Abstract
Biofilm resistance to biocides is becoming a global issue with an impact on many fields, including health care, agriculture, the environment, society and industry. Plants offer a virtually inexhaustible and sustainable resource of very interesting classes of biologically active, low-molecular-weight compounds (parvome). In the past, the plant parvomes were screened mainly for their lethal effects, disregarding concentrations and ecologically relevant functions of these molecules in the natural context. Testing sub-lethal concentrations of plant-derived compounds mimicking environmental levels may be critical to reveal mechanisms subtler than the killing activity, e.g. those influencing the multicellular behavior, offering an elegant way to develop novel biocide-free antibiofilm strategies. In a cross-disciplinary fashion, we illustrated recent successes of sub-lethal concentrations of plant-derived compounds, their ecological insight, pro et contra, future directions and impacts, envisioning implications for policy making and resource management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been estimated that at least 99 % of the world’s microbial biomass exists in form of biofilm, a complex differentiated surface-associated community embedded in a self-produced polymeric matrix enabling microorganisms to develop coordinated and efficient survival strategies. Although the inclination to colonize surfaces is advantageous from the microbial standpoint, it may cause chronic infections (Cegelski et al. 2008; Estrela et al. 2009), parasitism phenomena in animals and plants (Skamnioti and Gurr 2009), biodeterioration of historical and artistic objects (Giacomucci et al. 2011; Cappitelli et al. 2012), biodeterioration of engineered systems (Zhang et al. 2012), and fouling in food-processing equipments (Renier et al. 2011). Furthermore, biofilm injury has a profound socio-economic impact, incurring direct and indirect industrial costs that result in a huge financial burden for an already over-stretched economy.
For human societies, the most detrimental property of biofilms is the expression of specific characters that make sessile microorganisms more resistance to antimicrobial agents (up to 1,000-fold) than their planktonic counterparts (Høiby et al. 2010; Flemming 2011). As climate conditions change, natural and engineered ecosystems are increasingly reaching temperatures and humidity that are conducive to biofilm growth. Although increased biofilm biomass would lead to an increased use of biocides, questions concerning the biodegradability of biocides, their risk to human and animal health and their environmental impact, have increasingly discouraged biocide use. This is readily seen in the number of recent policies, directives, technical reports, strategies, recommendations and regulatory decisions designed to reduce antimicrobial agents consumption, ensuring the prudent use of these fragile strategies, and protect specific agents that are critically important for human and animal health and wellbeing (Directive 98/8/EC; Recommendation 2002/77/EC; SCENIHR report 2009; EFSA Summary Report 2012). Finally, the antimicrobial arena is experiencing a shortage of lead compounds progressing into both clinical and industrial trials and growing negative consumer perception against synthetic compounds has led to the search for natural-derived products (Lam 2007).
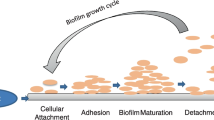
In the last few years, the efforts have been directed towards developing preventive strategies that can be used to disarm microorganisms without killing them (Cegelski et al. 2008; Rasko and Sperandio 2010). An innovative approach is the use of biocide-free antibiofilm agents with novel targets, unique modes of action and proprieties that are different from those of the currently used antimicrobials. In addition, as these substances do not exert their action by killing cells, they do not impose a selective pressure causing the development of resistance (Rasko and Sperandio 2010). Observing the processes of biofilm formation it is reasonable to expect that interfering with the key-steps that orchestrate genesis of virtually every biofilm could be a way for new preventive strategies that do not necessarily exert lethal effects on cells but rather sabotage their propensity for a sessile lifestyle (Fig. 1). For instance, interfering with the surface sensing process and mystifying intercellular signals, the biofilm cascade might be hampered.
These strategies might bring new products to the market and cover methodologies and novel approaches, making significant contributions to innovation and economic productivity in SMEs. They provide support for cross-cutting actions while offering new tools for society and policy makers.
Ecological insight of plant-derived antibiofilm compounds
The need for innovative antibiofilm technologies has led to renewed interest in the ways that organisms protect themselves against microbial colonization.
Plants lacking cell-based inducible immune responses and that live in nutrient-rich environments are continuously exposed to a broad array of potentially deleterious microorganisms leading to increased weight and friction, impeded trans-epidermal exchanges, altered color, smell, and contour (Wahl et al. 2012). This provides the driving force behind the evolution of a variety of sophisticated strategies to enhance plant fitness via chemical defenses against biofilms (de Nys and Steinberg 2002; Qian et al. 2010). In addition, one of the main advantages of plant-derived compounds with potential pharmaceutical and medical applications is the lack of shared pathogens between plants and mammals (Cichocka et al. 2010).
Both aquatic and terrestrial plants offer very interesting classes of biologically active, low-molecular-mass (<5 kDa) compounds (“parvome”, parv = small, -ome = group), like alkaloids, terpenoids, flavonoids and coumarins, peptides, glycosides, nucleosides and polyphenols. They may act in a variety of ways: antibiotics, allosteric regulators, catalysis, catalytic cofactors, regulatory activities at level of DNA, RNA and protein, pigments, mutagens, antimutagens, receptor agonists, antagonists, signal molecules, siderophores, detergents, metal complexing/transporting agents, pheromones, toxins and other interesting activities (Davies and Ryan 2012). However, during the intensive half-century of drug discovery, available natural compounds found in the plant parvome were screened mainly for their lethal effects, disregarding concentrations and ecologically relevant functions of these molecules in the natural environments. All that mattered were compounds effective in killing target microorganisms (inter alia Gibbons 2005; Puglisi et al. 2007; Quave et al. 2008; Mayavu et al. 2009; Tajkarimi et al. 2010; Artini et al. 2012; Falcão et al. 2012; Guedes et al. 2012). In contrast, few papers address the inhibition of biofilm formation by using compounds at sublethal concentrations.
In many cases, the killing activity of naturally-occurring compounds is primarily a laboratory property, since the concentrations of these agents available in nature would be insufficient to exert their lethal effects (Yim et al. 2007; Davies 2011). Several studies on marine plants highlighted a lack of correlation between antimicrobial activities and abundance of surface-associated microorganisms, suggesting that chemical defenses may function by mechanisms more subtle than the simple killing activities like those influencing the multicellular behavior by manipulating the expression of specific phenotypes that represent different stages of the biofilm process (Harder 2008).
The optimal defense theory asserts that organisms allocate resources to chemical defenses in a way that maximizes fitness and preserves their primary biological functions such as homeostasis maintenance, growth and reproduction (Ivanisevic et al. 2011). The production of toxic compounds might impose: (1) a significant metabolic burden to the plant in order to protect itself from autotoxicity (Heil and Baldwin 2002) and (2) ecological costs resulting from the myriad of interactions that a plant has with its biotic and abiotic environment (Heil and Baldwin 2002). In fact, it has been estimated that a considerable percentage of bacterial genomes is dedicated to shaping the organisms’ habitat and maintaining their community and niche in the ecosystem (Phelan et al. 2011). Thus, killing microorganisms is not advantageous for the plant as might affect local ecological relationship. Finally, sub-lethal concentration represents one mechanism by which the host minimizes the risk of counter adaptation, which would be likely to occur if secondary metabolites were toxic to associated microbes (Engel et al. 2002).
Testing sub-lethal concentrations of plant-derived compounds mimicking environmental levels may be critical to understand biological functions, highlighting different and valuable biological activities far from killing activities. As a consequence, one of the most pressing issues is the estimation of the sub-lethal concentrations of secondary metabolites experienced by microorganisms in nature. In the context of antibiofilm researches, this gap may be filled carrying out preliminary experiments to define the toxicological threshold zone for the selected model systems and then screening a wide range of sub-lethal concentrations at frequent intervals in order to identify the experimental space with the maximum antibiofilm activity. However, the efforts of industrial, academic, governmental actors are made to reduce time and costs of research programmes by testing few concentrations at standard conditions, demanding carefully designed experiments to explore in details and at reasonable cost the low-dose response and the cellular behavior in complex scenarios.
Determination of optimal sub-lethal concentrations
The design of experiments technique (DOE) could be successfully employed to clarify the antibiofilm performance of plant-derived compounds without testing many sub-lethal concentrations, but just performing a limited number of experiments according to rigorously formulated mathematical protocols (Franceschini and Macchietto 2008). With this multivariate approach it is possible to simulate cellular behavior in complex scenarios, considering effective factors, interactions and selecting optimum conditions that maximized the antibiofilm response (Leardi 2009).
Although DoE methods have been around since the mid-twentieth century, their application in the discovery of non-toxic antibiofilm compounds has only recently taken hold. DoE has been shown to perform excellently in a wide range of applications: chemical kinetics, process control, drug discovery, biological systems (e.g. fermentation and bio-kinetics), pharmacodynamics, process engineering etc. (inter alia Akhbari et al. 2011; Hu et al. 2012; Papaneophytou and Kontopidis 2012; Jibrail and Keat Teong 2013). However, to the best of our knowledge, only three works (carried out by the authors of the present paper) successfully modeled the antibiofilm performances of plant-derived compounds at sub-lethal concentrations exploiting High Throughput Screening techniques.
By using DoE coupled with microtiter biofilm assay Villa et al. (2011) observed that the best anti-biofilm performance of sub-lethal concentrations of the phenolic compound zosteric acid (secondary metabolite from the seagrass Zostera marina, Fig. 2a) against Candida albicans was obtained at a specific threshold level, which corresponds to the minimum point of the response surface model and not to the maximum concentration tested (Fig. 3). At this level, zosteric acid played a role in thwarting budded-to-hyphal-form transition, in reducing biofilm biomass and thickness, in extending the performance of antimicrobial agents and showed cytocompatibility towards soft and hard tissue (Fig. 4). The non-linear response patterns depicted by the surface response followed a parabola-like shape profile, resembling a hormetic property (the situation in which the response to an environmental stressor varies with the level of exposure). However, a biphasic profile is not new in the biofilm world: the biofilm mediators homoserine lactones act in a concentration-dependent manner, where upper and lower threshold concentrations trigger the formation of a biofilm (Rickard et al. 2006).
Three-D response surface model displaying the hormetic properties of zosteric acid, a secondary metabolite of the seagrass Z. marina tested against C. albicans biofilm. Plot shows interaction between zosteric acid and pH when time and temperature were 12 h and 25 °C respectively. The variables were coded in the range −1 (minimum selected value) and +1 (maximum selected value). Ranges in the legends represent the number of adhered cells. The graph shows that the best anti-biofilm performance of the plant-derived compound was obtained at a specific threshold level, which corresponds to the minimum point of the response surface model. Thus, the minimum number of adhered cells does not correspond to the high amount of zosteric acid. Minimum adhesion (that is the maximum response) corresponds to 10 mg/l of zosteric acid. The maximum response is predicted to be a reduction of fungal spores adhesion by 70 %
View of 3D reconstruction images of C. albicans biofilm grown without (a) and with sublethal dose of zosteric acid (b). Zosteric acid induces morphostructural alterations, thwarting budded-to-hyphal-form transition. Biofilms were stained with FUN-1 yeast viability stain (red-orange), indicating that zosteric acid treatment maintains metabolically active cells. Biofilm samples were visualized using a Leica TCS-SP2 AOBS confocal laser scanning microscope with excitation at 488 nm, and emission ≥530 nm (green and red channels). Images were captured with a 63 × 0.9 NA w water immersion objective and analyzed with the software Imaris (Bitplane Scientific Software, Zurich, Switzerland). (Color figure online)
Escherichia coli cells treated with zosteric acid were characterized by stress-associated (e.g. AhpC, OsmC, SodB, GroES, IscU, DnaK), motility-related (FliC), quorum-sensing-associated (LuxS) and metabolism/biosynthesis-related (e.g. PptA, AroA, FabD, FabB, GapA) proteins. This indicated that the antibiofilm compound targeted key steps involved in biofilm formation by modulating the threshold level of the extracellular signalling molecule autoinducer-2 (AI-2) and inducing a hypermotile phenotype unable to firmly adhere on surfaces (Villa et al. 2012b). The compound seems to act as an environmental stimulus or chemical manipulator that provides advance warning about environmental changes, allowing the microorganisms to prepare for adversity while conditions are still favorable. From an ecological perspective, the mechanism of action of the zosteric acid seems to portray the “xenohormesis theory”. According to the xenohormesis, heterotrophs (animals and microbes) are able to sense chemical stimuli synthesized by autotrophs (like plants) in response to stress to mount a preemptive defense response that increases their chance of survival (Howitz and Sinclair 2008). Interestingly, the synthesis of phenolic compounds is induced in plants by a variety of environmental stresses and the planktonic phenotype represents a life-extending physiological trait to escape from adversity improving the colonization of new favorable habitat. In a similar way, reacting to zosteric acid would allow the bacterial response to begin ahead of any direct damage or energy deficit, and, more importantly, would not stake the life of both the plant and the microorganism respecting the ecological relationships and leading to an extended lifespan of the involved counterparts.
Thus, exploring the effects of sub-lethal concentrations of plant-derived compounds on microbial behavior (e.g. adhesion, chemotaxis, swimming and swarming motility) has the potential not only to demonstrate interesting xenohormetic-like responses and the extent and the modality to which microbial surface colonization is chemically mediated, but also to unveil potent biocide-free antibiofilm mechanisms.
Recent successes of antibiofilm compounds from plants at sub-lethal concentrations
Vattem et al. (2007) have suggested that spices with renowned antibiotic properties could also possess antipathogenic activities, which may not be related to lethal effects on the target microorganism. The plant-derived compounds icariin and resveratrol, used in traditional Chinese medicine, were found potent antibiofilm molecules against Propionibacterium acnes (Coenye et al. 2012). Importantly, the antibiofilm activity was detected at sub-inhibitory concentrations. Similarly, extracts from Commiphora leptophloeos, Bauhinia acuruana and Pityrocarpa moniliformis demonstrated marked Staphylococcus epidermidis antibiofilm activity on polystyrene and glass surfaces without causing bacterial death (Trentin et al. 2011). The extract 220D-F2 from the root of Rubus ulmifolius was used to inhibit S. aureus biofilm formation to a degree that can be correlated with increased antibiotic susceptibility without limiting bacterial growth (Quave et al. 2012). Ursolic acid from the tree Diospyros dendo (Fig. 2b) is completely non-toxic towards E. coli, P. aeruginosa, Vibrio harveyi, and successfully inhibited the formation of these bacterial biofilms. Transcriptome analyses showed the induction of chemotaxis and motility genes in E. coli treated with the plant-derived compound, suggesting that ursolic acid may function as a signal that tells cells to remain too motile hindering cell adhesion or destabilizing already formed biofilm (Ren et al. 2005).
The methanolic extract obtained from Cuminum cyminum, a traditional food ingredient in South Indian dishes, was shown to act as quorum-sensing inhibitor. By interfering with the acyl-homoserine lactone activity, it inhibited the production of violacein pigment, swimming and swarming motility, production of the extracellular polymeric substances and biofilm formation in several bacterial pathogens (Issac Abraham et al. 2012). Also the extract of Capparis spinosa showed a high degree of anti-quorum sensing activity in a dose dependent manner without affecting the bacterial growth of Serratia marcescens, P. aeruginosa, E. coli and Proteus mirabilis. It also exhibited inhibition in swimming and swarming motility of the bacterial pathogens (Issac Abraham et al. 2011). Two synthetic furanones based on those produced by the marine macroalga Delisea pulchra (Fig. 2c) were shown to attenuate bacterial virulence in the mouse models of chronic lung infection by targeting Pseudomonas aeruginosa quorum-sensing without directly killing bacteria, not imposing a selective pressure for the development of bacterial resistance (Wu et al. 2004). A number of flavonoids found in citrus species, including naringenin (Fig. 2d), kaempferol (Fig. 2e), apigenin (Fig. 2f) and quercetin (Fig. 2g), which are antagonists of homoserine lactone and AI-2-mediated cell–cell signaling in V. harveyi, were able to inhibit biofilm formation by V. harveyi BB120 and E. coli O157:H7 in a dose-dependent manner (Vikram et al. 2010).
Recently, members of the transient receptor potential (TRP) channels have drawn large attention as versatile sensors to detect changes in the external environment being associated to sensation of heat, cold, noxious chemicals, pain, osmotic force, touch, vibration, proprioception and axon guidance (Vriens et al. 2008) in various animals and in man. Interestingly, fungal genomes present genes encoding a TRP-like structure. The mechanosensitive TRP channel in Saccharomyces cerevesiae (Yvc1 = TRPY1) has orthologs in other fungal genomes including TRPY2 of Kluyveromyces lactis and TRPY3 of C. albicans (Chang et al. 2010). Since several plant-derived taste-active substances are able to modulate/interact with these sensing channels, they are interesting bioactive molecules with new potential targets for the development of non-toxic strategies against biofilms. According to this chemosensory-based strategy, the efficacy of sub-lethal concentrations of Muscari comosum bulb extract in modulating yeast adhesion and subsequent biofilm development on abiotic surfaces and its role as extracellular signal responsible for biofilm dispersion was reported (Villa et al. 2012a) (Fig. 1).
Drawbacks in the advancement of plant-derived products production
Main reasons for the fact that plant-derived products research has not yet advanced to great lengths in the last 20 years include the incompatibility of natural product libraries with high-throughput screening, the marginal improvement in core technologies for natural product screening and natural product structure elucidation (Lam 2007). In addition, chemists have been sometimes frustrated by their inability to resolve complex mixtures at reasonable cost. However, an advantage of using mixture is that effects may be additive and synergistic, through their ability to affect multiple targets (Kirakosyan and Kaufman 2009), a smart strategy when dealing with the complex phenomenon such as biofilm formation in which different pathways are involved.
Recently, the development of new methodologies has revolutionized the screening of natural products: bio-prospecting, development of a streamlined screening process, improved natural product sourcing, advances in chemical methodologies, combinatorial biosynthesis and plant genomics (Lam 2007; Bohlin et al. 2010). For instance, rapid and more cost-effective genome sequencing technologies coupled with advanced computational power permits extracting chemical knowledge from genetic information more efficiently (Li et al. 2009). Less expensive DNA sequencing allows the identification of gene clusters known to be associated with a production of small molecules. In addition to identify new natural products, genome mining may certainly have an impact on the understanding the production of natural products (Clardy and Walsh 2004; Lam 2007).
When research leads to the commercialization of an agent, large quantities of the compound are required. The preferred option is synthesis of the compound. Combinatorial chemistry approaches are being applied based on phytochemical scaffolds to create screening libraries that closely resemble antibiofilm-like compounds. In silico techniques like quantitative structure–activity relationships (QSAR) analysis, pioneered by Hansch et al. (1962), helps to quantitatively correlate the activity or properties of compounds with their measured or computed physiochemical properties, playing crucial and rate accelerating steps for the better drug design in the modern era (Lill 2007; Verma et al. 2010; Kar and Roy 2012; Yao 2012). QSAR approaches have been developed and have demonstrated appealing advantages, including their low-cost and capability to scale up easily (Yao 2012). The main assumption in the QSAR approaches is that the all properties viz. physical, chemical and biological are purely depending on the molecular structure. QSAR is an attempt to remove the element of luck from drug design by establishing a mathematical relationship in the form of an equation between biological activity and measurable/computed physicochemical parameters. These equations may be used by the chemist to make a more informed choice as to which analogues to prepare. Currently, QSAR approach has been successfully applied to many data sets of plant-derived compounds (Wright et al. 2006; Chen and Li 2009; Nargotra et al. 2009; De-Eknamkul et al. 2011; Yao et al. 2011). Thus, by applying the QSAR technique, new organic synthetic methodologies and biotransformation for the modification of natural product leads would generate a novel, structurally diverse analogs with improved properties or new activities (Zhou et al. 2012).
However, owing to their structural complexity, some natural products are not currently produced on an industrial scale by chemical synthesis. Thus, another drawback lies in the sustainability of the use and management of plant resources, insuring that the population size and the availability of the extracted product do not decline as a result of harvesting (Gilliland et al. 2009). A solution is represented by microbial hosts engineered to express plant metabolic pathways as reported by Ajikumar et al. (2010) and the developing of a platform technology to isolate and culture cambial meristematic cells (CMCs, multipotent plant cells that give rise to the vascular tissues xylem and phloem) in the laboratory and then harvesting the desired products from the media in which they grow (Lee et al. 2010). Finally, tailoring efficient laboratory plant-systems to produce specific compounds can be an efficient and sustainable source of plant-derived products.
Concluding remarks
Plants represent a virtually inexhaustible and sustainable source of biocide-free antibiofilm agents with novel targets, unique modes of action and proprieties with potential for utilization in a plethora of medical, agricultural, and industrial fields. On the one hand, realization of this possibility has so far been hindered by insufficient fundamental research to comprehensively understand the ecologically relevant functions of plant-derived compounds in the real natural environments.
When testing the biocidal action of a naturally-occurring agent against biofilm-forming microorganisms, we should keep in mind that this might not be the modality whereby this molecule works in nature. The concept that the killing activity is not the only property of a compound can be traced back to the sixteenth century when the Swiss chemist and physician Paracelsus wrote: “All things are poison and nothing is without poison, only the dose permits something not to be poisonous”. Now the question is: what happens at sub-lethal concentrations?
This is a common failure of many studies in which the investigator is unaware of the microbial behavior at sub-inhibitory concentrations. Thus, it is possible that the use of plant-derived compounds as less toxic or non-toxic antibiofilm products has been neglected or even abandoned principally because the optimal sub-lethal concentrations and working conditions were not found and not because the agent was ineffective. This holistic approach provides risk managers and decision-makers with the evidence they need to prioritize their resources and efforts to develop new technologies to deal with the spread and recalcitrance of unwanted biofilms.
Sub-inhibitory concentrations of plant-derived compounds might offer an elegant way to interfere with specific key-steps that orchestrate biofilm formation, mitigating biofilm formation without affecting their existence, sidestepping drug resistance and extending the efficacy of the current arsenal of antimicrobial agents. This technology might pave the way to more innovative, resource efficient and competitive society that reconciles human wellbeing with the sustainable use of renewable resources for industrial purposes, while ensuring environmental protection.
References
Ajikumar PK, Xiao WH, Tyo KE, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G (2010) Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330:70–74
Akhbari A, Zinatizadeh AAL, Mohammadi P, Irandoust M, Mansouri Y (2011) Process modeling and analysis of biological nutrients removal in an integrated RBC-AS system using response surface methodology. Chem Eng J 168:269–279
Artini M, Papa R, Barbato G, Scoarughi GL, Cellini A, Morazzoni P, Bombardelli E, Selan L (2012) Bacterial biofilm formation inhibitory activity revealed for plant derived natural compounds. Bio org Med Chem 20:920–926
Bohlin L, Göransson U, Alsmark C, Wedén C, Backlund A (2010) Natural products in modern life science. Phytochem Rev 9:279–301
Cappitelli F, Salvadori O, Albanese D, Villa F, Sorlini C (2012) Cyanobacteria cause black staining of the National Museum of the American Indian Building (Washington, DC, USA). Biofouling 28:257–266
Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ (2008) The biology and future prospects of antivirulence therapies. Nat Rev Microbiol 6:17–27
Chang Y, Schlenstedt G, Flockerzi V, Beck A (2010) Properties of the intracellular transient receptor potential (TRP) channel in yeast, Yvc1. FEBS Lett 584:2028–2032
Chen KX, Li ZG (2009) Exploring the structural requirements for jasmonates and related compounds as novel plant growth regulators: a current computational perspective. Plant Signal Behav 4:1007–1009
Cichocka D, Claxton J, Economidis I, Högel J, Venturi P, Aguilar A (2010) European Union research and innovation perspectives on biotechnology. J Biotechnol 156:382–391
Clardy J, Walsh C (2004) Lessons from natural molecules. Nature 432:829–837
Coenye T, Brackman G, Rigole P, De Witte E, Honraet K, Rossel B, Nelis HJ (2012) Eradication of Propionibacterium acnes biofilms by plant extracts and putative identification of icariin, resveratrol and salidroside as active compounds. Phytomedicine 19:409–412
Council Recommendation (2001) Prudent use of antimicrobial agents in human medicine (2002/77/EC). http://antibiotic.ecdc.europa.eu/PDFs/l_03420020205en00130016.pdf
Davies J (2011) How to discover new antibiotics: harvesting the parvome. Curr Opin Chem Biol 15:5–10
Davies J, Ryan KS (2012) Introducing the parvome: bioactive compounds in the microbial world. ACS Chem Biol 7:252–259
De Nys R, Steinberg PD (2002) Linking marine biology and biotechnology. Curr Opin Biotechnol 13:244–248
De-Eknamkul W, Umehara K, Monthakantirat O, Toth R, Frecer V, Knapic L, Braiuca P, Noguchi H, Miertus S (2011) QSAR study of natural estrogen-like isoflavonoids and diphenolics from Thai medicinal plants. J Mol Graph Model 29:784–794
Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market. http://eur-lex.europa.eu/LexUriServ/site/en/consleg/1998/L/01998L0008-20070119-en.pdf
Engel S, Jensen PR, Fenical W (2002) Chemical ecology of marine microbial defense. J Chem Ecol 28:1971–1985
Estrela AB, Heck MG, Abraham WR (2009) Novel approaches to control biofilm infections. Curr Med Chem 16:1512–1530
European Food Safety Authority and European Centre for Disease Prevention and Control (2012) The European union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2010. EFSA J 10:2598 [233 pp]. www.efsa.europa.eu/efsajournal
Falcão MA, Fianco ALB, Lucas AM, Pereira MAA, Torres FC, Vargas RMF, Cassel E (2012) Determination of antibacterial activity of vacuum distillation fractions of lemongrass essential oil. Phytochem Rev. doi:10.1007/s11101-012-9255-3
Flemming HC (2011) Microbial biofouling: unsolved problems, insufficient approaches, and possible solutions. In: Flemming HC et al (eds) Biofilm highlights, Springer series on biofilms 5. Springer, Berlin
Franceschini G, Macchietto S (2008) Model-based design of experiments for parameter precision: state of the art. Chem Eng Sci 63:4846–4872
Giacomucci L, Bertoncello R, Salvadori O, Martini I, Favaro M, Villa F, Sorlini C, Cappitelli F (2011) Microbial deterioration of artistic tiles from the facade of the Grande Albergo Ausonia & Hungaria (Venice, Italy). Microb Ecol 62:287–298
Gibbons S (2005) Plants as a source of bacterial resistance modulators and anti-infective agents. Phytochem Rev 4:63–78
Gilliland SG, Gilchrist HG, Rockwell RF, Robertson GJ, Savard J-PL, Merkel F, Mosbech A, Lebreton J-D (2009) Evaluating the sustainability of harvest among northern common eiders Somateria mollissima borealis in Greenland and Canada. Wildlife Biol 15:24–36
Guedes AP, Franklin G, Fernandes-Ferreira M (2012) Hypericum sp.: essential oil composition and biological activities. Phytochem Rev 11:127–152
Hansch C, Maloney PP, Fujita T, Muir RM (1962) Correlation of biological activity of phenoxyacetic acids with Hammett substituent constants and partition coefficients. Nature 194:178–180
Harder T (2008) Marine epibiosis: concepts, ecological consequences and host defence. In Hans-Curt Flemming, P. Sriyutha Murthy, R. Venkatesan, Keith E. Cooksey (eds) Marine and industrial biofouling (Springer Series on Biofilms), edn. First, Springer, Berlin, pp 219–232
Heil M, Baldwin IT (2002) Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci 7:61–67
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332
Howitz KT, Sinclair DA (2008) Xenohormesis: sensing the chemical cues of other species. Cell 133:387–391
Hu SH, Kuo CH, Chang CM, Liu YC, Chiang WD, Shieh CJ (2012) Solvent selection and optimization of α-chymotrypsin-catalyzed synthesis of N-Ac-Phe-Tyr-NH2 using mixture design and response surface methodology. Biotechnol Prog 28(6):1443–1449
Issac Abraham SV, Palani A, Ramaswamy BR, Shunmugiah KP, Arumugam VR (2011) Antiquorum sensing and antibiofilm potential of Capparis spinosa. Arch Med Res 42:658–668
Issac Abraham SV, Palani A, Khadar Syed M, Shunmugiah KP, Arumugam VR (2012) Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res Int 45:85–92
Ivanisevic J, Thomas OP, Pedel L, Pénez N, Ereskovsky AV, Culioli G, Pérez T (2011) Biochemical trade-offs: evidence for ecologically linked secondary metabolism of the sponge Oscarella balibaloi. PLoS ONE 6:e28059
Jibrail K, Keat Teong L (2013) Process optimization and kinetic study for biodiesel production from non-edible sea mango (Cerbera odollam) oil using response surface methodology. Chem Eng J 214:157–164
Kar S, Roy K (2012) QSAR of phytochemicals for the design of better drugs. Expert Opin Drug Discov 7:877–902
Kirakosyan A, Kaufman PB (2009) Recent advances in plant biotechnology, 1st edn. Springer, Berlin
Lam KS (2007) New aspects of natural products in drug discovery. Trends Microbiol 15:279–289
Leardi R (2009) Experimental design in chemistry: a tutorial. Anal Chim Acta 652:161–172
Lee EK, Jin YW, Park JH, Yoo YM, Hong SM, Amir R, Yan Z, Kwon E, Elfick A, Tomlinson S, Halbritter F, Waibel T, Yun BW, Loake GJ (2010) Cultured cambial meristematic cells as a source of plant natural products. Nat Biotechnol 28:1213–1217
Li MH, Ung PM, Zajkowski J, Garneau-Tsodikova S, Sherman DH (2009) Automated genome mining for natural products. BMC Bioinformatics 10:185
Lill MA (2007) Multi-dimensional QSAR in drug discovery. Drug Discov Today 12:1013–1017
Mayavu P, Sugesh S, Ravindran VJ (2009) Antibacterial activity of seagrass species against biofilm forming bacteria. Res J Microbiol 4:314–319
Nargotra A, Sharma S, Koul JL, Sangwan PL, Khan IA, Kumar A, Taneja SC, Koul S (2009) Quantitative structure activity relationship (QSAR) of piperine analogs for bacterial NorA efflux pump inhibitors. Eur J Med Chem 44:4128–4135
Papaneophytou CP, Kontopidis GA (2012) Optimization of TNF-α overexpression in Escherichia coli using response surface methodology: purification of the protein and oligomerization studies. Protein Expr Purif 86:35–44
Phelan VV, Liu WT, Pogliano K, Dorrestein PC (2011) Microbial metabolic exchange–the chemotype-to-phenotype link. Nat Chem Biol 8:26–35
Puglisi MP, Engel S, Jensen PR, Fenical W (2007) Antimicrobial activities of extracts from Indo-Pacific marine plants against marine pathogens and saprophytes. Mar Biol 150:531–540
Qian P-Y, Xu Y, Fusetani N (2010) Natural products as antifouling compounds: recent progress and future perspectives. Biofouling 26:223–234
Quave CL, Plano LWR, Pantuso T, Bennett BC (2008) Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J Ethnopharmacol 118:418–428
Quave CL, Estévez-Carmona M, Compadre CM, Hobby G, Hendrickson H, Beenken KE, Smeltzer MS (2012) Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 7:e28737
Rasko DA, Sperandio V (2010) Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov 9:117–128
Ren D, Zuo R, González Barrios AF, Bedzyk LA, Eldridge GR, Pasmore ME, Wood TK (2005) Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl Environ Microbiol 71:4022–4034
Renier S, Hébraud M, Desvaux M (2011) Molecular biology of surface colonization by Listeria monocytogenes: an additional facet of an opportunistic Gram-positive foodborne pathogen. Environ Microbiol 13:835–850
Rickard AH, Palmer RJ Jr, Blehert DS, Campagna SR, Semmelhack MF, Egland PG, Bassler BL, Kolenbrander PE (2006) Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol Microbiol 60:1446–1456
SCENIHR (2009) The scientific committee on emerging and newly identified health risks report. http://ec.europa.eu/health/opinions/en/biocides-antibiotic-resistance/l-3/8-risk-assessment.htm
Skamnioti P, Gurr SJ (2009) Against the grain: safeguarding rice from rice blast disease. Trends Biotechnol 27:141–150
Tajkarimi MM, Ibrahim SA, Cliver DO (2010) Antimicrobial herb and spice compounds in food. Food Control 21:1199–1218
Trentin DS, Giordani RB, Zimmer KR, da Silva AG, da Silva MV, Correia MT, Baumvol IJ, Macedo AJ (2011) Potential of medicinal plants from the Brazilian semi-arid region (Caatinga) against Staphylococcus epidermidis planktonic and biofilm lifestyles. J Ethnopharmacol 137:327–335
Vattem DA et al (2007) Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 78:302–310
Verma J, Khedkar VM, Coutinho EC (2010) 3D-QSAR in drug design—a review. Curr Top Med Chem 10:95–115
Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS (2010) Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J Appl Microbiol 109:515–527
Villa F, Pitts B, Stewart PS, Giussani B, Roncoroni S, Albanese D, Giordano C, Tunesi M, Cappitelli F (2011) Efficacy of zosteric acid sodium salt on the yeast biofilm model Candida albicans. Microb Ecol 62:584–598
Villa F, Borgonovo G, Cappitelli F, Giussani B, Bassoli A (2012a) Sub-lethal concentrations of Muscari comosum bulb extract suppress adhesion and induce detachment of sessile yeast cells. Biofouling 28:1107–1117
Villa F, Remelli W, Forlani F, Vitali A, Cappitelli F (2012b) Altered expression level of Escherichia coli proteins in response to treatment with the antifouling agent zosteric acid sodium salt. Environ Microbiol 14:1753–1761
Vriens J, Nilius B, Vennekens R (2008) Herbal compounds and toxins modulating TRP channels. Curr Neuropharmacol 6:79–96
Wahl M, Goecke F, Labes A, Dobretsov S, Weinberger F (2012) The second skin: ecological role of epibiotic biofilms on marine organisms. Front Microbiol 3:292
Wright AD, de Nys R, Angerhofer CK, Pezzuto JM, Gurrath M (2006) Biological activities and 3D QSAR studies of a series of Delisea pulchra (cf. fimbriata) derived natural products. J Nat Prod 69:1180–1187
Wu H, Song Z, Hentzer M, Andersen JB, Molin S, Givskov M, Høiby N (2004) Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J Antimicrob Chemother 53:1054–1061
Yao L (2012) In silico search for drug targets of natural compounds. Curr Pharm Biotechnol 13:1632–1639
Yao C, Jiang B, Li T, Qin B, Feng X, Zhang H, Wang C, Tu S (2011) Design and an efficient synthesis of natural product-based cyclopenta[b]pyran derivatives with potential bioactivity. Bio org Med Chem Lett 21:599–601
Yim G, Wang HH, Davies J (2007) Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci 362:1195–1200
Zhang J, Loong WLC, Chou S, Tang C, Wang R, Fane AG (2012) Membrane biofouling and scaling in forward osmosis membrane bioreactor. J Memb Sci 403–404:8–14
Zhou W, Dai Z, Chen Y, Wang H, Yuan Z (2012) High-dimensional descriptor selection and computational QSAR modeling for antitumor activity of ARC-111 analogues based on support vector regression (SVR). Int J Mol Sci 13:1161–1172
Acknowledgments
This work was supported by the German DAAD and the Italian CRUI in the framework of the Vigoni project 2012 “Seagrass compounds inhibit biofilm formation—from the identification to the application”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villa, F., Cappitelli, F. Plant-derived bioactive compounds at sub-lethal concentrations: towards smart biocide-free antibiofilm strategies. Phytochem Rev 12, 245–254 (2013). https://doi.org/10.1007/s11101-013-9286-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-013-9286-4