity of the enzyme was expressed as enzyme unit per 1 mg protein (U mg−1 protein).
Total antioxidant capacity
Total antioxidant capacity was measured using the ability of antioxidants contained in the extract to reduce the cation ABTS+, as described by Morkunas et al. (2013). Typical antioxidants such as ascorbate and glutathione react very rapidly with ABTS+, thereby the decrease in absorbance of the solution containing the extract after 10 s is a measure of their contents in the sample. In turn, residues of tyrosine and tryptophan in proteins react more slowly; therefore, the decrease in absorbance of the solution containing the extract after 30 min is a measure of their contents. The calibration curve was plotted by adding 5 μl portions of 1 mM Trolox to the diluted ABTS+ and measuring the gradual decrease in absorbance. The final result of TAC was expressed as micromolar Trolox per gram of fresh weight.
Statistical analysis
All determinations were performed in three independent experiments, i.e., on plant material obtained from independent cultures. Analysis of variance (ANOVA) was applied to verify whether means from independent experiments within a given experimental variant were significant. Data shown in the figures are means of triplicates for each variant and standard errors of mean (SE). In individual figures significant differences are shown using asterisks.
Results
The effect of Acyrthosiphon pisum infestation on cytochemical localization and accumulation of flavonoids
A post-infestation increase in the accumulation of flavonoids was observed in epidermal cells of the leaves of pea seedling (Fig. 1). An intensive emission of green fluorescence was observed in the period from 48 to 96 h after infestation. A very high content of these metabolites was observed 72 h after infestation, particularly in leaves infested by 30 aphids. A strong accumulation of flavonoids was mainly detected in the cells, while in 48-h infested leaf cells a high content was recorded in the cell wall. In turn, at 96 h infestation, the intensity of green fluorescence was weaker than at 72 h infestation.
The effect of Acyrthosiphon pisum infestation on cytochemical localization and accumulation of flavonoids in seedling leaves of Pisum sativum L.cv. Cysterski. Green fluorescence came from diphenylboric acid 2-aminoethyl ester (DPBA), which is observed by a Zeiss LSM 510 confocal microscope, scale bar 20 μm
The effect of Acyrthosiphon pisum infestation on the level of pisatin
During 24 h of cultivation, the pisatin level decreased in control and infested seedlings (Fig. 2) with the exception of a slow increase in the level of this phytoalexin recorded in leaves of pea seedlings which were infested by 10 aphids. Starting from 48 h in leaves infested by 20 and 30 aphids, the concentration of pisatin increased markedly with time. This increase was proportional to population sizes of aphids colonizing pea seedlings. The highest concentration of pisatin was recorded in leaves infested by 30 aphids at 72 and 96 h; its concentration was 1.5 and 3.5 times, respectively, higher than in the control samples.
The effect of Acyrthosiphon pisum infestation on the level of flavonols
The LC-UV(DAD) chromatogram identified the following flavonols in the samples applied: kaempferol-diglucosides, kaempferol-triglucoside, kaempferol, quercetin-O-glucoside, and quercetin-3-O-glucoside. Leaves of pea seedlings infested by aphids were found to contain lower levels of kaempferol-diglucosides than the control at 24 and 48 h infestation (Fig. 3). In the leaves infested by 10 aphids levels of kaempferol-diglucosides were approximately 2 and 1.7 times lower at these timepoints in comparison to the control, whereas in the leaves infested by 20 and 30 aphids the levels were approximately 1.3 times lower than in the control. Between 72 and 96 h after infestation, a marked increase was observed in the concentrations of these metabolites (Fig. 3a, b, c). A 2.5- to 4.5-fold higher level of these compounds was recorded in leaves after 96 h A. pisum infestation in relation to the control. The highest concentration of kaempferol-diglucosides was observed in leaves infested by 30 aphids at this time point. The level of the flavonol kaempferol was lower at 24 h of feeding than in the control only (Fig. 3e), while at the successive time points of feeding, the concentration of this free aglycon in leaves of pea seedlings was generally higher than in the control. However, the level of kaempferol did not correlate with the number of aphids colonizing pea seedlings. The highest level of this metabolite was recorded at 96 h of feeding in leaves colonized by 10 aphids.
The effect of Acyrthosiphon pisum infestation on the level of flavonols, i.e., kaempferol-diglucosides (a,b,c), kaempferol-triglucosides (d) kaempferol (e), quercetin-3-O-glucoside (f), and quercetin-3-O-glucoside (g), in seedling leaves of Pisum sativum L. cv. Cysterski. Significance between the control and aphid infestations was determined by ANOVA and is represented by * (P < 0.05)
In analogy to kaempferol-diglucosides (Fig. 3a,b,c), the kinetics of the levels of quercetin-O-glucoside were similar in leaves of the control and infested seedlings (Fig. 3f). At 24 and 48 h infestation by A. pisum, the level of quercetin-O-glucoside was lower than in the control, while at 96 h of feeding by A. pisum the level of this compound was many times higher than in the control and the content correlated with the number of infesting aphids (Fig. 3f). The levels of quercetin-3-O-glucoside (Fig. 3g) and kaempferol-triglucoside (Fig. 3d) dropped drastically between 0 and 24 h culture in all the experimental variants, while this decrease was moderately at the successive time points.
The mRNA level in flavonoid biosynthetic pathway genes
The relative transcript levels of the flavonoid biosynthetic genes, i.e., chalcone synthase (CHS) and isoflavone synthase (IFS), in both the control and in aphid-infested leaves, were analyzed using semi-quantitative RT-PCR (Fig. 4). A strong post-infestation accumulation of the CHS transcription products after 48 h of infestation was found. In turn, already from 24 h after infestation, a considerable accumulation of the IFS transcription products was recorded in leaves infested by aphids, which was significantly higher at all time points than in the controls. The highest accumulation of the CHS and IFS transcription products was observed in the 48- and 96-h leaves infested by 30 aphids, respectively.
The effect of Acyrthosiphon pisum infestation on total antioxidant capacity
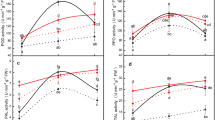
Infestation by A. pisum induced an increase in TAC (Fig. 5). The increase of TAC varied in time depending on the pool of slow- (residues of tyrosine and tryptophan in proteins) (Fig. 5a) and fast-acting (ascorbic acid or glutathione) antioxidants (Fig. 5b). Between 0 and 48 h feeding by A. pisum, a very strong increase was found in TAC, which was due to the accumulation of slow-acting antioxidants in leaves of pea seedlings infested by 20 and 30 aphids (Fig. 5a). Additionally, high content of slow-acting antioxidants was noted at 96 h of feeding in leaves colonized by 10, 20, and 30 aphids. These levels in TAC were significantly higher than in the control samples, but they did not correlate with the population size of feeding aphids. Strong increase in TAC dependent on the pool of fast antioxidants was also noted in pea response to A. pisum. The highest level was recorded at 48 h in pea seedling leaves infested by 10 aphids (Fig. 5b). Thus, the increase of aforementioned defensive antioxidants just like the flavonoid accumulation enhances the antioxidant capacity of pea leaf cells.
The effect of Acyrthosiphon pisum infestation on total antioxidant capacity dependent on the pool of slow- (tyrosine and tryptophan) (a) and fast-acting (ascorbic acid or glutathione) (b) antioxidants in seedling leaves of Pisum sativum L. cv. Cysterski. Significance between the control and aphid infestations was determined by ANOVA and is represented by * (P < 0.05)
The effect on Acyrthosiphon pisum on peroxidase activity
Analyses of peroxidase activity toward syringaldazine (POX) in leaves of pea seedlings showed a markedly higher activity of this enzyme in tissues infested by A. pisum than in the control (Fig. 6). The highest enzyme activity was observed in leaves of pea seedlings infested by 20 and 30 aphids, except for 48 h feeding. In these tissues, the first increase in POX activity was found during 0 to 24 h after A. pisum feeding, followed by the next increase starting from 48 h. The highest enzyme activity was recorded in leaves of pea seedlings exposed to feeding by 30 aphids at 96 h. In leaves subjected to feeding by 10 aphids, POX activity was found to increase in the period from 0 to 48 h feeding only. POX activity correlated with population sizes of pea aphids infesting leaves at 72 h. Also, it was highest in 96-h leaves infested by 30 aphids, but the difference between 10 and 20 aphids of feeding was minimal.
The effect of Acyrthosiphon pisum infestation on soluble sugar content
At 72 and 96 h infestation by A. pisum, lower total sugar content was observed in pea seedling leaves in comparison to control plants (Fig. 7a). The levels of sucrose and fructose already at 24 h infestation were significant lower than in the control, while the value at 48 h after infestation significantly increased (Figs. 7b, c). From 48 h, a decrease in the glucose content of pea seedling leaves infested by pea aphids was also noted (Fig. 7d). In parallel post-infestation reduction of soluble carbohydrates was accompanied by an increase in the level of flavonoids.
The effect of Acyrthosiphon pisum infestation on soluble sugar contents, i.e., total sugar (a), sucrose (b), fructose (c), and glucose (d), in seedling leaves of Pisum sativum L.cv. Cysterski. Significance between the control and aphid infestations was determined by ANOVA and is represented by * (P < 0.05)
The effect of Acyrthosiphon pisum infestation on the level of expression of genes encoding sugar transporters
Quantitative RT-PCR analysis indicates a two times higher sucrose transporter SUT1 transcript level upon infestation by 30 aphids in comparison to control samples and other samples in the period from 0 to 48 h (Fig. 8a). ANOVA results showed that SUT1 transcript levels significantly differ between the aphid-infested and the control plants (e.g., P = 1.83 × 10−6 at infestation by 30 aphids at 48 hpi). Starting from 72 h after feeding on pea seedling leaves, a lower SUT1 expression level was recorded in comparison to the control. These data show correlation with the number of feeding aphids. Moreover, a considerable post-infestation decrease within 24 to 72 h was observed in SUF4 transcript levels in comparison with the control plants. The lowest SUF4 transcript level was found in 72 h leaves of pea seedlings infested by 30 aphids (Fig. 8b). In turn, the SUF1 transcript level strongly fluctuated during feeding (Fig. 8c) and did not correlate with the number of aphids subjected to the seedlings. A considerable increase in SUF1 levels was observed between 24 and 72 h of feeding on leaves of pea seedlings infested by 10 aphids and between 48 and 72 h of feeding by 20 aphids. Moreover, decrease within 24 to 96 h in the transcript level encoding SUF1 in leaves of pea seedlings infested by 30 aphids was recorded in comparison with the control plants (Fig. 8c). At 96 h of feeding, the expression level of the SUF1 and SUT1 genes was significantly lower than in the control plants.
The effect of Acyrthosiphon pisum infestation on the level of expression of genes encoding sugar transporters, i.e., the sucrose transporter SUT1 (a) and sucrose facilitators (SUF4 and SUF1) (b, c). Significance between the control and aphid infestations was determined by ANOVA and is represented by * (P < 0.05)
Discussion
The results of this study for the first time show post-infestation accumulation of pisatin and the subcellular localization of flavonoids, in association with the changes in carbon metabolism and the expression of nuclear encoded sucrose transporter genes in the plant response to aphid infestation. Very high accumulation of flavonoids was particularly observed in leaf cells of pea seedlings at 72 h of infestation with 30 aphids (Fig. 1). Among the flavonoids, phytoalexin pisatin was observed to accumulate to high amounts in leaf cells after 48 h of colonization by aphids. Moreover, this increase correlated with the number of aphids colonizing pea seedlings (Fig. 2). The highest concentration of pisatin was observed in 72- and 96-h leaves infested by 30 aphids. Also, a considerable post-infestation accumulation was observed using semi-quantitative RT-PCR for transcripts of chalcone synthase (CHS) and isoflavone synthase (IFS). Results of research presented earlier revealed that activity of PAL and the expression of the gene encoding this enzyme in P. sativum seedling leaves were induced after A. pisum infestation (Mai et al. 2014). Additionally, this study also demonstrated that the levels of flavonols, such as kaempferol and flavonol glycosides, i.e., kaempferol-diglucosides, kaempferol-triglucosides, quercetin O-glucoside increased between 72 and 96 h after infestation. With the exception of kaempferol, the highest increase was observed in the leaves infested by 30 aphids (Fig. 3a–e). The level of flavonoid aglycons, such as kaempferol in pea seedling leaves colonized by aphids decreased from 48 h after infestation, but still was higher than in the control (Fig. 3e). Thereby, it is suggested that pisatin plays an important role in the defence responses of P. sativum toward A. pisum infestation. During their life cycle, plants respond actively to stress by producing phytoalexins and other stress metabolites (Dao et al. 2011; Jeandet et al. 2013). The accumulation of flavonoid aglycons such as quercetin, kaempferol, and isorhamnetin was observed in cultivated lines of cowpea (Vigna unguiculata L. Walp.) in response to Aphis fabae (Lattanzio et al. 2000). Additionally, quercetin and isorhamnetin have an inhibitory effect on aphid reproduction, as was demonstrated in in vitro bioassays. It was previously proven that flavonoids play a key role in plant responses to stress (Ateyyat et al. 2012).
At present, many studies on the function of flavonoids as natural insecticides focus mainly on chewing insects. Flavonoids from leaves of Annona squamosa (Kotkar et al. 2002) and Ricinus communis (Upasani et al. 2003) were found to arrest the population growth of the adzuki bean weevil, Callosobruchus chinensis, in mung bean (Vigna radiata L.). Flavonoids showed an ovicidal effect on bruchid eggs, while they also affect the number and weight of emerging adults (Salunke et al. 2005). Also, it has been reported that flavonoids can act as potential grain protectants through oviposition deterrence and ovicidal action. Moreover, these metabolites can modulate the feeding behavior of insects (Van Loon et al. 2002).
In this paper, we provide evidence using confocal microscopy for the subcellular accumulation of flavonoids in defence response to aphid infestation (Fig. 1). Therefore, an intense emission of green fluorescence was observed in infested leaf cells. Kinetics after infestation revealed that flavonoids are found both in the cell wall and in the cytoplasm of cells infested for 72 h by A. pisum, while they are mainly found in the cell wall at 48 h of infestation (Fig. 1). The accumulation of flavonoids in the cell wall as well as in the cytoplasm of cells is associated with the defence response of pea to A. pisum. Considering the fact that flavonoids may modify membrane fluidity/rigidity (Scheidt et al. 2004), it can be suggested that the accumulation of these metabolites in the cell wall and in the cytoplasm of cells of pea seedling leaves may hinder A. pisum puncturing of symplast and penetration of apoplast pathway. Moreover, flavonoids have been suggested to act as antioxidants, protecting plants from oxidative stress (Hernández et al. 2009). In addition, elevated levels of these metabolites in plant cells can act as a possible deterrent against pea aphid infestation, due to their cytotoxic properties.
It has also been demonstrated that a considerable increase in TAC was dependent on the pool of slow- and fast-acting antioxidants (Fig. 5) in the post-infestation stage of leaves in comparison to the control plants. A very high level of slow-acting antioxidants was found in leaves infested by A. pisum at 48 and 96 h. (Fig. 5b). The increased level of slow-acting antioxidants correlates with the increased pisatin content and the level of flavonols in aphid-infested leaves after 72 h post-infestation. Increased TAC levels revealed upon A. pisum infestation suggest an important protective role of the above-mentioned antioxidants in pea seedling leaves from oxidative stress. It may be related to free radical scavenging, acting as a defense against injury or antimicrobial activity. A phenol-containing amino acid such as tyrosine (Akerström et al. 2007) and tryptophan (Bourdon and Blache 2001) have been suggested to contribute to antioxidant functions. Increased TCA levels were previously reported only in plant response to pathogenic fungi ( Floryszak-Wieczorek et al. 2007; Morkunas et al. 2013).
An increased activity of POX, enzyme involved in many physiological processes in plants, including defence responses, was also measured in A. pisum infested leaves (Fig. 6). POX activity toward syringaldazine in leaves of P. sativum seedlings infested by A. pisum was markedly higher than in the control (Fig. 6). Several reports have shown that peroxidases present in tissues undergoing lignification display in vitro activity toward syringaldazine (Christensen et al. 1998; Quiroga et al. 2000). In our study, POX activity closely correlates with population sizes of A. pisum in leaves at 72 h after infestation. Also, at 96 hpi, the highest activity of these enzymes was in leaves infested by 30 aphids. It is proposed that these activities contribute to the oxidation of phenolic compounds, which are likely to be more toxic to aphids and/or engaged in the process of tissue lignifications. Peroxidases are involved mainly in detoxification of reactive oxygen species, but also have other functions in plant cells including the increase of the number of dehydrodiferulic bridges (greater cross-linking of saccharide chains in the cell wall) and the formation of suberins required in healing of wounds caused by injury (Quiroga et al. 2000; Morkunas and Gmerek 2007). They also provide downstream signaling molecules for other transduction pathways (Allison and Schultz 2004). Therefore, an increase in POX activity may be one of the crucial components of the pea defence strategy in response to aphid herbivory.
An increase in the total pool of flavonoids (Fig. 1) and the synthesis of pisatin (Fig. 2) in leaf cells of P. sativum after 48 h of A. pisum infestation were preceded (0–24 h) by a considerable reduction in the levels of sucrose (Fig. 7b) and fructose (Fig. 7c) in comparison to the control. At the same time, in leaves colonized by 30 aphids, a considerable increase in the expression level of the sucrose transporter 1 (SUT1) gene (Fig. 8a) was accompanied by the decrease in sucrose level up to 48 h (Fig. 7b). In parallel, reduction in the levels of sucrose and fructose in leaves colonized by 30 aphids between 0 and 48 hpi was accompanied by high level of glucose in these tissues. In turn, reduced glucose contents were observed in successive time points after feeding, i.e., between 48 and 96 h in comparison to the control (Fig. 7d). It is proposed that sucrose and the hexoses (glucose and fructose) may be redirected to the secondary metabolism in pea leaves resulting most likely in the increased content of flavonoids. The expression level of the SUF1 gene in pea leaves infested for 48 h by 10 aphids and for 72 h by 10 and 20 aphids (Fig. 8c) was approximately 1.5–2 times higher than in the control, whereas after 72 to 96 hpi the level of flavonols increased (Fig. 3a–c and e–f). These results may indirectly indicate to engage these transporters in redirecting to the secondary metabolism. Sugar transporters, mediating the transfer of sugars, can play an important role not only in the plant defence mechanism providing the carbon skeletons for the synthesis of secondary metabolites, but also indirectly in the carbon nutrition and osmoregulation of aphids. Novelty of this research is to determine the potential role of sugar transporters in defense strategy of P. sativum L.cv. Cysterski on A. pisum infestation.
Additionally, invertases are considered to be important in the defense mechanism of pea against A. pisum. These enzymes can operate both in the symplastic and/or apoplastic environment. As reported by Ferrieri et al. (2015), it seems that apoplastic cell wall invertase inhibitor (NaCWII) was strongly up-regulated in a jasmonate (JA)-dependent manner following a simulated attack by the specialist herbivore Manduca sexta. This observation indicates a key role in secondary metabolite synthesis. Additionally, it is well-known that some cell-wall invertases fulfilling important roles in carbohydrate partitioning (Lammens et al. 2008) collaborate with hexose transporters during the growth and development of plants (Sherson et al. 2003) and under biotic stresses (Fotopoulos et al. 2003). In Arabidopsis, M. persicae feeding induced the expression of STP4, a monosaccharide symporter that interacts with invertases to increase carbohydrate import and metabolism and contributes to the creation of nutrient sinks at aphid-feeding sites (Moran and Thompson 2001; Moran et al. 2002). Thus, some cell wall invertases collaborate with hexose importers under biotic stresses. At present, there is only very little information available on the activation of hexose transporters in plants in response to aphid attack. On the other hand, these transporters were well characterized in the same aphid A. pisum (Price and Gatehouse 2014).
Genes encoding sucrose transporter are induced after grazing by herbivores in rice seedlings, followed by suppression in the later stage (Chen et al. 2009). Kempema et al. (2007) reported the up-regulation of SUC1 in Arabidopsis thaliana after attack of silverleaf whitefly nymphs. During infestation, a general down-regulation of photosynthesis genes was observed, but transport genes, including SUC1, were up-regulated. A recent study on the expression pattern of sucrose transporters in A. thaliana during aphid (Myzus persicae) infestation showed a significant enhancement in the expression pattern for six out of nine sucrose transporters in response to aphid infestation (Dubey et al. 2013). In the first hours after infestation, the expressions of sucrose transporters genes were enhanced probably to compensate for the energy requirements of the damaged cell. The defence response is closely linked to the up-regulation of sucrose sink metabolism to satisfy the energy requirements and the activation of the cascade of defence reactions (Dubey et al. 2013). Moreover, Hodge et al. (2013) revealed that in response of Arabidopsis to M. persicae accumulation of trehalose dependent on a density of aphids was noted and this accumulation was systemic.
Thus, sucrose and stress-related signal transduction pathways are suggested to be integrated into the control of defence reactions (Rolland et al. 2006; Bolouri-Moghaddam et al. 2010). On the other hand, as reported earlier (Morkunas and Ratajczak 2014), sugar transporters are key elements, necessary for the formation of the secondary sink at the site of fungal pathogen invasion.
Moreover, 72 h after A. pisum infestation, a markedly lower SUT1 expression was observed in comparison to the control, which correlated with the number of feeding aphids (10, 20, and 30 aphids per plant) (Fig. 8a). A preceding decreased SUF1 expression within 24 to 72 h of infestation was determined (Fig. 8c). A significantly lower level of expression of the SUF4 gene was also observed compared to the control at 48 and 72 h in leaves infested by A. pisum at a varying population size (Fig. 8b).
The suppression of sucrose transporter and sucrose facilitator genes in the late response may be a defence strategy of the plant to repel aphids because at this stage of infestation aphids become a secondary sink (Dubey et al. 2013). It is proposed that the early stage of infestation is still characterized by efficient transportation of sucrose to energize the cells, but later with aphids acts as a secondary sink. Price and Gatehouse (2014) reported that when sucrose is utilized as a main respiratory substrate, the A. pisum can catabolizes 15–30 % of its ingested sucrose in oxidative pathways. A larger proportion, close to 50 % of the ingested sucrose, is incorporated into aphid tissues. While sucrose is the main carbon source for A. pisum, sucrose itself is not transported across the gut epithelium. Instead, sucrose is hydrolyzed in the gut lumen by the sucrase (an α-glucosidase, which is specific for the a-glucosyl residue of sucrose) to its constituent monosaccharides, glucose and fructose, and these hexose sugars are transported.
In response to pea aphid infestation, the levels of total sugar (Fig. 7a) and glucose (Fig. 7d) in pea seedling leaves were reduced with time, while those of sucrose (Fig. 7b) and fructose (Fig. 7c) increased 96 h after aphid infestation, although their levels at 24 h feeding were markedly lower than in the control. Moreover, Singh et al. (2011) reported that aphid (Myzus persicae) infestation of Arabidopsis leaves leads to a dramatic increase in sucrose and starch contents in source tissues despite pest feeding. These changes suggest a block of sugar export to the plant sinks. Infestation also induces an increase in trehalose levels. This change in trehalose metabolism promotes re-allocation of carbon into starch at the expense of sucrose, the primary energy source of the pest, and plant defence via the induction of the PHYTOALEXIN-DEFICIENT4 gene (Singh et al. 2011). Our research shows strong increase in the level of sucrose in 72-h leaves of pea seedlings infested by aphids and down-regulating the expression of SUT1 and SUF4 genes, which suggest the formation of a secondary sink in pea leaves to repelling aphids.
In conclusion, in the presented study, we revealed that the perception of A. pisum infestation by pea seedlings induces a whole sequence of potential defensive reactions (Fig. 9). It is proposed that the elevated expression of pea sucrose transporter genes at an early stage of A. pisum attack is most probably connected with an increased allocation of carbon in order to redirect carbon skeletons to secondary metabolism and the accumulation of flavonoids, which are important components of the defence system. Increased levels of flavonoids and slow- or fast-acting antioxidants enhance the total antioxidant capacity of leaf cells of pea. Additionally, an increase in the activity of peroxidase is suggested to contribute to oxidation of phenolic compounds which may be either more toxic to aphids and/or intensify the process of tissue lignification.
Abbreviations
- ABTS+ :
-
The 2,2'-azino-bis-(3-ethylbenzthiazoline-6-sulphonic acid) cation
- CHS:
-
Chalcone synthase
- DPBA:
-
Diphenylboric acid 2-aminoethyl ester
- G:
-
Gram
- Hpi:
-
Hours post infestation
- IFS:
-
Isoflavone synthase
- LC/UV/ESI/MS/MS:
-
Liquid chromatography/ultraviolet detection/electrospray/mass spectrometry (tandem mass spectrometry)
- POX:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SUT1:
-
Sucrose transporter
- SUF1, SUF4:
-
Sucrose facilitators
- SWEETs:
-
Sugar efflux carriers
- TAC:
-
Total antioxidant capacity
References
Akerström B, Maghzal GJ, Winterbourn CC, Kettle AJ (2007) The lipocalin 1-microglobulin has radical scavenging activity. J Biol Chem 282(43):31493–31503
Allison SD, Schultz JC (2004) Differential activity of peroxidase isozymes in response to wounding, gypsy moth, and plant hormones in northern red oak (Quercus rubra L.). J Chem Ecol 30(7):1363–137
Ateyyat M, Abu-Romman S, Abu-Darwish M, Ghabeish I (2012) Impact of flavonoids against woolly apple aphid, Eriosoma lanigerum (Hausmann) and its sole parasitoid, Aphelinus mali (Hald.). J Agric Sci 4(2):227–236
Berthier A, Desclos M, Amiard V, Morvan-Bertrand A, Demmig-Adams B, Adams WW 3rd, Turgeon R, Prud'homme MP, Noiraud-Romy N (2009) Activation of sucrose transport in defoliated Lolium perenne L.: an example of apoplastic phloem loading plasticity. Plant Cell Physiol 50(7):1329–1344
Bitterlich M, Krügel U, Boldt-Burisch K, Franken P, Kühn C (2014) The sucrose transporter SlSUT2 from tomato interacts with brassinosteroid functioning and affects arbuscular mycorrhiza formation. Plant J 78(5):877–89
Bolouri-Moghaddam MR, Le Roy K, Xiang L, Rolland F, Van den Ende W (2010) Sugar signalling and antioxidant network connections in plant cells. FEBS J 277:2022–2037
Bourdon E, Blache D (2001) The importance of proteins in defense against oxidation. Antioxid Redox Signal 3(2):293–311
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen S, Li XQ, Zhao A, Wang L, Li X, Shi Q, Chen M, Guo J, Zhang J, Qi D, Liu G (2009) Genes and pathways induced in early response to defoliation in rice seedlings. Curr Issues Mol Biol 11(2):81–100
Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, Frommer WB (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335:207–211
Chen LQ, Lin IW, Qu XQ, Sosso D, McFarlanec HE, Londoñoa A, Samuelsc AL, Frommer WB (2015) A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo. Plant Cell 27(3):607–619
Chincinska IA, Liesche J, Krugel U, Michalska J, Geigenberger P, Grimm B, Kühn C (2008) The sucrose transporter StSUT4 from Solanum tuberosum affects flowering, tuberization and shade avoidance response. Plant Physiol 146:515–528
Christensen JH, Bauw G, Welinder KG, Van Montagu M, Boerjan W (1998) Purification and characterization of peroxidases correlated with lignification in poplar xylem. Plant Physiol 118(1):125–135
Dao TT, Linthorst HJ, Verpoorte R (2011) Chalcone synthase and its functions in plant resistance. Phytochem Rev 10(3):397–412
Dibley K (2012) The functional characterisation of novel sucrose transporters. Dissertation. The University of Newcastle
Doidy J, Grace E, Kühn C, Simon-Plas F, Casieri L, Wipf D (2012) Sugar transporters in plants and in their interactions with fungi. Trends Plant Sci 17(7):413–422
Douglas AE (2006) Phloem-sap feeding by animals: problems and solutions. J Exp Bot 57(4):747–754
Dubey NK, Idris A, Verma AK, Chandrashekar K, Pandey KD (2013) Expression pattern of sucrose transporters in Arabidopsis thaliana during aphid (Myzus persiace) infestation. Am J Plant Sci 4:47–51
Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, Keller F, Baginsky S, Martinoia E, Schmidt UG (2006) Identification of a vacuolar sucrose transporter in Barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol 141:196–207
Ferrieri AP, Arce CC, Machado RA, Meza-Canales ID, Lima E, Baldwin IT, Erb M (2015) A Nicotiana attenuata cell wall invertase inhibitor (NaCWII) reduces growth and increases secondary metabolite biosynthesis in herbivore-attacked plants. New Phytol. doi:10.1111/nph.13475
Floryszak-Wieczorek J, Arasimowicz M, Milczarek G, Jeleń H, Jackowiak H (2007) Only an early nitric oxide burst and the following wave of secondary nitric oxide generation enhanced effective defence responses of pelargonium to a necrotrophic pathogen. New Phytol 175:718–730
Formela M, Samardakiewicz S, Marczak Ł, Nowak W, Narożna D, Bednarski W, Kasprowicz-Maluśki A, Morkunas I (2014) Effects of endogenous signals and Fusarium oxysporum on the mechanism regulating genistein synthesis and accumulation in yellow lupine and their impact on plant cell cytoskeleton. Molecules 19(9):13392–13421
Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanan AJ, Sauer N, Hall JL, Williams LE (2003) The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atbetafruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol 132(2):821–829
Gould KS, Lister C (2006) Flavonoid functions in plants. In: Andersen ØM, Markham KR (eds) Flavonoids: chemistry, biochemistry and applications. CRC Press, London, New York, Boca Raton, pp 397–441
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55(6):481–504
Hernández I, Alegre L, Van Breusegem F, Munné-Bosch S (2009) How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 14:125–132
Hodge S, Ward JL, Beale MH, Bennett M, Mansfield JW, Powell G (2013) Aphid-induced accumulation of trehalose in Arabidopsis thaliana is systemic and dependent upon aphid density. Planta 237:1057–1064
Jeandet P, Clément C, Courot E, Cordelier S (2013) Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int J Mol Sci 14(7):14136–14170
Jeandet P, Hébrard C, Deville MA, Cordelier S, Dorey S, Aziz A, Crouzet J (2014) Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 19(11):18033–18056
Kempema LA, Cui X, Holzer FM, Walling LL (2007) Arabidopsis transcriptome changes in response to phloem-feeding silver leaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol 143(2):849–865
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7:235–246
Kotkar HM, Mendki PS, Sadan SV, Jha SR, Upasani SM, Maheshwari VL (2002) Antimicrobial and pesticidial activity of partially purified flavonoids of Annona squamos. Pest Manag Sci 58(1):33–37
Kühn C (2010) Sucrose transporters in plant development. In: Geisler M, Venema K (ed) Signaling and Communication in Plants: Transporters and pumps in plant signaling, Springer Verlag Heidelberg, pp. 225-251
Kühn C, Grof CP (2010) Sucrose transporters of higher plants. Curr Opin Plant Biol 13(3):288–298
Kühn C, Hajirezaei MR, Fernie AR, Roessner-Tunali U, Czechowski T, Hirner B, Frommer WB (2003) The sucrose transporter StSUT1 localizes to sieve elements in potato tuber phloem and influences tuber physiology and development. Plant Physiol 131:102–113
Kuśnierczyk A, Winge P, Jørstad TS, Trończyska J, Rossiter JT, Bones AM (2008) Towards global understanding of plant defense against aphids—timing and dynamics of early Arabidopsis defense responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ 31(8):1097–1115
Lammens W, Le Roy K, Van Laere A, Rabijns A, Van den Ende W (2008) Crystal structures of Arabidopsis thaliana cell-wall invertase mutants in complex with sucrose. J Mol Biol 377(2):378–385
Lattanzio V, Arpaia S, Cardinali A, di Venere D, Linsalata V (2000) Role of endogenous flavonoids in resistance mechanism of Vigna to aphids. J Agric Food Chem 48:5316–5320
Lemoine R, La Camera S, Atanassova R, Dédaldéchamp F, Allario T, Portau N, Bonnemain J-L, Laloi M, Coutos-Thiévenot P, Maurousset L, Faucher M, Girousse C, Lemonnier P, Parrilla J, Durand M (2013) Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci 4(272):1–21
Lev-Yadun S, Gould KS (2009) Role of anthocyanins in plant defense. In: Gould KS, Davies KM, Winefield C (ed) Life's colorful solutions: the biosynthesis, functions and applications of anthocyanins, Springer-Verlag Berlin, pp. 21–48
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔC T method. Methods 25(4):402–408
Mai VC, Bednarski W, Borowiak-Sobkowiak B, Wilkaniec B, Samardakiewicz S, Morkunas I (2013) Oxidative stress in pea seedling leaves in response to Acyrthosiphon pisum infestation. Phytochemistry 93:49–62
Mai VC, Drzewiecka K, Jeleń H, Narożna D, Rucińska-Sobkowiak R, Kęsy J, Floryszak-Wieczorek J, Gabryś B, Morkunas I (2014) Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci 221–222:1–12
Moran PJ, Thompson GA (2001) Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol 125:1074–1085
Moran PJ, Cheng Y, Cassell JL, Thompson GA (2002) Gene expression profiling of Arabidopsis thaliana in compatible plant–aphid interactions. Arch Insect Biochem Physiol 51:182–203
Morkunas I, Gmerek J (2007) The possible involvement of peroxidase in defence of yellow lupine embryo axes against Fusarium oxysporum. J Plant Physiol 164(2):185–194
Morkunas I, Ratajczak L (2014) The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol Plant 36(7):1607–1619
Morkunas I, Marczak Ł, Stachowiak J, Stobiecki M (2005) Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol Biochem 43:363–373
Morkunas I, Kozłowska M, Ratajczak L, Marczak Ł (2007) Role of sucrose in the development of Fusarium wilt in lupine embryo axes. Physiol Mol Plant Pathol 70:25–37
Morkunas I, Stobiecki M, Marczak Ł, Stachowiak J, Narożna D, Remlein-Starosta D (2010) Changes in carbohydrate and isoflavonoid metabolism in yellow lupine in response to infection by Fusarium oxysporum during the stages of seed germination and early seedling growth. Physiol Mol Plant Pathol 75:46–55
Morkunas I, Mai VC, Gabryś B (2011a) Phytohormonal signaling in plant responses to aphid feeding. Acta Physiol Plant 33(6):2057–2073
Morkunas I, Narożna D, Nowak W, Samardakiewicz S, Remlein-Starosta D (2011b) Cross-talk interactions of sucrose and Fusarium oxysporum in the phenylpropanoid pathway and the accumulation and localization of flavonoids in embryo axes of yellow lupine. J Plant Physiol 168:424–433
Morkunas I, Formela M, Floryszak-Wieczorek J, Marczak Ł, Narożna D, Nowak W, Bednarski W (2013) Cross-talk interactions of exogenous nitric oxide and sucrose modulates phenylpropanoid metabolism in yellow lupine embryo axes infected with Fusarium oxysporum. Plant Sci 211:102–121
Mutti NS, Louis J, Pappan L, Pappan K, Begum K, Chen M-S, Park Y, Dittmer N, Marshall J, Reese JC, Reeck GR (2008) A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc Natl Acad Sci U S A 105(29):9965–9969
Payyavula RS, Tay KHC, Tsai C-J, Harding SA (2011) The sucrose transporter family in Populus: the importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. Plant J 65:757–770w
Peer WA, Murphy AS (2006) Flavonoid as signal molecules: targets of flavonoids action. In: Grotewold E (ed) The science of flavonoids. Springer, Berlin, pp 239–268
Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12(1):29–36
Price DR, Gatehouse JA (2014) Genome-wide annotation and functional identification of aphid GLUT-like sugar transporters. BMC Genomics 15:647
Proels RK, Huckelhoven R (2014) Cell-wall invertases, key enzymes in the modulation of plant metabolism during defence responses. Mol Plant Pathol 15(8):858–64
Quiroga M, Guerrero C, Botella MA, Barceló A, Amaya I, Medina MI, Alonso FJ, de Forchetti SM, Tigier H, Valpuesta V (2000) A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol 122(4):1119–1128
Reinders A, Sivitz AB, Starker CG, Gantt JS, Ward JM (2008) Functional analysis of LjSUT4, a vacuolar sucrose transporter from Lotus japonicus. Plant Mol Biol 68:289–299
Reinders A, Sivitz A, Ward J (2012) Evolution of plant sucrose uptake transporters. Front Plant Sci 3:1–12
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Salunke BK, Kotkar HM, Mendki PS, Upasani SM, Maheshwari VL (2005) Efficacy of flavonoids in controlling Callosobruchus chinensis (L.) (Coleoptera: Bruchidae), a post-harvest pest of grain legumes. Crop Prot 24(10):888–893
Sauer N (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581(12):2309–2317
Schaarschmidt S, Roitsch T, Hause B (2006) Arbuscular mycorrhiza induces gene expression of the apoplastic invertase LIN6 in tomato (Lycopersicon esculentum) roots. J Exp Bot 57(15):4015–23
Scheidt HA, Pampel A, Nissler L, Gebhardt R, Huster D (2004) Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angle spinning NMR spectroscopy. Biochim Biophys Acta 1663(1-2):97–107
Sherson SM, Alford HL, Forbes SM, Wallace G, Smith SM (2003) Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J Exp Bot 54(382):525–531
Shiratake K (2007) Genetics of sucrose transporter in plants. Genes, Genomes and Genomics Global Science Books 1(1):73-80
Simmonds MSJ (2003) Flavonoid–insect interactions: recent advances in our knowledge. Phytochemistry 6:21–30
Simmonds MSJ, Stevenson PC (2001) Effects of isoflavonoids from Cicer on larvae of Helicoverpa armigera. J Chem Ecol 27(5):965–977
Singh V, Louis J, Ayre BG, Reese JC, Shah J (2011) TREHALOSE PHOSPHATE SYNTHASE11-dependent trehalose metabolism promotes Arabidopsis thaliana defense against the phloem-feeding insect Myzus persicae. Plant J 67(1):94–104
Sivitz AB, Reinders A, Johnson ME, Krentz AD, Grof CPL, Perroux JM, Ward JM (2007) Arabidopsis sucrose transporter AtSUC9. High affinity transport activity, intragenic control of expression and early-flowering mutant phenotype. Plant Physiol 143:188–198
Smith CM, Chuang WP (2014) Plant resistance to aphid feeding: behavioral, physiological, genetic and molecular cues regulate aphid host selection and feeding. Pest Manag Sci 70(4):528–540
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140:637–646
Stobiecki M, Wojtaszek P, Gulewicz K (1997) Application of solid phase extraction for profiling of quinolisidine alkaloids and phenolic compounds in Lupinus albus. Phytochem Anal 8:153–158
Tjallingi WF (2006) Salivary secretions by aphids interacting with proteins of phloem wound responses. J Exp Bot 57(4):739–745
Upasani SM, Kotkar HM, Mendki PS, Maheshwari VL (2003) Partial characterization and insecticidal properties of Ricinus communis L. foliage flavonoids. Pest Manag Sci 59(12):1349–1354
Van Bel AJE, Hess PH (2008) Hexoses as phloem transport sugars: the end of a dogma? J Exp Bot 59(2):261–272
Van den Ende W (2013) Multifunctional fructans and raffinose family oligosaccharides. Front Plant Sci 4(247):1–11
Van Loon JJA, Wang CZ, Nielsen JK, Gols R, Qiu YT (2002) Flavonoids from cabbage are feeding stimulants for diamondback moth larvae additional to glucosinolates: chemoreception and behavior. Entomol Exp App 104:27–34
Voelckel C, Weisser WW, Baldwin IT (2004) An analysis of plant–aphid interactions by different microarray hybridization strategies. Mol Ecol 13:3187–3195
Wahl R, Wippel K, Goos S, Kämper J, Sauer N (2010) A novel high-affinity sucrose transporter is required for virulence of the plant pathogen Ustilago maydis. PLoS Biol 8(2):e1000303
Weschke W, Panitz R, Sauer N, Wang Q, Neubohn B, Wobus U (2000) Sucrose transport into barley seeds: molecular characterization of two transporters and implications for seed development and starch accumulation. Plant J 21:455–467
Wimmers LE, Turgeon R (1991) Transfer cells and solute uptake in minor veins of Pisum sativum leaves. Planta 186:2–12
Zhou Y, Qu H, Dibley KE, Offler CE, Patrick JW (2007) A suite of sucrose transporters expressed in coats of developing legume seeds includes novel pH-independent facilitators. Plant J 49(4):750–64
Acknowledgments
The study was supported by the Polish National Science Centre (NCN, grant no. 2011/01/B/NZ9/00074)
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Néstor Carrillo
Rights and permissions
About this article
Cite this article
Morkunas, I., Woźniak, A., Formela, M. et al. Pea aphid infestation induces changes in flavonoids, antioxidative defence, soluble sugars and sugar transporter expression in leaves of pea seedlings. Protoplasma 253, 1063–1079 (2016). https://doi.org/10.1007/s00709-015-0865-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0865-7