Abstract
Flavonoids make a relevant contribution to the response mechanisms of higher plants to a plethora of abiotic stresses. In addition to the long-reported functions as screeners of damaging short-wave solar radiation, flavonoids have been suggested as playing key functions as antioxidants in stressed plants, by inhibiting the generation and reducing reactive oxygen species (ROS) once formed. The ROS-scavenging properties of flavonoids are restricted to few structures, namely, the dihydroxy B-ring-substituted flavonoid glycosides. This structure–activity relationship conforms to the well-known stress-induced preferential biosynthesis of dihydroxy B-ring-substituted both flavones and flavonols. These flavonoids, especially the derivatives of quercetin, have been shown to greatly affect the movement of auxin at intra- and intercellular levels, and hence to tightly regulate the development of individual organs and the whole plant. The effectiveness of flavonoids to inhibit the activity of the auxin efflux facilitator proteins tightly depends on the chemical features that confer the antioxidant potential. In this review article, we discuss about (1) the effect of different abiotic stresses on the accumulation of individual flavonoids, (2) the potential role served by antioxidant flavonoids in the antioxidant machinery of plants exposed to severe stress conditions, and (3) the function of flavonoids as developmental regulators.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abiotic stresses
- Antioxidant enzymes
- Auxin movement
- Dihydroxy B-ring-substituted flavonoids
- Reactive oxygen species
- UV-radiation
1 Introduction
Flavonoids, the vast class of secondary metabolites encompassing more than 10,000 structures (Harborne and Williams 2000), have long been reported to display wide range of uses in plant–environment interactions (Winkel-Shirley 2002; D’Auria and Gershenzon 2005; Agati and Tattini 2010). In the recent past, the idea behind functioning of flavonoids primarily as attenuators of short solar wavelengths in plants exposed to UV-B or full solar irradiance has been questioned (Gerhardt et al. 2008; Agati and Tattini 2010; Agati et al. 2011; Akhtar et al. 2010), as flavonoids most responsive to UV-radiation are far from being the most effective UV-B absorbers among the thousands of polyphenol structures (Cockell and Knowland 1999; Harborne and Williams 2000). These suggestions are consistent with the early authoritative views of Swain (1986) and Stafford (1991) of flavonols, the most ancient and widespread of the flavonoids (Rausher 2006; Winkel 2006), having served key antioxidant or “internal regulatory” functions during the evolution of early terrestrial plants.
The biosynthesis of flavonoids is upregulated not only as a consequence of UV-radiation but also in response to a wide range of other abiotic (and biotic) stresses, ranging from nitrogen/phosphorus depletion to cold and salinity/drought stress (Tattini et al. 2004, 2005; Lillo et al. 2008; Olsen et al. 2009; Agati et al. 2011). Since different stresses have in common the generation of reactive oxygen species (Mittler et al. 2004; Mittler 2006), it has been postulated that flavonoids are synthesized to effectively counter the stress-induced oxidative damage. Flavonoids may accomplish their antioxidant functions by both preventing the generation of ROS (through their ability to chelate transition metal ions such as Fe and Cu, Brown et al. 1998; Melidou et al. 2005; Hernández et al. 2009; Agati and Tattini 2010) and scavenging ROS once formed (Ryan et al. 2002; Babu et al. 2003; Tattini et al. 2004; Agati et al. 2007; Jaakola and Hohtola 2010).
How flavonoids may perform their scavenging activity in an in vivo condition is still a matter of conflict, and major criticisms regarding the localization functional relationships (Halliwell 2009; Hernández et al. 2009) have been only partially addressed (Yamasaki et al. 1997; Agati et al. 2007; Agati and Tattini 2010; Akhtar et al. 2010). However, we note that flavonoids do not exclusively occur in the vacuoles of epidermal cells (as long been reported, Saunders and Mc Clure 1976), and hence far enough from the sites of ROS production. Relatively recent experiments have reported a large accumulation of flavonoids in mesophyll cells both in the vacuole (Agati et al. 2002, 2009, 2011; Gould et al. 2002; Tattini et al. 2005, 2006; Kytridis and Manetas 2006) and in the chloroplasts (Agati et al. 2007). This subcellular distribution of flavonoids is, therefore, consistent with a their putative role as ROS-quenchers. Chloroplast flavonoids (chloroplasts have been reported to be both capable of flavonoid biosynthesis and may represent an important site of flavonoid accumulation), (Oettmeier and Heupel 1972; Saunders and Mc Clure 1976; Takahama 1982; Takahama and Oniki 1997; Zaprometov and Nikolaeva 2003) have been shown to effectively quench singlet oxygen generated upon excess blue-light irradiance (Agati et al. 2007). A vacuolar distribution of mesophyll flavonoids may be of key significance in reducing hydrogen peroxide (H2O2) that may freely escape from the chloroplast under severe stress conditions, as also hypothesized to occur for flavonoids located in the vacuoles of epidermal cells (Yamasaki et al. 1997; Sakihama et al. 2000). Nevertheless, the matter is far being conclusively elucidated and poses serious concerns from an analytical and technical point of view, mostly concerning the simultaneous visualization of reactive oxygen species and flavonoid distribution at inter and intracellular levels (Hernández et al. 2009; Agati and Tattini 2010).
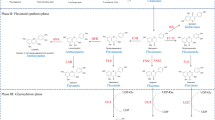
We highlight that even though polyphenols, particularly flavonoids have long been shown to scavenge various forms of reactive oxygen “in vitro”, the flavonoids usually encountered in plants, e.g., in leaf tissues, are the glycosylated structures, as glycosylation both increases the solubility of carbon-based metabolites in an aqueous cellular milieu and preserve the most reactive functional groups from autooxidation (Pearse et al. 2005). Actually, few glycosylated flavonoids are effective antioxidants, whereas most flavonoid aglycones (i.e., lacking the sugar moiety) are actually capable of quenching ROS (Rice-Evans et al. 1996). Quercetin 3-O- and luteolin 7-O-glycosides that posses a catechol group (ortho-dihydroxy B-ring substitution, Fig. 9.1) in the B-ring of the flavonoid skeleton, but not kaempferol 3-O- or apigenin 7-O-glycosides, display an appreciable antioxidant activity, in the molar concentration-range likely encountered in plant cells (Tattini et al. 2004).
Scheme of the main flavonoid pathway leading to the most common flavanones, flavones, dihydroflavonols, flavonols, and flavonol glycosides. Enzymes – CHS chalcone synthase, CHI chalcone isomerase, FNS I/II flavone synthase I and II, F3′H flavonoid 3′-hydroxylase, FHT flavanone 3β-hydroxylase, FLS flavonol synthase, GT glycosyl transferases
Noticeably, stress-responsive flavonoids have the greatest antioxidant potential, and the ratio of “effective antioxidant” to “poor antioxidant” flavonoids has been conclusively shown to increase steeply in response to a plethora of abiotic stresses (Kolb et al. 2001; Schmitz-Hoerner and Weissenböck 2003; Tattini et al. 2004; Lillo et al. 2008; Kotilainen et al. 2008; Jaakola and Hohtola 2010; Agati et al. 2009, 2011). The issue of “how significant are flavonoids as antioxidants in plants” has been recently explored by Hernández et al. (2009): these authors have suggested of minor significance the contribution of flavonoids to the highly integrated and constitutive antioxidant defense system (which includes antioxidant enzymes, ascorbic acid, and glutathione) operating in plants suffering from various abiotic stresses (as early authoritatively suggested by Halliwell 2009).
Nevertheless, the first line of defense against stress-induced enhancement in ROS concentration – the antioxidant enzymes – have been reported to be ineffective to protect cells from oxidative damage during severe stress conditions (Polle 2001; Apel and Hirt 2004; Hatier and Gould 2008). Hatier and Gould (2008) have recently suggested that the very conditions that lead to the accumulation of flavonoids are those that may inactivate key antioxidant enzymes (Casano et al. 1997; Streb et al. 1997). In other words, the actual significance of stress-responsive “antioxidant” flavonoids in the context of the well-coordinated antioxidant defenses operating in stressed plants has to be explored on the basis of the stress severity at which plants are faced with.
Flavonoids with the greatest antioxidant potential have the additional capacity to inhibit the polar auxin transport (PAT, Jacobs and Rubery 1988; Brown et al. 2001; Besseau et al. 2007; Peer and Murphy 2007), and, hence, capable of regulating the development of individual organs and the whole plant (Taylor and Grotewold 2005; Lazar and Goodman 2006; Agati and Tattini 2010). Flavonoids have long been shown to inhibit the activity of PIN and MDR P-glycoproteins, that regulate the cellular auxin homeostasis and the cell-to-cell auxin transport (Geissler et al. 2005; Peer and Murphy 2007; Friml and Jones 2010). In this regard, flavonoids do not perform any reducing activity (i.e., antioxidant activity sensu stricto), but the chemical features that confer the antioxidant potential are required to effectively interact with the auxin transport proteins (Peer and Murphy 2006; Agati and Tattini 2010). These “internal regulatory” or “physiological” functions of flavonoids, early hypothesized by Stafford (1991) as the most prominent in planta, have to be regarded with special attention. In fact, an increasing body of evidence suggests that the health-promoting effect of flavonoids in mammals depends not only upon their ability to scavenge a wide array of reactive oxygen species – free radicals and H2O2 – but on their affinity with several proteins (including the mitogen activated protein kinases, MAPK) that supersede key steps of cell growth and differentiation (Williams et al. 2004; Taylor and Grotewold 2005; Peer and Murphy 2006; Lamoral-Theys et al. 2010). Flavonoids might behave, therefore, as “signaling molecules” (Peer and Murphy 2006) or “developmental regulators” (Taylor and Grotewold 2005) in plants, as well as in animals, and their functional roles going well beyond their ability to merely scavenge reactive oxygen.
In this article, we (1) review the pertinent literature on the effect of most common abiotic stresses on the biosynthesis of “UV-absorbing” flavonoids (2) discuss on the potential contribution of flavonoids in the antioxidant machinery of plants under severe stress conditions and (3) their functional roles in the control of plant growth.
2 Stress-Induced Biosynthesis of Flavonoids
A brief summary of stress-induced changes in secondary metabolism, particularly in the biosynthesis of flavonoids has been reported in Table 9.1.
2.1 UV-Radiation
Hundreds of experiments conducted over the last three decades have conclusively shown that flavonoid biosynthesis is mostly upregulated as a consequence of high UV-B and/or full solar irradiance (it is noted that UV-B radiation does not account for more than 5% of the total UV-radiation at mid-latitudes, Ballaré 2003). It is interesting to note that the biosynthesis of other phenolics compounds, both hydroxycinnamic acid derivatives and poly-galloyl derivatives (i.e., hydrolyzable tannins) that are in constitutively greater concentrations than flavonoids under low-light conditions, is very poorly affected by an increase in UV-radiation (Ollson et al. 1999; Burchard et al. 2000; Hofmann et al. 2003; Tattini et al. 2004, 2005, 2006). Since hydroxycinnamic acid derivatives and some hydrolyzable tannins have been reported to display a superior molar extinction coefficient than flavonoids over the 280–320 nm region of the solar spectrum (Harborne and Williams 2000; Tattini et al. 2004), it may be questioned that flavonoids play the primary role as UV-B attenuators in response to UV-B or full solar irradiance (Cockell and Knowland 1999; Agati and Tattini 2010). Similarly, the loss of mycosporin-like aminoacid (MAA) in favor of flavonoid (actually flavonol) metabolism during the colonization of land by plants was likely for fulfilling several uses, as MAA are effective UV-B absorbers (Cockell and Knowland 1999). The old statement that flavonoids are UV-screening pigments that allow the penetration of photosynthetic active radiation in photosynthetic cells is of limited significance (Caldwell et al. 1983), as all phenolics display the same physical–chemical properties (Harborne and Williams 2000). Instead, the UV-B-induced biosynthesis of anthocyanins (actually flavonoids sensu stricto) is hard to be explained on the basis of their UV-screening features (Mendez et al. 1999; Manetas 2006; Kytridis and Manetas 2006), as most anthocyanins (with the exception of few acylated forms) do not appreciably absorb in the 280–390 (UV-waveband) region of the solar spectrum (Harborne and Williams 2000).
Furthermore, UV-induced biosynthesis of flavonoids is actually restricted to very few structures, i.e., the dihydroxy B-ring-substituted flavonoid glycosides, such as quercetin 3-O or luteolin 7-O-glycosides. UV-induced increase in the ratio of dihydroxy to monohydroxy B-ring substituted flavonoid glycosides, e.g., the luteolin to apigenin or quercetin to kaempferol ratios, has long been reported in different plant species exposed to various proportions of UV-radiation (Markham et al. 1998; Ryan et al. 1998, 2002; Schmitz-Hoerner and Weissenböck 2003; Kotilainen et al. 2008). Gerhardt et al. (2008) have shown that the ratio of quercetin glycosides to acylated kaempferol glycosides, which are capable to effectively absorb in both the UV-B and UV-A region of the solar spectrum, increased greatly as a consequence of UV-B irradiance. This differential accumulation of flavonoid glycosides has been explained by different authors in terms of the large variations in the free radical scavenger properties of mono with respect to dihydroxy B-ring substituted flavonoids (Rice-Evans et al. 1996; Tattini et al. 2004; Gerhardt et al. 2008; Akhtar et al. 2010).
It has been reported that glycosylation, firstly in 3-postion in the case of flavonols or in 7-position in the case of flavones (Fig. 9.1), is necessary to make these compounds soluble in the aqueous cellular milieu, in addition to preserving the most reactive groups from autooxidation (Pearse et al. 2005). The catechol group in the B-ring of the flavonoid skeleton is mostly responsible to conferring “antioxidant” capacity to glycosylated flavonoids in the concentration range that may be actually encountered in cell compartments (Rice-Evans et al. 1996; Yamasaki et al. 1997; Tattini et al. 2004; Agati et al. 2009; Agati and Tattini 2010). Luteolin and quercetin glycosides may effectively chelate Fe and Cu-ions, thus preventing the generation of ROS (Brown et al. 1998; Melidou et al. 2005), in addition to reducing a wide range of ROS, from singlet oxygen (Agati et al. 2007) to superoxide anion (Tattini et al. 2004), and the stable hydrogen peroxide (Takahama and Oniki 1997; Yamasaki et al. 1997). Tattini et al. (2004) reported that upon high solar irradiance the ratio of the antioxidant luteolin 7-O-glucoside to the poor antioxidant luteolin 4′-O-glucoside was steeply enhanced, suggesting a fine tuning exerted by high light in the flavonoid metabolism to specifically synthesize antioxidant metabolites, as also reported for the light-induced increase in chlorogenic to other mono-hydroxy hydroxycinnamates (Grace et al. 1998). Recently, Jaakola and Hohtola (2010) have reported the latitude-induced enhancement in the quercetin to kaempferol ratio in different species was for protecting plants from cold-induced oxidative damage rather than for merely absorbing UV-B-radiation. Flavonoids with the greatest antioxidant potential have been reported to accumulate in response to high solar irradiance, either in the presence or in the absence of UV-radiation (Kolb et al. 2001; Agati et al. 2009, 2011). Berli et al. (2010) have shown that in grape leaves exposed to UV-B radiation some of the key components of the antioxidant machinery, e.g., CAT, APX, and carotenoids were unaltered, while the biosynthesis of quercetin derivatives mostly upregulated. It is noted that hydroxycinnamic acid derivatives, including caffeic acid, were unaffected by UV-B irradiance. These findings once again confirm that UV-B irradiance did not enhance the biosynthesis of effective UV-B attenuators (i.e., hydroxycinnamates) but that of ROS-scavenging compounds, i.e., the flavonoids. On the whole, these findings strongly support the hypothesis of a key antioxidant function served by flavonoids in photoprotection (Agati and Tattini 2010).
It may be hypothesized that the flavonoid biosynthesis is upregulated under high-sunlight irradiance, irrespective of the proportion of various solar wavelengths reaching the leaf surface, as a consequence of changes in ROS and/or REDOX homeostasis. This hypothesis is further corroborated by the observation that R2R3MYB transcription factors that regulate the flavonol biosynthesis are themselves REDOX-controlled (Taylor and Grotewold 2005; Dubos et al. 2010).
2.2 Nitrogen-Depletion and Cold
Low nitrogen availability and cold have been reported to have a strong impact on flavonoid, particularly flavonol metabolism. Bilger et al. (2007) have shown that UV-absorbing compounds, particularly flavonoids, accumulated to a great extent as a consequence of a decrease in temperature – from 19 to 11°C – in the epidermal cells of various species. Since this increase was detected in plants exposed to light irradiance in the absence of UV-radiation, the authors suggested for flavonoids an antioxidant function in response to cold-induced photooxidative damage. Similar conclusions were also drawn by Korn et al. (2008), who observed a positive correlation between the concentration of flavonoids and the cold-tolerance of Arabidopsis thaliana accessions, even though these authors did not discharge a protective function of flavonoids in membrane stability (Erlejman et al. 2004). These findings are consistent with those coming from the experiments of Hannah et al. (2006), as the expression of CHS, CHI, DFR, FLS1, and F3′H, i.e., the whole set of genes involved in the biosynthesis of di-hydroxylated B-ring flavonols (Fig. 9.1) was induced to a substantially greater degree in cold-tolerant than in cold-sensitive accessions, and positively correlated with the biosynthesis of quercetin derivatives and anthocyanins.
Several experiments conducted by the Cathrine Lillo’s group have shed new light on the impact of nitrogen depletion and low T on the biosynthesis of flavonoids, particularly flavonols. Kaempferol glycosides were less responsive to nitrogen depletion than corresponding quercetin derivatives, and the interactive effects of nitrogen and low T were significantly greater on the accumulation of quercetin as compared with the kaempferol accumulation (Olsen et al. 2009). It is interesting to note that in Arabidopsis, the mere depletion of nitrogen in plants growing under low light irradiance was unable to substantially affect the biosynthesis of quercetin, which was at trace level in leaves growing under normal greenhouse conditions (Lillo et al. 2008). Lillo et al. (2008) have suggested that a second factor, such as low temperature or high light, seems to be actually required to trigger the biosynthesis of the antioxidant quercetin derivatives. This suggestion have to be considered, however, with some cautions, as most experiments have been conducted under growth conditions typical of the understorey or mild-to-deep shaded environments, since light irradiance did not exceed 200 μmol quanta m−2 s−1. However, results are in partial agreement with the recent suggestion reported in Jaakola and Hohtola (2010), for the effect of latitude on the flavonol biosynthesis. In detail, Albert et al. (2009) and Schmidt et al. (2010) observed that the increase in quercetin to kaempferol ratio was strongly correlated with a decrease in external temperature in Arnica montana and Brassica oleracea, respectively, under natural conditions. It has been suggested that the enhancement in the quercetin to kaempferol ratio depended more on the decrease of T than on the increase of UV-B radiation because of an increase in latitude (Jaakola and Hohtola 2010).
On the whole, cold and nitrogen deprivation affect the biosynthesis of flavonoids and lead to a preferential biosynthesis of the antioxidant quercetin derivatives with respect to that of the corresponding monohydroxy B-ring-counterparts, i.e., kaempferol glycosides. These effects are very similar to those exerted by high light irradiance, irrespective of the portions of various solar wavebands reaching the leaf surface (Lillo et al. 2008; Jaakola and Hohtola 2010). Even more, the synergic effect of cold and high light in inducing the expression of both the flavonoid and the flavonol-branch biosynthesis is similar to that found in response to nitrogen and cold treatments on the biosynthesis of quercetin derivatives (Soitamo et al. 2008; Løvdal et al. 2010).
The issue, however, deserves further investigation, as an in-depth analysis of the stress-induced effects on the regulation of flavonoid biosynthesis has not been supported with a detailed analysis on the sites of flavonoid accumulation, a prerequisite to conclusively address their “antioxidant” functions in vivo.
2.3 Drought and Salinity Stress
There are few, but interesting experiments that have detailed the impact of “osmotic stress”, as drought and salinity, on the flavonoid metabolism in higher plants. Walia et al. (2005) have reported an upregulation of the flavonoid biosynthetic genes, as a consequence of NaCl stress. The increase in the expression of F3′H, which leads to the biosynthesis of ortho-dihydroxylated B-ring “antioxidant flavonoids”, was superior in the salt-sensitive genotype than in the salt-tolerant rice genotype. The enhancement in flavonoid biosynthesis was paralleled with the increase in glutathione-S-transferase, which is involved in the transport of flavonoids to the vacuole (Zhao and Dixon 2009). The increase in carbon allocated to myricetin and quercetin glycosides, two well-known antioxidant flavonols, was significantly greater in the salt-sensitive Myrtus communis than in the salt-tolerant Pistacia lentiscus (Tattini et al. 2006), and supposed to be involved in the peroxidase-catalyzed reduction of H2O2, based on their large accumulation in the palisade parenchyma cells. Recently, Agati et al. (2011) have shown that root-zone salinity stress had a very similar effect on flavonoid metabolism of that exerted by UV-radiation. In both cases the biosynthesis of quercetin 3-O-glycosides was mostly upregulated, while the biosynthesis of apigenin 7-O-glycosides (mono-hydroxy B-ring flavones) remaining unaffected by mild NaCl stress. It was additionally observed that salinity stress and UV-radiation greatly enhanced the biosynthesis of luteolin 7-O-glycosides. These findings support the idea that different osmotic stressors lead to a similar plant response: the upregulation of the biosynthesis of those flavonoids, as the dihydroxy B-ring-substituted flavones, like luteolin 7-O-glycosides, which are effective antioxidants (Tattini et al. 2004). A fine tuning exerted by root-zone salinity on the flavonoid metabolic pathway was observed, as the ratio of luteolin 7-O-glucoside to the monohydroxy B-ring-substituted luteolin 4′-O-glucoside steeply increased passing from control to NaCl-treated plants. We note that luteolin 4′-O-glucoside lacking the catechol group in the B-ring of the flavonoid skeleton does not display effective free radical scavenger activity in the molar concentration range that may reasonably occur in leaf cells. Vasquez-Robinet et al. (2008) have found that the maximum flavonoid gene expression (CHS and GST) was observed during the period of more intense drought stress, which suggests for flavonoids a key protective role against water stress. The decrease of maximum carbon assimilation (A max) induced by osmotic stresses exacerbates the deleterious effects of high solar irradiance, as it has been found in some Mediterranean species (Guidi et al. 2008, 2011; Melgar et al. 2009; Agati et al. 2011). In grape berries, Castellarin et al. (2007a, b) found a steep induction of the whole set of genes involved in the biosynthesis and transport of flavonoids because of water stress. These data are consistent with those of Tattini et al. (2004) in Ligustrum vulgare leaves, as both water stress and sunlight irradiance led to a steep enhancement in the biosynthesis of flavonoids with an orthodihydroxy B-ring substitution. Although drought stress and sunlight irradiance have been shown to synergistically enhance the expression of flavonoid biosynthetic genes, drought-stressed plants growing at 35 or 100% sunlight irradiance in the field had a significantly smaller concentration of both quercetin 3-O- and luteolin 7-O-glycosides than the well-watered counterparts. It was shown that drought stress depressed steeply the amount of “newly assimilated” or “fresh” carbon available to flavonoid biosynthesis. The matter needs to be explored in depth as, transcript or expression abundance may not directly translate in protein abundance, i.e., enzyme activity (Sweetlove and Fernie 2005), and the tissue flavonoid accumulation may additionally largely depend upon the extent to which different stresses may affect carbon gain and tissue specific concentration in nonstructural carbohydrates. Since it is the molar concentration of flavonoids in different plant tissues coupled with their intracellular distribution that supersede the potential ROS-scavenging functions in vivo, the issue merits further investigation (Turtola et al. 2005; Melgar et al. 2009; Remorini et al. 2009).
2.4 Ozone
Ozone, a secondary pollutant formed in the troposphere by the interaction of hydrocarbons, nitrogen oxides and sunlight, is a powerful oxidizing agent capable of reacting with any biomacromolecule, although it is neither a free radical species nor a reactive oxygen species, such as H2O2 (Mustafa 1990). It is not clear whether oxidative burst occurs and whether visible lesions are caused by ozone through programmed cell death (Sandermann 1996), but the upregulation of aromatic secondary metabolism, including the shikimate and the flavonoid pathways, has been commonly reported during O3-stress (Janzik et al. 2005; Betz et al. 2009; Iriti and Faoro 2009).
In Arabidopsis, ozone induced mRNA levels of PAL within 3 h from exposure, whereas increases in the mRNA levels of antioxidant enzymes (e.g., a neutral peroxidase and a cytosolic CuZn-superoxide dismutase) were found after 12 h (Sharma and Davis 1994). Other works report an induction of PAL and glutathione S-transferase (GST, which conjugates most flavonoids and allowed transport to different cellular compartments, Agati and Tattini 2010; Zhao and Dixon 2009) transcription and activity within 2–3 h from treatment (Eckey-Kaltenbach et al. 1994; Sgarbi et al. 2003; Guidi et al. 2009), followed by a twofold increase of flavone glycoside concentration (Eckey-Kaltenbach et al. 1994). An increase of chalcone synthase (CHS) and chalcone isomerase (CHI), the enzymes involved in the first committed steps of flavonoid biosynthesis (Fig. 9.1), has been reported after ozone fumigation (Kangasjarvi et al. 1994; Paolacci et al. 2001) in a variety of plant species. Kanoun et al. (2003) detected a linear relationship between O3-levels and the accumulation of kaempferol 3-O-glucuronide, and Betz et al. (2009) found an increase of kaempferol 3-O-glycoside in ozone-treated beeches. Higher concentration of quercetin derivates were found in O3-treated birch growing at ambient CO2 concentration (Peltonen et al. 2005). Interestingly, ozone both decreased total phenolics and did not display any significant effects on kaempferol biosynthesis, while steeply enhancing the biosynthesis of quercetin derivatives in leaves of Ginkgo biloba (He et al. 2009). Saviranta et al. (2010) have recently reported a specific induction of flavonoid biosynthesis in response to mild ozone stress, and supposed to be involved in countering O3-induced oxidative damage. As previously reported, ozone enters the leaf through stomata, which are located, in most dicotyledonous species, on the lower side of the leaf lamina, where the density of glandular trichomes producing epicuticular flavonoids is also high (Valkama et al. 2003). Moreover, flavone aglycones have antioxidant activity (Rice-Evans et al. 1997) and can react directly with O3 deposited on leaf surface.
2.5 Heavy Metals
Other potential abiotic stresses that may expose plants to oxidative damage have been investigated with respect to flavonoid metabolism. The functional roles of flavonoids in the response mechanisms to heavy-metal stress have received some attention. Kováĉik et al. (2009) have reported an increase in both flavonoids and caffeic acid in Matricharia camomilla leaves as a consequence of high Ni2+-supply, whereas coumaric acid derivatives and phenolic acids did not vary. These findings led authors to hypothesize an antioxidant function of phenolics under heavy-metal stress. Ali et al. (2006) have shown an increase in flavonoid content and free radical scavenging activity in root suspension culture because of excess Cu2+. Excess-Cu2+ ions have been reported to stimulate the biosynthesis of flavonoids, mostly luteolin-glycosides, in the absence of UV-irradiance, closely resembling the effect to exposing plants to simulated solar irradiance in leaves of Lemna gibba (Babu et al. 2003). These authors showed a positive correlation between the stress-induced increase in ROS concentration and the accumulation of flavonoids with the greatest antioxidant potential. It may be, therefore, postulated that the flavonol metabolism responds to alterations in ROS homeostasis and flavonoids contributed to its restoration, to maintain ROS concentration at a sublethal level. Similar conclusions were drawn by Schützendübel et al. (2001) for the role of root polyphenols in plants suffering from a severe Cd2+-stress. These authors found that the accumulation of polyphenols was inversely related with the activities of key antioxidant enzymes, that declined steeply as Cd2+-stress progressed, and hypothesized for polyphenols a ROS-scavenging functions to compensate for the decrease in the activity of primary antioxidant defenses. More recently, Potters et al. (2007) have suggested that flavonoids may exert their beneficial effects on Cd2+-stress by affecting the movement of auxin and hence exert a tight control on the root architecture.
3 Antioxidant Flavonoids and the Antioxidant Machinery of Plants
3.1 Stress-Induced Alterations in the Antioxidant Enzymes System
There is a large consensus that a well-coordinated system of constitutive antioxidant defenses is activated in plants upon a plethora of abiotic stresses (for a recent review, see Gill and Tuteja 2010). Superoxide dismutase (SOD), the well-known first-line of defense against ROS generation (aimed at removing the highly reactive superoxide anion) (O −2 ), and both ascorbate peroxidase (APX) and catalase (CAT), the enzymes that are devoted at detoxifying the “relatively stable” H2O2, have long been reported to play a key role in protecting plants from stress-induced oxidative injuries (Schwanz and Polle 2001a, b; Polle 2001). The extent to which the activity of antioxidant enzymes increases upon stress imposition has been widely reported to correlate positively with tolerance to various abiotic stresses (Hernández et al. 1999, 2000, 2003; Tattini et al. 2005; Sekmen et al. 2007), although it has been also reported that the constitutive activity of antioxidant enzymes is correlated with stress tolerance (Pasqualini et al. 2001; Schwanz and Polle 2001a, b; Guidi et al. 2010). However, under prolonged stress, the activities of key components of the antioxidant machinery of stress-sensitive species have been reported to decline (Hatier and Gould 2008). For example, high doses of UV-B radiation have been reported to decrease the activity of both SOD and APX in Ulva fasciata (Shiu and Lee 2005). Several papers of the Andrea Polle’s group have conclusively reported of a depression in the activity of antioxidant enzymes in plants exposed to the concomitant action of two or more stresses (Peltzer and Polle 2001; Polle 2001; Peltzer et al. 2002). These findings lead to the hypothesis that the stress severity, which depends on both the intensity and duration of the stress imposed, in addition to the species-specific ability to counter the stress-induced impairments of the photosynthetic machinery, may detrimentally affect the first line of defense against the generation of ROS (Schwanz and Polle 2001a, b; Schützendübel et al. 2001; Wang et al. 2007, 2008).
On the whole, these findings do not support the general view that the whole set of constitutive antioxidant defenses is activated as a consequence of stressful conditions of different origin, and poses the question of the extent to which key components of the antioxidant machinery may actually integrate to counter stress-induced ROS generation. The action of antioxidant enzymes have long been suggested to need of being be complemented by the action of other antioxidant defenses on a long-term basis, when the severity of stress increases (Apel and Hirt 2004).
3.2 Antioxidant Enzymes and Antioxidant Flavonoids: Is There a Relation?
Hatier and Gould (2008) has recently suggested that flavonoids may serve an important antioxidant function when the activity of other components of the antioxidant machinery is steeply depressed under severe conditions of excess light. Their hypothesis is based upon the observation that the very conditions that lead to enzyme inactivation are those responsible for the maximum biosynthesis of anthocyanins.
Indeed, the biosynthesis of antioxidant flavonoids is mostly upregulated under excess light stress, both in the absence and in the presence of UV-irradiance. Kolb et al. (2001) have reported a great increase in the biosynthesis of quercetin derivatives with respect to kaempferol derivatives in response to visible sunlight irradiance. Agati et al. (2009) in L. vulgare, a sun-sensitive species (Tattini et al. 2005), detected a sevenfold increase in the concentration of quercetin 3-O-rutinoside passing from 20 to 100% sunlight irradiance in the absence of UV-radiation. Even more, the ratio of phenylpropanoids with catechol group in the B-ring of the flavonoid skeleton (quercetin 3-O- plus luteolin 7-O-glycosides) or in the benzene ring (echinacoside, a caffeic acid derivative) to monohydroxy substituted counterparts (p-coumaric, apigenin 7-O-glycosides) increased as much as 360% because of an increase in PAR irradiance. Babu et al. (2003) have reported of a preferential biosynthesis of luteolin as compared with apigenin derivatives in L. gibba as a consequence of excess Cu2+ in the absence of UV-radiation. In addition to the known ROS-related signaling pathway leading to the induction of flavonoids, a recent work showed the existence of a second retrograde signaling pathway that operates during various stress situation and influences flavonoid biosynthesis (Akhtar et al. 2010). This second signaling pathway has been related to photosynthetic electron transport chain (PETC) redox state. The PETC redox signal can operate independently of ROS and override the effects of ROS on flavonoid biosynthesis (Akhtar et al. 2010).
Few experiments have been conducted to specifically address the issue of flavonoid biosynthesis as a consequence of stress-induced alteration in antioxidant enzyme activity. Xu et al. (2008) have reported a greater accumulation of antioxidant enzyme proteins in a soybean line with reduced flavonoid content. Aguilera et al. (2002) and Shiu and Lee (2005) have shown that in U. fasciata exposed to high UV-B doses, a condition that leads to the maximal flavonoid accumulation, the activity of antioxidant enzymes, particularly CAT and APX, declined greatly. Under severe excessive light stress, the expression of genes involved in the biosynthesis and conjugation of flavonoids were mostly upregulated, whereas the activity of SOD was unaltered (Soitamo et al. 2008). In two Oleaceae species differing in their ability to withstand excessive sunlight radiation (estimated in terms of chlorophyll loss and the leaf lipid peroxidation), which exhibited a constitutively different antioxidant enzyme activity, the activity of phenylalanine ammonia-lyase and the biosynthesis of antioxidant quercetin glycosides was steeply greater in the sun-sensitive than in the sun-tolerant species. Agati et al. (2011) have recently reported that the accumulation of epidermal flavonoids was completed after 2 weeks of treatment, when the activities of key antioxidant enzymes declined, as a consequence of UV-irradiance (Guidi et al. 2011).
On the whole, these findings may lead to the hypothesis that the biosynthesis of flavonoids is mostly upregulated under severe stress conditions, when the activities of antioxidant enzymes decline, and, hence, flavonoids may complement the action of other ROS-scavenging systems. Flavonoids have, therefore, to be regarded as a “secondary” antioxidant system, activated upon a severe ROS/REDOX unbalance because of the depletion of primary antioxidant defense systems. This hypothesis is supported by the observation that the greatest antioxidant flavonoid biosynthesis is correlated with the greatest oxidative damage, which conforms to the depletion of antioxidant defenses primarily, also in terms of time, aimed at detoxifying ROS. Under high solar radiation, a greater increase of di-hydroxylated flavonoids was in plants of L. vulgare, a shade-tolerant species, when compared to Phillyrea latifolia, a sun-requiring species (Tattini et al. 2005). The greater shift toward the flavonoid biosynthetic pathway detected in L. vulgare than in P. latifolia was related to the greater need of the former species to counter oxidative damage. Similarly, when M. communis and P. lentiscus were exposed to high solar radiation and root-zone salinity, the allocation of carbon to flavonoid metabolism increased more in the former than in the latter species, and appeared to be related to leaf oxidative damage (Tattini et al. 2006).
It may not be a mere coincidence that flavonols, the most ancient and widespread of flavonoids, are effective antioxidants. Excess light stress is experienced by plants not only on a seasonal, but also on a daily basis, under natural conditions (Li et al. 2009), and the flavonol metabolism has been highly conserved from the colonization of land by plants, even though the evolution of flavonoid metabolism have produced more than 10,000 structures.
The issue of how flavonoids may perform their reducing activity if confined in cellular compartments, the vacuole, far from the chloroplast, the main source of ROS still generates conflict (Hernández et al. 2009). The old view that flavonoids accumulate almost exclusively in the vacuole of epidermal cells has been recently confuted by a series of experiments in which flavonoids have been shown to largely accumulate in the vacuole of mesophyll cells, and, hence, in the proximity of ROS generation centers. Gould et al. (2002) have shown that vacuolar anthocyanins in the mesophyll may quench H2O2 generated upon mechanical injury. Antioxidant quercetin and luteolin glycosides have been reported to accumulate in the vacuole of mesophyll cells exposed to excess PAR irradiance, and it has been speculated that they may help reducing hydrogen peroxide. It is noted that ascorbic acid is a very poor substrate for vacuolar peroxidases that may act to reduce H2O2 using flavonoids as preferential substrates (Yamasaki et al. 1997). Ascorbic acid has long been reported to serve as a secondary reducing agent to recycle the flavonoid radicals to their original forms (Sakihama et al. 2000). In a recent experiment Zechmann et al. (2011) have interestingly noted that the pool of vacuolar ascorbate increased dramatically as a consequence of excess light stress, and it may be speculated to be involved in the peroxidase-catalyzed reduction of H2O2 using flavonoids as substrates.
Recently, Agati et al. (2007) have reported of a chloroplastic distribution of antioxidant flavonoids using three-dimensional deconvolution microscopy. These findings conform to early views of chloroplast localization of flavonoids (Oettmeier and Heupel 1972; Saunders and Mc Clure 1976; Takahama 1982), and of chloroplast being capable of flavonoid biosynthesis (Zaprometov and Nikolaeva 2003). Chloroplast quercetin and luteolin 7-O-glycosides were effective in quenching singlet oxygen generated upon excess visible light in vivo. Feucht et al. (2004) and Polster et al. (2006) have reported of the occurrence of flavonoids in the nucleus of emerging leaflets of various species, which conforms to a nuclear distribution of both chalcone synthase (CHS) and chalcone isomerase (CHI) (Saslowsky et al. 2005). Hernández et al. (2009) have suggested nuclear flavonoids being capable to chelate transition metal ions and, hence, to inhibit the generation of H2O2. This function is to be considered as an antioxidant function, even though there is not a reducing activity here, as it prevents the oxidative damage (Halliwell 2009).
Nevertheless, the issue of the subcellular distribution of flavonoids is far from being conclusively addressed. Flavonoids do not display fluorescence when dissolved in the aqueous cellular milieu and, hence, they need to become pseudofluorescent upon the addition of “fluorescent” probes. The largely used Naturstoff reagent (NR), 2-amino ethyl diphenyl borinic acid, has long been reported to have difficulty to enter cellular compartments, because of its acidic nature (Shehan et al. 1998), and, it is highly specific for dihydroxy B-ring-substituted flavonoid glycosides (Agati et al. 2009). By contrast, the alkalinization of flavonoid solutions with NH3 under UV-excitation is not specific for flavonoid fluorescence (Kolb et al. 2001; Agati et al. 2002). We recall here that the fluorescence signature of NR-stained tissue under blue light excitation at 488 nm, as commonly used in confocal laser scanning microscopy (CLSM), has to be considered with some cautions.
4 “Antioxidant” Flavonoids as Developmental Regulators
The term “developmental regulators” for flavonoids has been proposed recently by Taylor and Grotewold (2005), and resemble closely that of “internal regulators” early coined by Stafford (1991). Stafford speculated that in early terrestrial plants the concentration of flavonoids had to be relatively low, and, hence, their UV-B screening functions of relatively scarce significance, as flavonoid concentrations capable to effectively attenuating UV-radiation need to be in the mM range (Edwards et al. 2008; Agati and Tattini 2010). By contrast, flavonoid concentration in the high nM to low μM range may be effective in regulating the auxin movement (Besseau et al. 2007) and quenching reactive oxygen species (Tattini et al. 2004). Recent findings of the localization of the auxin efflux facilitator protein, PIN5, to the endoplasmic reticulum (Friml and Jones 2010), the site of flavonoid biosynthesis, are of particular interest. The localization of the moss PIN proteins to the endoplasmic reticulum leads to the hypothesis that the ancestral functions of these “short” PIN proteins was likely to mediate the cellular auxin homeostasis, which further reinforces the Stafford’s hypothesis.
Flavonoids are, in fact, well-known endogenous regulators of auxin movement (Jacobs and Rubery 1988; Brown et al. 2001; Peer and Murphy 2007), and, interestingly, antioxidant flavonoids display the greatest ability to regulate the transport of auxin in vivo (Taylor and Grotewold 2005; Besseau et al. 2007). Quercetin aglycone (which lacks the glycosyl moiety in the 3-postion of the flavonoid skeleton, and hence, display the greatest antioxidant potential, Rice-Evans et al. 1996) is more effective than both kaempferol aglycone and quercetin 3-O-rutinoside to inhibiting the basipetal transport of auxin (PAT, Jacobs and Rubery 1988; Besseau et al. 2007). Flavonoids may profoundly alter the tissue- and cell-specific auxin concentrations by tightly affecting the IAA-oxidation (Mathesius 2001), not just by modulating its intra- and intercellular movements. Monohydroxy B-ring flavonoids have long been shown to behave as cofactors and dihydroxy B-ring flavonoids as inhibitors of the peroxidase-mediated oxidation of auxin (Galston 1969). Jansen (2002) has provided strong evidence that the increase in quercetin to kaempferol ratio may confer UV-tolerance because of the strikingly different affinities of the two flavonols on class III peroxidases (Yamasaki et al. 1997).
Plants suffering from different abiotic stresses display a marked redistribution of growth (Baena González 2010), the so-called stress-induced morphogenic responses (SIMR) (Potters et al. 2007). This “unspecific” response of plants suffering from different stressful conditions is a part of their acclimation strategy to “flight” away from unfavorable environments (Potters et al. 2007). It has become clear that ROS-production and altered phytohormone transport and/or metabolism, that are traits common to different stresses, are involved in SIMR (for review articles, Pritzschke and Hirt 2006; Peer and Murphy 2006; Beveridge et al. 2007), thus making flavonoids as ideal candidates to greatly impact on stress-induced redistribution of growth (Thibaud-Nissen et al. 2003; Lazar and Goodman 2006). The involvement of phenolics in the tolerance mechanisms to Cd2+-stress (Schützendübel et al. 2001; Potters et al. 2007) depends on their ability to both scavenging ROS (as a consequence of Cd2+-induced depression in the activities of antioxidant enzymes, Schützendübel et al. 2001) and inhibiting the basipetal transport of auxin and hence the redirection of root growth (Potters et al. 2007).
It may not be a coincidence, therefore, that stress-responsive flavonoids are effective antioxidants. The chemical features that confer reducing ability against a wide array of free radicals and H2O2 allow flavonoids to also display the greatest affinity for proteins involved in key processes of growth and development (Peer and Murphy 2006). Flavonoids have long been reported to behave as transcript regulators in eukaryotic cells mostly through the inhibition of phosphorylation signaling cascades or specific kinases (Peer and Murphy 2006; Lamoral-Theys et al. 2010). This effect is believed to be responsible for the flavonoid-induced inhibition in the activity of PIN/MDR-PGP auxin transport proteins (Muday and DeLong 2001; Taylor and Grotewold 2005), and the catechol group in the B-ring of the flavonoid skeleton is the key feature responsible for the high affinity of flavonoids for different protein kinases (Williams et al. 2004; Lamoral-Theys et al. 2010). As a consequence, stress-responsive dihydroxy B-ring-substituted flavonoids may have a dual role on SIMR, behaving as regulators of both ROS (H2O2) concentration and ROS-induced MAPK signaling cascades.
5 Conclusion and Future Perspective
Flavonoids have long been shown to be involved in the response of plants to a plethora of stressful agents of both abiotic and biotic origin. This general finding is consistent with flavonoids being capable of displaying a wide range of functional roles in stressed plants. However, few flavonoid structures are capable of multiple functions, ranging from UV-screening, ROS scavenging, and inhibition of the activity of auxin efflux facilitator proteins. These flavonoids, which are the most responsive to various abiotic stresses, display the greatest potential to behave as antioxidants. The flavonol metabolism, which was at work in early terrestrial plants, has remained intact for millions of years despite the evolution of flavonoid metabolism and has produced more than 12,000 structures.
To serve such a variety of functional roles, antioxidant flavonols have to be distributed in different tissues and cellular compartments. Flavonols have been detected in the nucleus and suggested to protect DNA from damage, in the vacuole of mesophyll cells as well as in the chloroplasts, and suggested to scavenge highly reactive free radicals and the relatively stable H2O2. Flavonols have also been detected at the plasma membrane and hence optimally located to interfere with PIN/PGP-glycoproteins that mediate the cell-to-cell movement of auxin. Even more, flavonoids, which are synthesized in the endoplasmic reticulum, may exert a tight control on the cellular auxin homeostasis, through their interaction with ER-located “short” PIN proteins.
Nevertheless, there are still relevant issues that need to be deeply explored to address the actual relevance of stress-responsive flavonoids in an in planta situation. In our opinion, the relative contribution of flavonoids into the well-coordinated antioxidant machinery “activated” as a consequence of different abiotic stress is to be primarily assessed. In this regard, time-course experiments aimed not only at determining the transcript abundance or the gene expression of different antioxidant components but also at quantifying their activities or concentrations are actually required. At the same time, the stress-induced alterations on the inter- and intracellular distribution of key components of the antioxidant machinery, with special emphasis to flavonoids and ascorbic acid, should be routinely estimated as the severity of stress increases.
References
Agati G, Tattini M (2010) Multiple functional roles of flavonoids in photoprotecion. New Phytol 186:786–793
Agati G, Galardi C, Gravano E, Romani A, Tattini M (2002) Flavonoid distribution in tissues of Phyllyrea latifolia as estimated by microspectrofluorometry and multispectral fluorescence microimaging. Photochem Photobiol 76:350–360
Agati G, Matteini P, Goti A, Tattini M (2007) Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol 174:77–89
Agati G, Stefano G, Biricolti S, Tattini M (2009) Mesophyll distribution of antioxidant flavonoids in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann Bot 104:853–861
Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M (2011) The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J Plant Physiol 168:204–212
Aguilera J, Dummermuth A, Karsten U, Schriek R, Wiencke C (2002) Enzymatic defenses against photooxidative stress induced by ultraviolet radiation in Arctic marine macroalgae. Polar Biol 25:432–441
Akhtar TA, Lees HA, Lampi MA, Enstone D, Brain RA, Greenberg BM (2010) Photosynthetic redox imbalance influences flavonoid biosynthesis in Lemna gibba. Plant Cell Environ 33:1205–1219
Albert A, Sareedenchai V, Heller W, Seidlitz HK, Zidorn C (2009) Temperature is the key to altitudinal variation of phenolics in Arnica montana L. c.v. ARBO. Oecologia 160:1–8
Ali RM, Singh N, Shohael AM, Hahn EJ, Paek K-Y (2006) Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci 17:147–154
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Babu TS, Akhtar TA, Lampi MA, Tripuranthakam S, Dixon DG, Greenberg BM (2003) Similar stress responses are elicided by copper and ultraviolet radiation in the aquatic plant Lemna gibba: implication of reactive oxygen species as common signals. Plant Cell Physiol 44:1320–1329
Baena González E (2010) Energy signaling in the regulation of gene expression during stress. Mol Plant 3:300–313
Ballaré CL (2003) Stress under the sun: spotlight on ultraviolet-B responses. Plant Physiol 132:1725–1727
Berli FJ, Moreno D, Piccoli P, Hespanhol-Viana L, Silva MF, Bressan-Smith R, Cavagnaro JB, Bottini R (2010) Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ 33:1–10
Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M (2007) Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19:148–162
Betz GA, Gerstner E, Stich S, Winkler B, Welzl G, Kremmer E, Langebartels C, Heller W, Sandermann H, Ernst D (2009) Ozone affects shikimate pathway genes and secondary metabolites in saplings of European beech (Fagus sylvatica L.) grown under greenhouse conditions. Trees 23:539–555
Beveridge CA, Mathesius U, Rose RJ, Gresshoff PM (2007) Common regulatory themes in meristem development and whole-plant homeostasis. Curr Opin Plant Biol 10:44–51
Bilger W, Rolland M, Nybakken L (2007) UV screening in higher plants induced by low temperature in the absence of UV-B radiation. Photochem Photobiol Sci 6:190–195
Brown JE, Khodr H, Hider RC, Rice-Evans CA (1998) Structural dependence of flavonoid interactions with Cu2+ ions: implication for their antioxidant properties. Biochem J 330:1173–1178
Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126:524–535
Burchard P, Bilger W, Weissenböck G (2000) Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV-A and UV-B radiation in developing rye primary leaves as assessed by ultraviolet-induced chlorophyll fluorescence measurements. Plant Cell Environ 23:1373–1380
Caldwell MM, Robberecht R, Flint SD (1983) Internal filters: prospects for UV-acclimation in higher plants. Physiol Plant 58:445–450
Casano LM, Gómez LD, Lascano HR, Gonzáles CA, Trippi VS (1997) Inactivation and degradation of CuZn–SOD by active oxygen species in wheat chloroplasts exposed to photooxidative stress. Plant Cell Physiol 38:433–440
Castellarin S, Matthews MA, Gaspero GD, Gambetta GA (2007a) Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 227:101–112
Castellarin S, Pfeiffer A, Sivilotti P, Degan M, Peterlunger E, Di Gaspero G (2007b) Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ 30:1381–1399
Cockell CS, Knowland J (1999) Ultraviolet radiation screening compounds. Biol Rev 74:311–345
D’Auria JC, Gershenzon J (2005) The secondary metabolism of Arabidopsis thaliana: growing like a weed. Curr Opin Plant Biol 8:308–316
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15:573–581
Eckey-Kaltenbach HE, Ernst D, Heller W, Sandermann H (1994) Biochemical plant response to ozone (IV. Cross-induction of defensive pathways in parsley (Petroselinum crispum L.) plants). Plant Physiol 104:67–74
Edwards WR, Hall JA, Rowlan AR, Schneider-Barfield T, Sun TJ, Patil MA, Pierce ML, Fuclher RG, Bell AA, Essenberg M (2008) Light filtering by epidermal flavonoids during the resistant response of cotton to Xanthomonas protects leaf tissue from light-dependent phytoalexin toxicity. Phytochemistry 69:2320–2328
Erlejman AG, Verstraiten SV, Fraga CG, Oteiza PI (2004) The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radic Res 38:1311–1320
Feucht W, Treutter D, Polstre J (2004) Flavanol binding of nuclei from tree species. Plant Cell Rep 22:430–436
Friml J, Jones AR (2010) Endoplasmic reticulum: the rising compartment in auxin biology. Plant Physiol 154: 458–462
Galston AW (1969) Flavonoids and photomorphogenesis in peas. In: Harborne JB, Swain T (eds) Perspectives in phytochemistry. Academic Press, New York, pp 193–204
Geissler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Peer WA, Bailly A, Richards EL, Edjendal KF, Smith AP, Baroux C, Grossniklaus U, Muller A, Hrycyna CA, Dudler R, Murphy AS, Martinoia E (2005) Cellular efflux of auxin by catalyzed by the Arabidopsis MDR/RGP transporter AtPGP1. Plant J 44:179–194
Gerhardt KE, Lampi MA, Greenberg BM (2008) The effects of far-red on plant growth and flavonoid accumulation in Brassica napus in the presence of ultraviolet B radiation. Photochem Photobiol 84:1445–1454
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gould KS, McKelvie J, Markham KR (2002) Do anthocyanins function as antioxidant in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environ 25:1261–1269
Grace SC, Logan BA, Adams WW (1998) Seasonal differences in foliar content of chlorogenic acid, a phenylpropanoid antioxidant in Mahonia repens. Plant Cell Environ 21:513–521
Guidi L, Degl’Innocenti E, Remorini D, Massai R, Tattini M (2008) Interaction of water stress and solar irradiance on the physiology and biochemistry of Ligustrum vulgare. Tree Physiol 28:873–883
Guidi L, Degl’Innocenti E, Martinelli F, Piras M (2009) Ozone effects on carbon metabolism in sensitive and insensitive Phaseolus cultivars. Environ Exp Bot 66: 117–125
Guidi L, Degl’Innocenti E, Giordano C, Biricolti S, Tattini M (2010) Ozone tolerance in Phaseolus vulgaris depends on more than one mechanism. Environ Pollut 158:3164–3171
Guidi L, Degl’Innocenti E, Remorini D, Biricolti S, Fini A, Ferrini F, Nicese FP, Tattini M (2011) The impact of UV-radiation on the physiology and biochemistry of Ligustrum vulgare exposed to different visible-light irradiance. Environ Exp Bot 70:88–95
Halliwell B (2009) The wanderings of a free radical. Free Radic Biol Med 46:531–542
Hannah MA, Weise D, Freund S, Fiehn O, Heyer AG, Hincha DK (2006) Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol 142:98–112
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Hatier JHB, Gould KS (2008) Foliar anthocyanins as modulators of stress signals. J Theor Biol 253:625–627
He X, Huang W, Chen W, Dong T, Liu C, Chen Z, Xu S, Ruan Y (2009) Changes of main secondary metabolites in leaves of Ginkgo biloba in response to ozone fumigation. J Environ Sci 21:199–203
Hernández JA, Campillo A, Jimenez A, Alarcon JJ, Sevilla F (1999) Response of antioxidant systems and leaf water relations to NaCl stress in pea plants. New Phytol 141:241–251
Hernández JA, Jimenez A, Mullineaxu P, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23:853–862
Hernández JA, Aguilar AB, Portillo B, Lopez-Gomez E, Beneyto JM, Garcia Legaz MF (2003) The effect of calcium on the antioxidant enzymes from salt-treated loquat and anger plants. Funct Plant Biol 30: 1127–1137
Hernández I, Alegre L, van Breusegem F, Munné-Bosch S (2009) How relevant are flavonoids as antioxidant in plants? Trends Plant Sci 14:125–132
Hofmann RW, Campbell BD, Bloor SJ, Swinny EF, Markham KR, Ryan KG, Fountain DW (2003) Responses to UV-B radiation in Trifolium repens L. – physiological links to plant productivity and water availability. Plant Cell Environ 26:603–612
Iriti M, Faoro F (2009) Chemical diversity and defence metabolism: how plants cope with pathogens and ozone pollution. Int J Mol Sci 10:3371–3399
Jaakola L, Hohtola A (2010) Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ 33:1239–1247
Jacobs M, Rubery PH (1988) Naturally occurring auxin transport regulators. Science 241:346–349
Jansen M (2002) Ultraviolet-B radiation effects on plants: induction of morphogenic responses. Physiol Plant 116:423–439
Janzik I, Preiskowski S, Kneifel H (2005) Ozone has dramatic effects on the regulation of the prechorismate pathway in tobacco. Planta 223:20–27
Kangasjarvi J, Talvinen J, Utriainen M, Karjalainen R (1994) Plant defence system induced by ozone. Plant Cell Environ 17:783–794
Kanoun M, Goulas P, Biolley JP (2003) Describing and modelling ozone-dependent variation in flavonoid content of bean (Phaseolus vulgaris cv. Bergamo) leaves: a particular dose–response relationship analysis. Funct Plant Biol 30:561–570
Kolb CA, Käser MA, Kopecky J, Zotz G, Riederer M, Pfündel EE (2001) Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol 127:863–875
Korn M, Peterek S, Petermock H, Heyer AG, Hincha DK (2008) Heterosis in the freezing tolerance, and sugar and flavonoid contents of crosses between Arabidopsis thaliana accessions of widely varying freezing tolerance. Plant Cell Environ 31:313–327
Kotilainen T, Tegelberg R, Julkunen-Tiitto R, Lindfors A, Aphalo PJ (2008) Metabolic specific effects of solar UV-A and UV-B on alder and birch leaf phenolics. Glob Chang Biol 14:1294–1304
Kováĉik J, Klejdus B, Baĉkor M (2009) Phenolic metabolism of Matricaria chamomilla plants exposed to nickel. J Plant Physiol 166:1460–1464
Kytridis VP, Manetas Y (2006) Mesophyll versus epidermal anthocyanins as potential in vivo antioxidants: evidence linking the putative antioxidant role to the proximity of oxy-radical source. J Exp Bot 57:2203–2210
Lamoral-Theys D, Pottier L, Dufrasne F, Nève J, Dubois J, Kornienko A, Kiss R, Ingrassia L (2010) Natural polyphenols that display anticancer properties through inhibition of kinase activity. Curr Med Chem 17:812–825
Lazar G, Goodman HM (2006) MAX1, a regulator of flavonoid pathway, controls vegetative bud outgrowth in Arabidopsis. Proc Natl Acad Sci U S A 103:472–476
Li Z, Wakao S, Fusher BB, Niyogi KK (2009) Sensing and responding to excess light stress. Annu Rev Plant Biol 50:239–260
Lillo C, Lea US, Ruoff P (2008) Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ 31:587–601
Løvdal T, Olsen KM, Slimestad R, Verheul M, Lillo C (2010) Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 71:605–613
Manetas Y (2006) Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora 201:163–177
Markham KR, Ryan KG, Bloor SJ, Mitchell KA (1998) An increase in luteolin: apigenin ratio in Marchantia polymorpha on UV-B enhancement. Phytochemistry 48:791–794
Mathesius U (2001) Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J Exp Bot 52:419–426
Melgar JC, Guidi L, Remorini D, Agati G, Degl’Innocenti E, Castelli S, Baratto MC, Faraloni C, Tattini M (2009) Antioxidant defences and oxidative damage in salt-treated olive plants under contrasting sunlight irradiance. Tree Physiol 29:1187–1198
Melidou M, Riganakos K, Galaris D (2005) Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: the role of iron chelation. Free Radic Biol Med 39:1591–1600
Mendez M, Gwynn Jones D, Manetas Y (1999) Enhanced UV-B radiation under field conditions increases anthocyanin and reduces the risk of photoinhibition but does not affect growth in the carnivorous plant Pinguicula vulgaris. New Phytol 144:275–282
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) The reactive oxygen gene network in plants. Trends Plant Sci 9:490–498
Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6:535–542
Mustafa MG (1990) Biochemical basis of ozone toxicity. Free Radic Biol Med 9:245–265
Oettmeier W, Heupel A (1972) Identification of flavonoids and cinnamic acid derivatives from spinach chloroplast preparations. Zeitschrift für Naturforschung B 27:177–183
Ollson LC, Veit M, Bornman JF (1999) Epidermal transmittance and phenolic composition of atrazine-tolerant and atrazine-sensitive cultivars of Brassica napus grown under enhanced UV-B radiation. Physiol Plant 107:259–266
Olsen KM, Slimestad R, Lea US, Brede C, Løvdal T, Ruoff P, Verheul M, Lillo C (2009) Temperature and nitrogen effects on regulators and products of the flavonoid pathway: experimental and kinetic model studies. Plant Cell Environ 32:286–299
Paolacci AR, D’ovidio R, Marabottini R, Nali Lorenzini G, Abanavoli MR, Badiani M (2001) Ozone induces a differential accumulation of phenylalanine ammonia-lyase, chalcone synthase and chalcone isomerase RNA transcripts in sensitive and resistant bean cultivars. Aust J Plant Physiol 28:425–428
Pasqualini S, Batini P, Ederli L, Porceddu A, Piccioni C, De Marchis F, Antonielli F (2001) Effects of short-term ozone fumigation on tobacco plants: response of the scavenging system and expression of the glutathione reductase. Plant Cell Environ 24:245–252
Pearse IS, Heath KD, Cheeseman JM (2005) Biochemical and ecological characterization of two peroxidise isoenzymes from the mangrove, Rhizophora mangle. Plant Cell Environ 28:612–622
Peer WA, Murphy AS (2006) Flavonoids as signal molecules. In: Grotewold E (ed) The science of flavonoids. Springer, New York, pp 239–267
Peer WA, Murphy AS (2007) Flavonoids and auxin transport: modulators or regulators. Trends Plant Sci 12:556–563
Peltonen PA, Vapaavouri E, Julkunen-Tiitto R (2005) Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Glob Chang Biol 11:1305–1324
Peltzer D, Polle A (2001) Diurnal fluctuations of antioxidant systems in leaves of field-grown beech trees (Fagus sylvatica): response to light and temperature. Physiol Plant 111:158–164
Peltzer D, Dreyer E, Polle A (2002) Differential temperature dependencies of antioxidative enzymes in two contrasting species: Fagus sylvatica and Coleus blumei. Plant Physiol Biochem 40:141–150
Polle A (2001) Dissecting the superoxide dismutase–ascorbate–glutathione–pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol 126:445–462
Polster J, Dithmar H, Burgemeister R, Friedemann G, Feucht W (2006) Flavonoids in plant nuclei: detection by laser microdissection and pressure catapulting (LMPC), in vivo staining, and UV–visible spectroscopic titration. Plant Physiol 128:163–174
Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MA (2007) Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci 12:98–105
Pritzschke A, Hirt H (2006) Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiol 141:351–356
Rausher MD (2006) The evolution of flavonoids and their genes. In: Grotewold E (ed) The science of flavonoids. Springer, New York, pp 175–211
Remorini D, Melgar JC, Guidi L, Degl’Innocenti E, Castelli S, Traversi ML, Massai R, Tattini M (2009) Interaction effects of root-zone salinity and solar irradiance on the physiology and biochemistry of Olea europaea. Environ Exp Bot 65:210–219
Rice-Evans CA, Miller NJ, Papanga G (1996) Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Ryan KG, Markham KR, Bloor SJ, Bradley JM, Mitchell KA, Jordan BR (1998) UV-B radiation induces increase in quercetin: kaempferol ratio in wild-type and transgenic lines of Petunia. Photochem Photobiol 68:323–330
Ryan KG, Swinny EE, Markham KR, Winefield C (2002) Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry 59:23–32
Sakihama Y, Mano J, Sano S, Asada K, Yamasaki H (2000) Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochem Biophys Res Commun 279:949–954
Sandermann H (1996) Ozone and plant health. Annu Rev Phytopathol 34:347–366
Saslowsky DE, Warek U, Winkel BSJ (2005) Nuclear localization of flavonoid enzymes in Arabidopsis. J Biol Chem 25:23735–23740
Saunders JA, Mc Clure JN (1976) The distribution of flavonoids in chloroplasts of twenty five species of vascular plants. Phytochemistry 15:809–810
Saviranta NMM, Julkunen-Tiitto R, Oksanen E, Karjalainen RO (2010) Leaf phenolic compounds in red clover (Trifolium pratense L.) induced by exposure to moderately elevated ozone. Environ Pollut 158:440–446
Schmidt S, Zietz M, Schreiner M, Rohn S, Kroh LW, Krumbein A (2010) Genotypic and climatic influences on the concentration and composition of flavonoids in kale (Brassica oleracea var. sabellica). Food Chem 119:1293–1299
Schmitz-Hoerner R, Weissenböck G (2003) Contribution of phenolic compounds to the UV-B screening capacity of developing barley primary leaves in relation to DNA damage and repair under elevated UV-B levels. Phytochemistry 64:243–255
Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld R, Heyser D, Godbold L, Polle A (2001) Cadmium induced changes in antioxidative system, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 127:878–898
Schwanz P, Polle A (2001a) Differential stress response of oxidative system to drought in pedunculate oak (Quercus robur) and maritime pine (Pinea pineaster) grown under high CO2 concentration. J Exp Bot 52:133–143
Schwanz P, Polle A (2001b) Growth under elevated CO2 ameliorates defenses against photo-oxidative stress in poplar (Populus alba x tremula). Environ Exp Bot 45:43–53
Sekmen AH, Turkan I, Takio S (2007) Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physiol Plant 131: 399–411
Sgarbi E, Baroni Fornasiero R, Lins AP, Medeghini Bonatti P (2003) Phenol metabolism is differentially affected by ozone in two cell lines from grape (Vitis vinifera L.) leaf. Plant Sci 165:951–957
Sharma YK, Davis KR (1994) Ozone-induced expression of stress related genes in Arabidopsis thaliana. Plant Physiol 105:1089–1096
Shehan JJ, Cheong H, Rechnitz GA (1998) The colorless flavonoids of Arabidopsis thaliana (Brassicaceae). I. A model system to study the orthodihydroxy structure. Am J Bot 85:467–475
Shiu CT, Lee TM (2005) Ultraviolet-B-induced oxidative stress and responses of the ascorbate–glutathione cycle in a marine macroalga Ulva fasciata. J Exp Bot 56:2851–2865
Soitamo A, Piippo M, Allahverdiyeva Y, Battchikova N, Aro EM (2008) Light has a specific role in modulating Arabidopsis gene expression at low temperature. BMC Plant Biol 8:13
Stafford HA (1991) Flavonoid evolution: an enzymic approach. Plant Physiol 96:680–685
Streb PF, Feierebend J, Bigney R (1997) Resistance to photoinhibition of photosystem II and catalase and antioxidative protection in high mountain plants. Plant Cell Environ 20:1030–1040
Swain T (1986) Plant flavonoids in biology and medicine. In: Cody V, Middleton E Jr, Harborne JB (eds) Progress in clinical and biological research 213. Liss, New York, NY, pp 1–14
Sweetlove LJ, Fernie AR (2005) Regulation of metabolic networks: understanding metabolic complexity in the systems biology era. New Phytol 168:9–24
Takahama U (1982) Suppression of carotenoids photobleaching by kaempferol in isolated chloroplasts. Plant Cell Physiol 23:859–864
Takahama U, Oniki T (1997) A peroxidase/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells. Physiol Plant 101:845–852
Tattini M, Galardi C, Pinelli P, Massai R, Remorini D, Agati G (2004) Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol 163:547–561
Tattini M, Guidi L, Morassi Bonzi L, Pinelli P, Remorini D, Degl’Innocenti E, Giordano C, Massai R, Agati G (2005) On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phyllirea latifolia to high solar radiation. New Phytol 167:457–470
Tattini M, Remorini D, Pinelli P, Agati G, Saracini E, Traversi ML, Massai R (2006) Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytol 170:779–794
Taylor LP, Grotewold E (2005) Flavonoids as developmental regulators. Curr Opin Plant Biol 8:317–323
Thibaud-Nissen F, Shealy RT, Khanna A, Vodkin LO (2003) Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiol 132:118–136
Turtola S, Rousi M, Pusenius J, Yamaji K, Heiska S, Tirkkonens V, Meier B, Julkunen Tiitto R (2005) Clone-specific responses in leaf phenolics of willows exposed to enhanced UVB radiation and drought stress. Glob Chang Biol 11:1655–1663
Valkama E, Salminen JP, Koricheva J, Pihlaia H (2003) Comparative analysis of leaf trichome structure and composition of epicuticular flavonoids in Finnish birch species. Ann Bot 91:643–655
Vasquez-Robinet C, Mane SP, Ulanov AV, Watkinson JI, Stromberg VK, de Koeyer DD, Schafleitner R, Willmot DB, Bonierbale M, Bohnert HJ, Grene R (2008) Physiological and molecular adaptations to drought in Andean potato genotypes. J Exp Bot 59: 2109–2123
Walia H, Wilson C, Condamine P, Liu X, Ismail AM, Zeng L, Wamaker SI, Mandal J, Xu J, Cui X, Close TM (2005) Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol 139:822–835
Wang R, Chen S, Deng L, Fritz E, Hüttermann A, Polle A (2007) Leaf photosynthesis, fluorescence response to salinity and the relevance to chloroplast salt compartmentation and anti-oxidative stress in two poplars. Trees 21:581–591
Wang R, Chen S, Zhou X, Shen X, Deng L, Zhu H, Shao J, Shi Y, Dai S, Fritz E, Hüttermann A, Polle A (2008) Ionic homeostasis and reactive oxygen species control in leaves and xylem sap of two poplars subjected to NaCl stress. Tree Physiol 28:947–957
Williams RJ, Spencer JPE, Rice-Evans CA (2004) Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med 36:838–849
Winkel BSJ (2006) The biosynthesis of flavonoids. In: Grotewold E (ed) The science of flavonoids. Springer, New York, pp 71–95
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effect of stress. Curr Opin Plant Biol 5:218–223
Xu C, Sullivan JH, Garrett WM, Caperna TJ, Natarajan S (2008) Impact of solar Ultraviolet-B on the proteome in soybean lines differing in flavonoid contents. Phytochemistry 69:38–48
Yamasaki H, Sakihama Y, Ikeara N (1997) Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol 115:1405–1412
Zaprometov MN, Nikolaeva TN (2003) Chloroplast isolated from kidney bean leaves are capable of phenolic compound biosynthesis. Russ J Plant Physiol 50:623–626
Zechmann B, Stumpe M, Mauch F (2011) Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta 233:1–12
Zhao J, Dixon RA (2009) The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci 14:72–80
Zietz M, Weckmuller A, Schmidt S, Rohn S, Schreiner M, Krumbein A, Kroh LW (2010) Genotypic and climatic influence on the antioxidant activity of flavonoids in kale (Brassica oleracea var. sabellica). J Agric Food Chem 58:2123–2130
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Di Ferdinando, M., Brunetti, C., Fini, A., Tattini, M. (2012). Flavonoids as Antioxidants in Plants Under Abiotic Stresses. In: Ahmad, P., Prasad, M. (eds) Abiotic Stress Responses in Plants. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-0634-1_9
Download citation
DOI: https://doi.org/10.1007/978-1-4614-0634-1_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-0633-4
Online ISBN: 978-1-4614-0634-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)