Abstract
In this study, we determined the neuroprotective effect of aucubin on diabetes and diabetic encephalopathy. With the exception of the control group, all rats received intraperitoneal injections of streptozotocin (STZ; 60 mg/kg) to induce type 1 diabetes mellitus (DM). Aucubin (1, 5, 10 mg/kg ip) was used after induction of DM (immediately) and diabetic encephalopathy (65 days after the induction of diabetes). The diabetic encephalopathy treatment groups were divided into short-term and long-term treatment groups. Treatment responses to all parameters were examined (body weight, plasma glucose, Y-maze error rates and proportion of apoptotic cells). In diabetic rats, aucubin controlled blood glucose levels effectively, prevented complications, and improved the quality of life of diabetic rats. In diabetic encephalopathy, aucubin significantly rescued neurons in the hippocampal CA1 subfield and reduced working errors during behavioral testing. The significant neuroprotective effect of aucubin could be seen not only in the short term (15 days) but also in the long term (45 days), which was a highly encouraging finding. These data suggest that aucubin may be a potential neuroprotective agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a metabolic disease caused by either insufficient production of insulin or the inability of the body to respond to insulin produced within the system. The disease can be classified into three separate categories: type 1, type 2 and gestational diabetes [1]. Type 1 diabetes is caused by the loss of beta cells in the pancreas. Type 2 diabetes is generally characterized by the body’s resistance to insulin. Gestational diabetes can have damaging effects on both the mother and the fetus. DM is a complex disease that can produce a wide array of diabetic complications [2–5]. Most of the diabetic complications are known to be serious and are often fatal if left untreated [6]. For this reason, it is extremely important for diabetic patients to seek immediate medical treatment as soon as the disease is diagnosed [7]. There is substantial experimental and clinical evidence that diabetes may lead to impairments in learning, memory, problem solving, and mental and motor speed [8]. The type of central nervous damage caused by DM is also known as diabetic encephalopathy [9, 10]. Diabetic encephalopathy encompasses characteristic biochemical, electrophysiological, and morphological changes that may lead to cognitive deficits in diabetic patients [11, 12] and diminish quality of life.
Diabetic encephalopathy is associated with various structural brain abnormalities, such as synaptic and neuronal degeneration, neuron loss, dilated and fragmented endoplasmic reticulum, increased microtubuli, and irregular nuclei [13–15]. The hematoxylin-eosin (HE) or TUNEL staining of serial coronal hippocampi sections from diabetic animals was examined, with a focus on the CA1 pyramidal cell layer of the hippocampus [16–19]. Diabetic encephalopathy is characterized by damaged cognitive function and neurobehavioral deficits [20, 21]. The impairment affects the process of learning, memory, problem solving, retrieval of learned information, and mental and motor speed. Impaired performances on the Morris water maze and Y-maze are characteristic of streptozotocin (STZ)-induced diabetic rats [22].
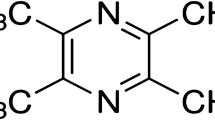
The iridoid monoterpene aucubin is found in traditional Chinese medicinal herbs, such as Plantago asiatica [23], Eucommia ulmoides [24], and Plantago lanceolata L. [25]. Aucubin has many pharmacological effects, including anti-inflammatory, blood pressure reduction, liver protection, anti-microbial, and analgesic and antitumor properties [26]. It has also been reported that aucubin is a potent free radical scavenger. Studies in vitro and in vivo have shown that aucubin is a powerful scavenger of superoxide anions, hydroxyl radicals, and nitrogen dioxide [18, 27–29] and that it also prevents lipid peroxidative damage [18, 28]. In diabetes, aucubin can suppress blood glucose levels and increase the antioxidant status of pancreatic β-cells [17, 28]. Based on this background information, the aim of this study was to determine whether aucubin prevents diabetic encephalopathy and treats diabetic encephalopathy in rats.
Materials and methods
Chemical and chemical doses
Analytical grade aucubin (product >99 % purity) was purchased from Genay Extrasynthêse (France) and dissolved in physiological saline (Heilongjiang Kelun Pharmaceutical Co. Ltd., China) for administration to rats. Aucubin was administered to normal Wistar rats (eight in each group) at 1, 5, 10, 20, 50, and 100 mg/kg doses by a single intraperitoneal injection, and the effects of the drug on the rats’ behavior were observed. At the 100 mg/kg dose, the mortality rate was 0 %, although the rats that survived suffered paralysis. At the 50 mg/kg dose, the mortality rate was 0 %, and behavior was normal. At the 1, 5, 10 and 20 mg/kg doses, the rats did not show any abnormalities. The molecular structure of aucubin is very similar to that of catalpol, which has been reported as having neuroprotective effects [30]. On the basis of these findings, doses of aucubin below 10 mg/kg were also chosen for this study.
Animals
Male Wistar rats (12 weeks of age; 230–250 g) were acquired from the Dalian Medical University Experimental Animal Center, Dalian, China. The rats were maintained (4 rats/cage) in an air-conditioned room (24 ± 1 °C) with a 12-h light/12-h dark cycle and ad libitum access to food and water and were allowed to adapt to laboratory facilities for 1 week before the experiment began. All experimental procedures were conducted in accordance with institutional guidelines for the care and use of laboratory animals in Dalian Medical University, Dalian, China, and international guidelines on the ethical use of animals (NIH publications No 80–23, revised 1996). Throughout the study, rats were assessed daily for distress, and body weight and blood glucose levels were monitored.
Induction of diabetes and diabetic encephalopathy
Type 1 DM rats were induced by intraperitoneal injections of streptozotocin (STZ; 60 mg/kg body weight). STZ was dissolved in a freshly prepared 0.01 M citrate buffer (pH 4.5). Blood was drawn 72 h later from the tail plexus of conscious rats using a heparinized inoculator. Blood glucose concentrations were tested using the Span Diagnostic kit with Jinque test strips. Rats with blood glucose >11.1 mmol/L were considered diabetic [31].
Diabetic encephalopathy was induced in the rats. Briefly, the initial cognitive capacity of the rats was assessed using a standardized Y-maze protocol as described in 2.5. To prevent the potentially significant differences in cognition between the rats from affecting the results, the rats were excluded from the experiment according to the results of pre-diabetic training. At day 65 after STZ administration, a subset of encephalopathy-positive DM rats was stratified by cognitive capability as measured in a Y-maze.
Aucubin treatment protocols
Experiment 1: The effectiveness study of aucubin treatment in diabetic rats
This experiment was undertaken to investigate the efficacy of three different doses of aucubin in protecting against the loss of CA1 neurons and cognitive impairment in diabetic rats. Five experimental groups were designated: a normal group (controls), a diabetic group treated with saline, and three groups of diabetic rats treated with aucubin, respectively, at doses of 1, 5 and 10 mg/kg. Aucubin and saline were administered via intraperitoneal injections twice daily for 65 days. All control rats were injected with saline alone. Blood glucose concentrations were measured using the Span Diagnostic kit with Jinque test strips along with body weight at day 0, 7, 20, 30, 40, 50, and 65. Each group contained ten rats. All rats were tested in the Y-maze on day 66.
Experiment 2: The short-term efficacy of aucubin treatment in diabetic encephalopathy rats
The experimental groups were designed to include a normal group (controls), a diabetic encephalopathy group treated with saline and a diabetic encephalopathy group treated with intraperitoneal injections of aucubin at doses of 5 mg/kg. Injections were administered twice daily for 10 days and then once daily for another 5 days. Each group contained ten rats. All experimental rats were assessed with a Y-maze test on day 16 and then euthanized.
Experiment 3: The long-term efficacy of aucubin treatment in diabetic encephalopathy rats
To examine the long-term or short-term protective effect of aucubin, this experiment was performed on the diabetic encephalopathy rats treated with intraperitoneal injections of aucubin (5 mg/kg). The long-term group was administered intraperitoneal injections twice daily for 35 days and then once daily for another 10 days. The group contained ten rats. The experimental rats were assessed with a Y-maze test on day 46 and then euthanized.
Behavioral testing
Behavioral testing was performed in Y-maze by two investigators who were blind to the animals of experiment 1, experiment 2 and experiment 3, as described previously [32]. The Y-maze was placed in a darkened room, and the rats were trained to choose between entering the randomly bright branch or the darkened branch. The bright branch was correct and the darkened branch was incorrect. Twenty consecutive training trials were performed for each rat once every 24 h, and the training procedure was performed on 7 consecutive days. An 80 % success rate (8 correct choices in 10 consecutive training trials) served as the learning criterion. The number of correct choices was recorded.
Histological evaluation
At the end of the study, each rat was anaesthetized deeply with ethyl ether and decapitated. Each brain was fixed with 4 % (v/v) paraformaldehyde in PBS, pH 7.2, for histological examinations. Serial coronal sections (5 μm thick) were prepared from the hippocampi of each rat in each group and stained with HE. The CA1 field of each brain slice was identified according to Paxinos and Watson [33], and images were captured and analyzed using a morphometric analysis system equipped with an Olympus IX71 microscope (Japan) and SPSS 13.0 Production Mode Facility. The number of intact pyramidal cells was quantified at 400× magnification by a blinded investigator according to a previously described method [34]. The neuronal density was expressed as the number of cells per 1 mm of the CA1 field of the hippocampus.
Statistical analysis
All results are expressed as the mean ± S.D. Comparisons were made using the unpaired t test. P values less than 0.05 were considered statistically significant.
Results
Effect of aucubin on body weight and blood glucose level in diabetic rats
The STZ-induced rats exhibited significant increases in blood glucose levels and decreases in body weight compared with the control rats. With long-term intraperitoneal injections of aucubin (at doses of 1, 5 or 10 mg/kg) in diabetic rats, the blood glucose levels were significantly lower and the body weight was significantly higher in comparison with the diabetic rats. On day 65, aucubin used at 10 mg/kg doses did not show a significant influence on blood glucose levels compared with the control group (P > 0.05). On days 7, 20, and 30, body weight was not significantly higher in the aucubin-treated rats than in the control rats. Body weight values were similar between 5 and 10 mg/kg dose groups. Blood glucose levels and body weight are presented in Tables 1 and 2.
Effects of aucubin on neuroprotection in diabetic rats
Cognitive function was assessed using the Y-maze. The results showed that the cognitive function of STZ-induced diabetic rats was markedly impaired. How ever, with long-term intraperitoneal injections of aucubin in diabetic rats at doses of 1, 5 or 10 mg/kg, the error rates were similar to the control rats. The cognitive ability of the long-term group did not show a significant reduction (Fig. 2a).
Light microscopic examination of the hippocampal logical structures revealed damage in the CA1 region of the hippocampus in the brains of DM rats. Pyramidal neurons were densely stained and appeared shrunken with minimal or no cytoplasm. By contrast, the majority of CA1 neurons appeared healthy in the aucubin-treated DM rats (Fig. 1). Surviving neuronal cells in the CA1 region numbered 192 ± 10 cells/mm in the control rats and only 65 ± 9 cells/mm in the DM group (Control group > DM; P < 0.001) and 172 ± 8, 181 ± 4, 185 ± 9 cells/mm in the variable dose aucubin-treated diabetic group (1, 5 or 10 mg/kg), respectively (P < 0.001). Treatment means did not differ (P > 0.05) between the aucubin-treated groups (1, 5 or 10 mg/kg) and the DM group (Fig. 2b).
Morphological staining of paraffin sections with HE after treatment with aucubin (400×). The pyramidal neurons of DM rats showed a densely stained shrunken appearance with minimal or no cytoplasm. The control pyramidal neurons presented clear and evident nuclei and nucleoli. Apoptotic cells are indicated by the arrow. Control rats (non-DM), DM (STZ-induced diabetic) rats receiving saline and STZ-induced DM rats receiving aucubin concentrations of 1, 5 or 10 mg/kg (DM-1, DM-5 and DM-10). Scale bars = 25 μm
Effect of aucubin on the behavior and survival of neurons in the CA1 subfield of the hippocampus in untreated rats (controls), in rats treated with streptozotocin (STZ) to induce diabetes mellitus and given no further treatment (DM), and in STZ-induced diabetic rats subsequently administered aucubin concentrations of 1, 5 or 10 mg/kg (DM-1, DM-5 and DM-10). Values shown are the mean ± S.D. (n = 10). Significance levels are represented by a P < 0.001 versus DM; b P > 0.05 versus control
The short-term efficacy of aucubin in diabetic encephalopathy rats
In the diabetic encephalopathy rats treated with aucubin at 1, 5 or 10 mg/kg for 15 days, the CA1 neuronal cells numbered 1412 ± 199, 2172 ± 102 and 2639 ± 210 cells/mm2, respectively. The data have been published by Xue et al. [18]. In this article, neuronal density was expressed as the number of cells per 1 mm. The surviving neurons in the CA1 region were 190 ± 11, 51 ± 6, and 142 ± 13 cells/mm in the control group, the DE group, and the 5 mg/kg aucubin-treated group, respectively. The data are shown in Figs. 3 and 4b. In the diabetic encephalopathy rats treated with aucubin at 5 mg/kg for 15 days, the cognition of all rats was tested in the Y-maze, and the results are shown in Fig. 4a.
Histological examination of hippocampal CA1 regions in control rats treated with saline (control), diabetic encephalopathy rats given saline (DE), diabetic encephalopathy rats treated with intraperitoneal injections of aucubin at a dose of 5 mg/kg for 15 days (short-term), and diabetic encephalopathy rats treated with intraperitoneal injections of aucubin at a dose of 5 mg/kg for 45 days (long-term). Arrows indicate positive pyramidal neurons. Scale bars = 25 μm
Short-term or long-term effect of aucubin on the behavior and survival neurons in CA1 subfield of control rats treated with saline (control), diabetic encephalopathy rats given saline (DE), and aucubin-treated DE groups (aucubin). The data presented are the mean ± S.D. (n = 10). Significance levels are represented by a P < 0.01, b P < 0.001 versus control; **P < 0.001, *P < 0.001 versus DE; #P < 0.05 versus aucubin treated group for 15 days
The long-term efficacy of aucubin in diabetic encephalopathy rats
To determine the long-term efficacy of aucubin, all long-term aucubin-treated rats were tested in the Y-maze. The number of errors was 4 ± 2, and the number of healthy cells in the CA1 area was 195 ± 17 cells/mm for the control group. The survival neuronal density and cognitive function were all markedly decreased in the diabetic encephalopathy rats. Only 45 ± 8 cells/mm were preserved, while the number of errors reached as high as 34 ± 13. Treatment with aucubin for 45 days significantly increased the number of healthy cells to 160 ± 15 cells/mm and decreased the number of errors to 5 ± 3. All results are shown in Figs. 3 and 4.
Discussion
All types of diabetes are treatable, but type 1 and type 2 diabetes last a lifetime as there is no known cure. If diabetes is not adequately controlled, patients have a significantly higher risk of developing complications, such as hypoglycemia, ketoacidosis, and nonketotic hyperosmolar coma [35, 36]. Longer term complications may involve cardiovascular disease, retinal damage, chronic kidney failure, nerve damage, poor wound healing, foot gangrene (which may lead to amputation), and erectile dysfunction [37–39]. Hyperglycemia and weight loss are the most important characteristics of diabetes mellitus, and therefore, elevated blood glucose levels and body weight are important in determining whether diabetes is controlled at any given time. Our previous studies showed that aucubin could decrease the blood glucose concentration in diabetic encephalopathy rats [17, 28]. In this study, the results are similar to previous findings; aucubin also decreased blood glucose concentrations and increased body weight in diabetic rats. This type of STZ-induced diabetic animal model has been used extensively in studies on the pathophysiology of diabetes and its complications.
Diabetic encephalopathy has a major impact on the quality of life of diabetic patients. The impaired was performed in Morris’ water maze in STZ-treated diabetic rats after 10 weeks of disease induction. The cognitive function of STZ-induced diabetic rats was shown to be impaired [21]. Our previous studies also found that 65 days after the disease was induced with STZ, the diabetic rats showed cognitive deficits [17]. In this study, aucubin was intraperitoneally injected immediately after diabetes for 65 days. Blood glucose levels were controlled at a specific time, and the diabetic rats did not show cognitive deficits. Cognitive ability is associated with neuronal death in the CA1 region of the hippocampus [16]. Our results from HE staining showed that neuronal apoptosis did not occur in the CA1 region of aucubin-treated rats with STZ-induced diabetes mellitus. The experimental data are likely to show that aucubin may be helpful for preventing diabetic encephalopathy.
In our previous results, short-term (15 days) intraperitoneal injections of aucubin ameliorated cognitive deficits, reduced oxidative stress, modulated the expressions of Bcl-2 and Bax genes and reduced working errors in diabetic encephalopathy rats. In this study, we also discussed the long-term (45 days) efficacy of aucubin in diabetic encephalopathy rats. The results indicated that aucubin had long-term effectiveness. Long-term intraperitoneal aucubin treatment significantly decreased blood glucose levels, increased body weight, ameliorated cognitive deficit, attenuated neuronal damage and rescued neuronal cells. These data imply that the hypoglycemic activity and neuroprotective effect of aucubin are dose and time dependent. The study further showed that long-term intraperitoneal aucubin treatment is more effective in attenuating neuronal damage and reducing working errors than short-term intraperitoneal aucubin treatment. Aucubin is a small compound that is derived from traditional Chinese medicine. Its herbal toxicity and adverse reactions are minimal, it is relatively inexpensive, it can pass through the blood–brain barrier, and it has vast potential for development. Thus, due to its low side-effect profile and long history of safe use, aucubin may find clinical applications in treating neuronal disturbances in diabetic patients.
In conclusion, aucubin decreased blood glucose levels and maintained body weight in diabetic rats and diabetic encephalopathy rats. The aucubin-treated diabetic rats did not develop diabetic encephalopathy. Long-term treatment with aucubin may ameliorate neuronal injury and cognitive deficiency in diabetic encephalopathy rats, with better long-term than short-term efficacy. Our results suggest that aucubin may have therapeutic value for the treatment or prevention of neuronal disturbances in diabetic patients.
References
Hoybergs YMJJ, Meert TF (2007) The effect of low-dose insulin on mechanical sensitivity and allodynia in type I diabetes neuropathy. Neurosci Lett 417:149–154
Alberti KG, Zimmet PZ (1998) New diagnostic criteria and classification of diabetes-again? Diabet Med 15:535–536
Krentz AJ, Clough G, Byrne CD (2007) Interactions between microvascular and macrovascular disease in diabetes: pathophysiology and therapeutic implications. Diabetes Obes Metab 9(6):781–791
Rosolova H, Petrlova B, Simon J, Sifalda P, Sipova I, Sefrna F (2008) Macrovascular and microvascular complications in type 2 diabetes patients. Vnitr Lek 54(3):229–237
Scheffel RS, Bortolanza D, Weber CS, Costa LA, Canani LH, Santos KG et al (2004) Prevalence of micro and macroangiopatic chronic complications and their risk factors in the care of out patients with type 2 diabetes mellitus. Rev Assoc Med Bras 50(3):263–267
Bleyer AJ, Sedor JR, Freedman BI, O’Brien A, Russell GB, Graley J et al (2008) Risk factors for development and progression of diabetic kidney disease and treatment patterns among diabetic siblings of patients with diabetic kidney disease. Am J Kidney Dis 51(1):29–37
Polich J, Herbst KL (2000) P300 as a clinical assay: rationale, evaluation, and findings. Int J Psychophysio 38(1):3–19
Biessels GJ, Kappelle AC, Bravenboer B, Erkelens DW, Gispen WH (1994) Cerebral function in diabetes mellitus. Diabetologia 37:634–650
Li ZG, Sima AA (2004) C-peptide and central nervous system complications in diabetes. Exp Diabesity Res 5:79–90
Seyyed AF (2009) Neuroprotection in diabetic encephalopathy. Neurodegener Dis 6:213–218
Li ZG, Zhang W, Grunberger G, Sima AAF (2002) Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res 946:221–231
Trudeau F, Gagnon S, Massicotte G (2004) Hippocampal synaptic plasticity and glutamate receptor regulation: influences of diabetes mellitus. Eur J Pharmacol 490:177–186
Kodl CT, Seaquist ER (2008) Cognitive dysfunction and diabetes mellitus. Endocr Rev 29(4):494–511
van den Reijmer YD, Berg E, Ruis C, Kappelle LJ, Biessels GJ (2010) Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev 26:507–519
Cooray G, Nilsson E, Wahlin A, Laukka EJ, Brismar K, Brismar T (2011) Effects of intensified metabolic control on CNS function in type 2 diabetes. Psych Endocrinol 36(1):77–86
Li ZG, Zhang W, Sima AAF (2005) The role of impaired insulin/IGF action in primary diabetic encephalopathy. Brain Res 1037:12–24
Xue H, Jin L, Jin L, Zhang P, Li DQ, Xia YQ, Lu YN, Xu YP (2008) Neuroprotection of aucubin in primary diabetic encephalopathy. Sci China C Life Sci 51:495–502
Xue HY, Jin L, Jin LJ, Li XY, Zhang P, Ma YS, Lu YN, Xia YQ, Xu YP (2009) Aucubin prevents loss of hippocampal neurons and regulates antioxidative activity in diabetic encephalopathy rats. Phytother Res 23(7):980–986
Wang CF, Li DQ, Xue HY, Hu B (2010) Oral supplementation of catalpol ameliorates diabetic encephalopathy in rats. Brain Res 1307:158–165
Sima AAF, Zhang WX, Li ZG, Kamiya H (2008) The effects of C-peptide on type 1 diabetic polyneuropathies and encephalopathy in the BB/Wor-rat. Exp Diabetes Res 2008:1–13
Gispen WH, Biessels GJ (2000) Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci 23:542–549
Biessels GJ, Kamal A, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH (1998) Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res 800:125–135
Pailer M, Haschke-Hofmeister E (1969) Inhaltsttoffe aus Plantago major. Planta Med 17:139–145
Bianco A, Iavarone C, Trogolo C (1974) Structure of eucommiol, a new cyclopentenoid-tetraol from Eucommia ulmoides. Tetrahedron 130:4117–4121
Tamura Y, Nishibe S (2002) Changes in the concentrations of bioactive compounds in plantain leaves. J Agric Food Chem 50(9):2514–2518
An SJ, Pae HO, Oh GS, Choi BM, Jeong S, Jang SI, Oh H, Kwon TO, Song CE, Chung HT (2002) Inhibition of TNF-a, IL-1h, and IL-6 productions and NF-kB activation in lipopolysaccharide-activated RAW 264.7 macrophages by catalposide, an iridoid glycoside isolated from Catalpa ovata G. Don (Bignoniaceae). Int Immunopharmacol 2:1173–1181
Ho J, Lee YH, Lee YD, Jun WJ, Kim HK, Hong BS, Shin DH, Cho HY (2005) Inhibitory effect of Aucubin isolated from Eucommia ulmoides against UVB-induced matrix metalloproteinase-1 production in human skin fibroblasts. Biosci Biotechnol Biochem 43:500–506
Jin L, Xue HY, Jin LJ, Li SY, Xu YP (2008) Antioxidant and pancreas-protective effect of aucubin on rats with streptozotocin induced diabetes. Eur J Pharmacol 582:162–167
Xue HY, Gao GZ, Lin QY, Jin LJ, Xu YP (2011) Protective effects of aucubin on H2O2-induced apoptosis in PC12 cells. Phytother Res. doi:10.1002/ptr.3562
Li DQ, Li Y, Liu YX, Bao YM, Hu B, An LJ (2005) Catalpol prevents the loss of CA1 hippocampal neurons and reduces working errors in gerbils after ischemia-reperfusion injury. Toxicon 46:845–851
Tomlinson KC, Gardiner SM, Hebden RA, Bennett T (1992) Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol Rev 44:103–150
Rao Y, Xiao P, Xu ST (2001) Effects of intrahippocampal aniracetam treatment on Y-maze avoidance learning performance and behavioral long-term potentiation in dentate gyrus in rat. Neurosci Lett 298:183–186
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, Sydney
Bae HJ, Lee YS, Kang DW, Gu JS, Yoon BW, Ron JK (2000) Neuroprotective effect of low dose riluzole in gerbil model of transient global ischemia. Neurosci Lett 294:29–32
Baker FJ II, Rosen P, Coppleson LW, Evans T, Fauman B, Segal MB (1976) Diabetic emergencies: hypoglycemia and ketoacidosis. J Am Coll Emerg Physicians 5(2):119–122
Connery LE, Coursin DB (2004) Assessment and therapy of selected endocrine disorders. Anesthesiol Clin N Am 22(1):93–123
Takahashi H, Goto T, Shoji T, Tanito M, Park M, Chihara E (2006) Diabetes-associated retinal nerve fiber damage evaluated with scanning laser polarimetry. Am J Ophthalmol 142(1):88–94
Ritchie C, Ekundayo OJ, Muchimba M, Campbell RC, Stuart JF, Liu B, Aban IB, Ahmed A (2009) Effects of diabetes mellitus in patients with heart failure and chronic kidney disease: a propensity-matched study of multimorbidity in chronic heart failure. Int J Cardiol 134(3):330–335
Nather A, Bee CS, Huak CY, Chew JLL, Lin CB, Neo S, Sim EY (2008) Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complicat 22(2):77–82
Acknowledgments
Financial support for this research from the Anhui Provincial Natural Science Foundation (10040606Q65; 11040606M44), the Foundation of Anhui Excellent Youth Talents in University (2011SQRL158ZD), and the Natural Science Research Project of Education Office of Anhui Province (KJ2011B175) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xue, H.Y., Lu, Y.N., Fang, X.M. et al. Neuroprotective properties of aucubin in diabetic rats and diabetic encephalopathy rats. Mol Biol Rep 39, 9311–9318 (2012). https://doi.org/10.1007/s11033-012-1730-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1730-9