Abstract
Purpose
This work investigated the effect of relative humidity (RH) on bipolar electrostatic charge profiles of dry powder inhaler aerosols using the Bipolar Charge Analyzer (BOLAR).
Methods
Two commercial products, Pulmicort® (400 μg, budesonide) and Bricanyl® (500 μg, terbutaline sulfate) Turbuhaler®, were used as model dry powder inhalers (DPIs) in this study. Three individual doses from each Turbuhaler® were sampled at 15, 40, 65 and 90% RH. Subsequently, charge and mass profiles were determined for each dispersion.
Results
The aerosols from these two Turbuhaler® DPI were bipolarly charged, with larger particles carrying negative charge and smaller particles positive charge. Particles changed polarity around 2.60–4.17 μm and 0.95–2.60 μm for Pulmicort® and Bricanyl®, respectively. The effect of RH on particles differed between DPIs even though the mass output was not significantly affected. The net charge profiles of Pulmicort® were relatively independent of RH, whereas those of Bricanyl® showed a reduction in the charge magnitude with increasing RH. Both positive and negative charge profiles followed a similar trend with the change in RH and individually they had higher magnitudes than the measured net charge.
Conclusions
This study showed drug-specific bipolar charging of the Turbuhaler® DPI aerosols at varied RHs. Bricanyl® was more susceptible to RH and showed decreased bipolar and net charge levels with increasing RH, in comparison to Pulmicort®.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The last few decades the saw rapid development of dry powder inhalers (DPIs) for treating pulmonary diseases. While electrostatic effects in DPIs were poorly understood due to its complexity, electrostatic charge remains of interest because it affects aerosol deposition in the lungs (1). Deposition of inhaled particles can be enhanced by electrical charge (2), and the deposition of particles increased significantly when a threshold charge (qc) was exceeded. This interaction is generally governed by image forces between a particle and the airway wall, which is also dependent on the magnitude of charge carried by each individual particle (3). A few theoretical and experimental works revealed that deposition of inhaled particles are under potential influence of electrostatic charge (4,5,6,7,8,9,10). However, how magnitude and polarity of charged aerosols affect the total and regional lung deposition is yet to be established. Investigation of electrostatic charge in inhalable pharmaceutical aerosols is a prerequisite for understanding aerosol charging in drug delivery to the lungs.
Relative humidity (RH) is of significant importance in aerosolization performance of powders as adsorbed water on the surface affects capillary forces, electrical conductivity and, under certain circumstances, surface chemistry (11). The effect of RH on particle electrostatic charging is complex and depends on many factors (12). Although most studies have shown that the electrostatic charge decreased with increasing RH (12,13,14,15), there is a lack of data on how RH affects bipolar charge profiles of dry powder aerosols. A bipolar next generation impactor (bp-NGI) was designed to investigate the bipolar electrostatic properties and formulation performance of lactose with budesonide DPI mixture, and the results showed that with an increase in RH resulting in a decrease in the aerosol particle charge (16). However, the bipolar charge properties of drug-alone formulations are unclear.
Pharmaceutical aerosols carry bipolar charges. There are several published studies on using the electrical low pressure impactor (ELPI) and electrical Next Generation Impactor (eNGI) to analyze the charge and mass distributions of aerosols from DPIs and MDIs (17,18,19,20,21). The ELPI and eNGI provided information on the net charge within defined particle size fractions, but could not give details of the bipolar characteristics. Since the performance and outcome of inhaled powders are determined by charge on individual particles (22), rather than the net charge within a particle size fraction (which may contain both positively and negatively charged particles), bipolar charge measurement is necessary to provide a better understanding of the influence of electrostatic charge in inhalation therapy. Previous studies used instruments such as the Bipolar Charge Measurement System, Phase Doppler Anemometry and Electrical Single-Particle Aerodynamic Relaxation Time (ESPART) to analyze bipolar charge using indirect measurements of charge-to-mass ratio (23,24). Furthermore, ESPART measured distribution of the aerosol particles by number instead of by mass. The Bipolar Charge Analyzer (BOLAR) was designed by Dekati Ltd. to overcome these limitations (25). The BOLAR is the first commercially available instrument capable of separating and detecting bipolar charge of particles within an aerodynamic size fraction, which also allows direct mass assay for the calculation of charge-to-mass ratio. The BOLAR offers a new platform for direct measurement of bipolar electrostatic charge in pharmaceutical aerosols (26,27,28).
The aim of the current study is to use the novel BOLAR to investigate the effect of RH on bipolar charging profiles of Pulmicort® and Bricanyl®, two commercially available Turbuhaler® products, both of which are drug-only DPIs containing no carriers or excipients. In addition, the net charge data generated from the study are compared with those previously obtained from ELPI measurements for cross verification.
Materials and Methods
Materials
Two commercial DPI Turbuhaler® products were tested in the current study: Pulmicort® (400 μg budesonide; AstraZeneca, Sydney, Australia) and Bricanyl® (500 μg terbutaline sulfate; AstraZeneca, Sydney, Australia). All inhalers were used before the expiration date and kept inside the original packaging under ambient prior to experiment.
Deionized water was obtained from Modulab Type II Deionization System (Continental Water System, Sydney, Australia). High-performance liquid chromatography (HPLC) grade methanol was supplied by Honeywell (Burdick & Jackson, Ulson, Korea) and sodium dodecyl sulfate was purchased from J.T. Baker (New Jersey, USA). Silicone release spray (Dow Corning Molykote, Mount Waverly, Australia) was used for coating the impactor collection plates. 45 mm glass fiber filters were sourced from MicroAnalytix, Pty. Ltd. (Sydney, Australia).
Scanning Electron Microscopy (SEM)
Particle morphology was assessed by scanning electron microscopy (SEM, Carl Zeiss SMT AG, Oberkochen, Germany). Samples from each turbuhaler were collected from the reservoir and then dispersed onto sticky carbon tapes mounted on SEM stubs, sputter-coated with 15 nm thick gold using a K550X sputter coater (Quorum Emitech, Kent, UK). The images were captured at 3 kV using secondary electron detector.
Charge Measurement
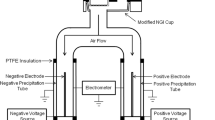
Figure 1 exhibits a schematic diagram of the BOLAR. Briefly, the drug from the Turbuhaler® was aerosolized at an air flow rate of 60 L/min and delivered through a standard USP induction port into the flow divider, which evenly separated the aerosol into six outlet branches. Five of the air streams were drawn through individual bipolar detection tubes, which were coupled with pre-separator impactors (sprayed with silicone to minimize particle bounce) and had a defined cutoff diameter, as well as a reference chamber containing a simple Faraday pail and glass fiber filter. The bipolar detection tubes were composed of two concentric cylinders, with the inner one maintained at a high positive potential and the outer one grounded, creating a voltage difference in the gap between the cylinders. When the aerosol traveled through this gap, particles having positive and negative charge would be attracted to the outer and inner tubes, respectively, while the neutral particles passed straight through the gap and deposited on the filter stage at the bottom. The measured bipolar charge data was verified by comparing the sum of charges from the five detection tubes with the net charge generated by aerosols that deposited in the reference chamber.
The BOLAR was enclosed in a polycarbonate box to maintain conditions at the required RH and ambient temperature using a custom made temperature and humidity controller (Active Instrument Services Pty Ltd., Sydney, Australia), and an electric fan (Matsushita Electric, Osaka, Japan). Four RHs (15, 40, 65 and 90%) were chosen in this study and were maintained at ±5% of the required RH value. Each time before dispersion, the examed inhaler (upright with the cap closed) would be placed inside the polycarbonate box to condition at the targeted RH for about one hour before loading the inhaler and conducting the experiment. A single dose was dispersed into the BOLAR for each single measurement and all the measurements were conducted three times, and the operator did not wear gloves when using the Turbuhalers® in order to simulate real patient use. The BOLAR was operated under the manufacture’s recommendations: a self-check test was first conducted before measurement to make sure there was no systematic error (25). Subsequently, the Turbuhaler® was loaded inside the box and attached tightly to a standard USP induction port using a rubber adapter. When the automated measurement sequence was initiated, the internal valves were opened and air flowed through Turbuhaler® to disperse the powder into the BOLAR. Each measuring sequence was about 60 s, and the Turbuhaler® remained attached to the USP induction port for the duration of the measurement. Blank measurements (with an unloaded Turbuhaler®) were conducted in the same way to allow subtraction from the powder measurements, so as to determine the actual charge value. Immediately after dose sampling, the inhaler was disconnected from the adaptor and the cap was tightly replaced. The detection tubes were carefully dismantled for mass assay.
Mass Assay
The depositions of Pulmicort® (budesonide) or Bricanly® (terbutaline sulfate) particle inside the BOLAR were exhaustively washed with known volumes of a 40:60 (v/v) water: methanol co-solvent: 3 ml each for the adaptor and impactor stages; 5 ml each for the USP throat, flow divider inlet, reference filter holder and glass fiber filter; 10 ml for the flow divider; 15 ml for the inner detection tube and 20 ml for the outer detection tube. An aliquot of sample from the glass fiber filters was further centrifuged at a speed of 13,400 rpm for 10 min (Minispin, Eppendorf, Westbury, USA) for the collection of the supernatant. High performance liquid chromatography (HPLC) was used for quantifying the drug content. The HPLC system consisted of a Shimadzu CBM-20A controller, LC-20AT pump, SIL-20A HT auto sampler, SPD-20A UV/VIS detector (Shimadzu, Kyoto, Japan) and a Nova-Park® C18 4 μm 3.9 × 150 mm column (Waters, Massachusetts, USA). The mobile phase for Pulmicort® (budesonide) consisted of deionized water and methanol at 25:75% v/v ratio. Additionally, the injection volume for each sample was 50 μL and the flow rate was 1 mL/min. For Bricanyl® (terbutaline sulfate), the mobile phase consisted of 0.25% (w/v) sodium dodecyl sulfate aqueous solution and methanol at 40:60% v/v ratio. The injection volume was 100 μL and flow rate was 1 mL/min. Prior to each HPLC run, fresh standards were prepared to generate calibration curves.
Data Analysis
Particles that entered each detection tube were below the specific cutoff diameter of the upstream impactor stages. Thus, the charge and mass properties of particles within a particular size range can be obtained by the subtraction of values between two consecutive detection tubes (Table I). Since the flow divider separated the aerosol cloud into six even air streams, the actual magnitude of charge and mass for a specific particle size fraction was calculated by multiplying the measured value by a factor of six. The mid-point diameter for each size range was used as suggested by the manufacture to facilitate graphical presentations and discussion (25).
The emitted dose (ED, μg) was defined as the total mass of drug that was collected from all parts except the inhaler device. The fine particle dose (FPD, μg) was defined as the total mass of particles with an aerodynamic diameter less than 4.2 μm, which was the mass of particles collected in detection tube 3 multiplied by six. The fine particle fraction (FPF, %) was defined as the percentage of FPD in the ED. The specific charge or charge-to-mass ratio (q/m) was the quotient of the charge (either positive or negative) and mass assayed from the corresponding detection tube (outer and inner, respectively), while the net charge-to-mass ratio was calculated by dividing the net charge (sum of positive and negative charges) by the total drug mass amount (sum of mass assayed from both inner and outer detection tubes and the glass fiber filter).
Results
Particle Morphology
Figure 2 shows SEM images of particles from Pulmicort® and Bricanyl® Turbuhaler®. The primary particles from both products were similar, mostly 2–3 μm with some sub-micro particles attached on the surface. The surface morphology between them was notably different. The Pulmicort® particles showed a smooth and rigid surface structure, whereas those from the Bricanyl® were plate-like and hollow.
Charge and Mass Profiles
The recovery of the total mass from all measurements were within the range of 85–115%, which was considered acceptable according to the 75–125% range stated in the US Pharmacopoeia (29). Deposition of powder on certain components of the BOLAR were too low to be detected by HPLC. The ED, FPD and FPF across four different RHs are shown in Fig. 3. The ED values for both Pulmicort® and Bricanyl® were not significantly affected by the RH. Overall, the FPD and FPF results for both DPIs agreed with those previously measured by ELPI (12).
Figures 4 and 5 shows the in vitro bipolar electrostatic charge, mass deposition and charge-to-mass ratio profiles of the Pulmicort® and Bricanyl®, respectively. Particles with both positive and negative charge were detected in all size fractions.
Across different RHs, Pulmicort® particles were charged positively, with the magnitude changed from +48 to +985 pC, and negatively, from −71 to −838 pC, resulted in −23 to +146 pC of the net charge. The lower magnitudes of the net charge came from the cancellation of high magnitudes of positive and negative charges in the particles within a specific size fraction. The net charge profiles obtained at all four RHs showed a bipolar trend where small particles (size fraction ≤2.60 μm) were positively charged and large particles were negatively charged, which were comparable with the results from ELPI (particles larger than 2.0 μm were negatively charged) (12). The charge scales of both positively and negatively charged particles from Pulmicort® at the same size fraction remained relatively constant regardless of the RH. At the mid-point aerodynamic diameter of 1.78 μm (range 0.95 to 2.60 μm), the magnitudes of both positively and negatively charged particles were significantly higher than those of other size ranges, which can potentially enhance drug deposition in the lung by electrostatic attraction. Similarly, the net charge of the aerosols at different RHs was similar, and reached the highest magnitude also at the same size fraction of 0.95–2.60 μm. The total recovered dose and the mass of positively and negatively charged particles do not show much difference with increasing RH either. After correcting the charge for mass, the q/m values of particles with positive charge were from +11.35 to +43.66 pC/μg and from −14.19 to −33.91 pC/μg for particles with negative charge, which resulted in the net q/m values being −1.08 to +5.65 pC/μg. In general, the electrostatic charge profiles were correlated with the mass, and RH did not affect the qualitative q/m values of Pulmicort®. While the charge profiles were reproducible, it is important to note that there were large variations in mass deposition which led to large variations in q/m particularly at 90% RH.
In contrast, the charge-RH relationship for Bricanyl® was simpler. The bipolar charge, as well as the net charge profiles, showed a decreasing trend with increasing RH for each particle size fraction, which indicated that RH affected electrostatic charging across all particle size fractions. At the mid-point diameter of 1.78 μm, both positive and negative charge magnitudes reached a maximum. While the magnitude of the net charge reached the highest positive value of +176 pC at the mid-point diameter of 0.52 μm, and changed polarity to reach the lowest negative value of −70.2 pC at the mid-point diameter of 1.78 μm. The net charge of Bricanyl® also showed that smaller particles (size fraction ≤0.96 μm) were positively charged and large particles were negatively charged. These values were similar to those previously obtained on the ELPI (i.e., particles larger than 0.96 μm were negatively charged) (12). The FPD and FPF for Bricanyl® followed a similar trend, except at 15% RH, which was higher compared to other RHs. The net charge-to-mass ratio of Bricanyl® fine particles below 0.96 μm was decreased monotonically with increasing RH.
Discussion
This study was to investigate the RH effect on the bipolar charge profiles of the Pulmicort® and Bricanyl® Turbuhaler®. The results showed that the drug particles had much higher positive and negative charge values than those of the net charge. For Pulmicort®, the bipolar charge was not affected by RH, resulting in the net charge being independent of RH. In contrast, the bipolar charge for Bricanyl® varied in response to changing RH such that the net charge was also RH dependent. The positive and negative particle charge-size profiles were almost mirror images, and these trends are similar across different RH for both Pulmicort® and Bricanyl®. While similar behavior was reported previously (25,26,27,28), the reason behind this is still not clear. One explanation could be that each particle acquires the same amount of charge but with opposite polarity after contact charging (26). Thus, while the net charge of the measured aerosols may be close to neutral, individual particles actually carry charges at a sufficiently high level that could affect their dispersion and lung deposition (26). Charging is a complex process and can be affected by several physicochemical factors. During powder aerosolization, vigorous movement of particles in the airstream caused multiple physical interactions inside the inhaler device. Drug particles not only contacted with each other but also with the inhaler’s interior surfaces, from the powder reservoir to the channels in the mouthpiece of the Turbuhaler®. Therefore, the particles could be instantly charged after emitting from the reservoir, even prior to aerosolization (30).
The mass output generated from the BOLAR was similar to those in a previous study on the Turbuhalers® which was conducted under similar experimental conditions using a modified ELPI that operated at 60 L/min (12). Drug particles generated from Pulmicort® and Bricanyl® both carried charges while their profiles showed different responses to RH and some of these differences were attributed to the drug-dependent physico-chemical characters including hygroscopicity, crystallinity and resistivity (12).
The net q/m values for Bricanyl® obtained from the BOLAR were similar to those measured by ELPI (12). For Pulmicort®, the q/m values were similar despite the increasing RH, while results from ELPI showed a trough at 40% RH (12). Since the net charge profiles are comparable between the ELPI and the BOLAR measurements, the difference in q/m values could be due to the difference between mass measurements, which will be discussed below in the limitation of the BOLAR.
Charged aerosols generated from the Pulmicort® (100 μg) and Bricanyl® (500 μg) Turbuhalers® were previously characterized using an aerosol sampling electrometer unit (30). The fine particles dispersed from the Pulmicort® under ambient temperature and RH were positively charged (30). Although the dose of budesonide (100 μg vs 400 μg in the present study) is different, the polarity in both measurements are consistent, showing most of the particles in the fine size fraction were highly positively charged. The present BOLAR results have further demonstrated that bipolar electrification of the Pulmicort® powder during aerosolization was not dependent on the RH, whereas the charge profile of Bricanyl® showed a decreasing trend with increasing RH. Budesonide in Pulmicort® is a hydrophobic glucocorticosteroid, while terbutaline sulfate in the Bricanyl® is a salt that possesses hygroscopicity and electrical conductivity. Moisture uptake could facilitate charge dissipation due to an increase of conductivity on the particle surface. Bulk conductivity would also be altered after absorbing moisture at high RHs. On the contrary, the effects of RH are negligible on the charging of solids that are less hygroscopic (31,32,33). The difference in morphology between budesonide and terbutaline sulfate particles could also play an important role in their diverse response to RH. Given the same mass, spherical particles have been reported to charge less easily and also carry less charge than particles that are elongated (34).
A main limitation of the BOLAR is the large variations in the mass profile measured within specific particle size ranges. Compared with the ELPI, the charge and mass data of particles at a certain size fraction in the BOLAR are obtained from the subtraction of two consecutive detection tubes which were assembled in parallel. Since the dose emitted from the Turbuhaler was distributed evenly into six components including a reference chamber and five detection tubes, each tube only received one-sixth of the original dose. This reduced dose together with the relatively large surface area per tube to be washed for the drug assay were likely to have contributed to the large mass variation, which in turn could also raise uncertainty in the calculation of the q/m ratio. More sophisticated instruments such as liquid chromatography mass spectrometry (LCMS) would be necessary to improve the chemical quantification. Besides these possible limitations, the BOLAR was capable of analyzing the bipolar charge properties and performance of pharmaceutical aerosol products at different RH. The fundamental information obtained on bipolar electrostatic charge from the pharmaceutical powder aerosols might impact the development of optimized DPI products and possibly regulatory requirements on the characterizations of electrostatic properties.
Conclusion
This study investigated the effect of RH on bipolar charging of two different commercial Turbuhaler® aerosols using the BOLAR. The results showed that the tested inhalers carried significantly different positive and negative charges, which had higher magnitudes than the measured net charge. The net charge results indicated that these two drug-specific DPIs showed different responses to the RH influence. The Bricanyl® was more sensitive to RH than the Pulmicort® in particle charging. The similar net charge profiles across four RHs obtained by BOLAR and ELPI verified that the specific charge measurements between the two instruments were comparable in their overlapped size range. The simultaneous measurement of the electrical properties and mass distribution using the BOLAR has helped to provide more information of bipolar charging in the pharmaceutical aerosols.
Abbreviations
- BOLAR:
-
Bipolar charge analyzer
- bp-NGI:
-
Bipolar next generation impactor
- DPI:
-
Dry powder inhaler
- ELPI:
-
Electrical low pressure impactor
- eNGI:
-
Electrical next generation impactor
- ESPART:
-
Electrical single particle aerodynamic relaxation time
- FPD:
-
Fine particle dose
- FPF:
-
Fine particle fraction
- HPLC:
-
High performance liquid chromatography
- LCMS:
-
Liquid chromatography mass spectrometry
- MDI:
-
Metered dose inhaler
- RH:
-
Relative humidity
- SEM:
-
Scanning electron microscopy
- USP:
-
United State Pharmacopeia
References
Hinds WC. Aerosol technology: properties, behavior, and measurement of airborne particles. New York: Wiley-Interscience; 1982. p. 442.
Wilson IB. The deposition of charged particles in tubes, with reference to the retention of therapeutic aerosols in the human lung. J Colloid Sci. 1947;2(2):271–6.
Prodi V, Mularoni A. Electrostatic lung deposition experiments with humans and animals. Ann Occup Hyg. 1985;29(2):229–40.
Bailey A. The inhalation and deposition of charged particles within the human lung. J Electrost. 1997;42(1):25–32.
Balachandran W, Machowski W, Gaura E, Hudson C. Control of drug aerosol in human airways using electrostatic forces. J Electrost. 1997;40:579–84.
Chan T, Yu C. Charge effects on particle deposition in the human tracheobronchial tree. Ann Occup Hyg. 1982;26(1):65–75.
Majid H, Winker-Heil R, Madl P, Hofmann W, Alam K. Effect of oral pathway on charged particles deposition in human bronchial airways. J Aero Med Pulmonary Drug Del. 2016;29(1):24–9.
Melandri C, Prodi V, Tarroni G, Formignani M, De Zaiacomo T, Bompane G, et al. On the deposition of unipolarly charged particles in the human respiratory tract. Inhaled Particles. 1975;4:193–201.
Tarroni G, Melandri C, Prodi V, Zaiacomo TD, Formignani M, Basso P. An indication on the biological variability of aerosol total deposition in humans. Am Indust Hygiene Assoc J. 1980;41(11):826–31.
Yu C, Chandra K. Precipitation of submicron charged particles in human lung airways. Bull Math Biol. 1977;39(4):471–8.
Young PM, Sung A, Traini D, Kwok P, Chiou H, Chan H-K. Influence of humidity on the electrostatic charge and aerosol performance of dry powder inhaler carrier based systems. Pharm Res. 2007;24(5):963–70.
Kwok PCL, Chan H-K. Effect of relative humidity on the electrostatic charge properties of dry powder inhaler aerosols. Pharm Res. 2008;25(2):277–88.
Eilbeck J, Rowley G, Carter P, Fletcher E. Effect of contamination of pharmaceutical equipment on powder triboelectrification. Int J Pharm. 2000;195(1):7–11.
Elajnaf A, Carter P, Rowley G. Electrostatic characterisation of inhaled powders: effect of contact surface and relative humidity. Eur J Pharm Sci. 2006;29(5):375–84.
Elajnaf A, Carter P, Rowley G. The effect of relative humidity on electrostatic charge decay of drugs and excipient used in dry powder inhaler formulation. Drug Dev Ind Pharm. 2007;33(9):967–74.
Rowland M. Electrostatic properties of particles for inhalation: University of Bath; 2015.
Glover W, Chan H-K. Electrostatic charge characterization of pharmaceutical aerosols using electrical low-pressure impaction (ELPI). J Aerosol Sci. 2004;35(6):755–64.
Hoe S, Traini D, Chan H-K, Young PM. Measuring charge and mass distributions in dry powder inhalers using the electrical next generation impactor (eNGI). Eur J Pharm Sci. 2009;38(2):88–94.
Kwok PCL, Glover W, Chan HK. Electrostatic charge characteristics of aerosols produced from metered dose inhalers. J Pharm Sci. 2005;94(12):2789–99.
Kwok PCL, Collins R, Chan H-K. Effect of spacers on the electrostatic charge properties of metered dose inhaler aerosols. J Aerosol Sci. 2006;37(12):1671–82.
Wong J, Kwok PCL, Noakes T, Fathi A, Dehghani F, Chan H-K. Effect of crystallinity on electrostatic charging in dry powder inhaler formulations. Pharm Res. 2014;31(7):1656–64.
Balachandran W, Kulon J, Koolpiruck D, Dawson M, Burnel P. Bipolar charge measurement of pharmaceutical powders. Powder Technol. 2003;135:156–63.
Ali M, Mazumder MK, Martonen TB. Measurements of electrodynamic effects on the deposition of MDI and DPI aerosols in a replica cast of human oral-pharyngeal-laryngeal airways. J Aerosol Med Pulmonary Drug Del. 2009;22(1):35–44.
Beleca R, Abbod M, Balachandran W, Miller PR. Investigation of electrostatic properties of pharmaceutical powders using phase Doppler anemometry. IEEE Trans Ind Appl. 2010;46(3):1181–7.
Yli-Ojanperä J, Ukkonen A, Järvinen A, Layzell S, Niemelä V, Keskinen J. Bipolar charge analyzer (BOLAR): a new aerosol instrument for bipolar charge measurements. J Aerosol Sci. 2014;77:16–30.
Leung SSY, Chiow ACM, Ukkonen A, Chan H-K. Applicability of bipolar charge analyzer (BOLAR) in characterizing the bipolar electrostatic charge profile of commercial metered dose inhalers (MDIs). Pharm Res. 2016;33(2):283–91.
Wong J, Lin Y-W, Kwok PCL, Niemelä V, Crapper J, Chan H-K. Measuring bipolar charge and mass distributions of powder aerosols by a novel tool (BOLAR). Mol Pharm. 2015;12(9):3433–40.
Wong J, Kwok PCL, Niemelä V, Heng D, Crapper J, Chan H-K. Bipolar electrostatic charge and mass distributions of powder aerosols–effects of inhaler design and inhaler material. J Aerosol Sci. 2016;95:104–17.
Pharmacopoeia U, editor 30/NF25. US Pharmacopoeial Convention, Rockville; 2007.
Byron PR, Peart J, Staniforth JN. Aerosol electrostatics I: properties of fine powders before and after aerosolization by dry powder inhalers. Pharm Res. 1997;14(6):698–705.
Grosvenor M, Staniforth J. The influence of water on electrostatic charge retention and dissipation in pharmaceutical compacts for powder coating. Pharm Res. 1996;13(11):1725–9.
Nomura T, Satoh T, Masuda H. The environment humidity effect on the tribo-charge of powder. Powder Technol. 2003;135:43–9.
Rowley G, Mackin L. The effect of moisture sorption on electrostatic charging of selected pharmaceutical excipient powders. Powder Technol. 2003;135:50–8.
Chakrabarty RK, Moosmuller H, Garro MA, Arnott WP, Slowik JG, Cross ES, et al. Morphology based particle segregation by electrostatic charge. J Aerosol Sci. 2008;39(9):785–92.
Acknowledgments and Disclosures
This research was supported under Australian Research Council’s Discovery Projects funding scheme (DP150103953). H-KC thanks Richard Stenlake for his generous donation which was used as part of a University of Sydney PhD scholarship to financially support JY. The authors also thank Dr. Philip Chi Lip Kwok for valuable comments on the equipment setup and the Australian Centre for Microscopy & Microanalysis for providing imaging facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, J., Wong, J., Ukkonen, A. et al. Effect of Relative Humidity on Bipolar Electrostatic Charge Profiles of dry Powder Aerosols. Pharm Res 34, 1707–1715 (2017). https://doi.org/10.1007/s11095-017-2178-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2178-3