Abstract
Purpose
At present, there is no published data examining the effect of relative humidity on the electrostatic charges of dry powder inhaler aerosols. The charging behaviour of two commercial products, Pulmicort® and Bricanyl® Turbuhalers®, were investigated using an electrical low pressure impactor (ELPI).
Methods
ELPI was successfully modified to disperse the aerosols at 60 l/min. Four doses from each new inhaler were sampled at 15, 40, 65, and 90% RH. Particles deposited on the impactor stages according to their aerodynamic diameters and their charges were measured simultaneously by the electrometers. The drug in each size fraction was quantified using HPLC.
Results
Both products generated bipolar charges. The charging behaviour of the two types of inhaler showed different humidity dependence although the mass output was not significantly affected. The absolute specific charge of budesonide fine particles from Pulmicort® was the lowest at 40% RH but increased at lower and higher RHs. In contrast, the terbutaline sulfate fine particles from Bricanyl® followed the expected trend of charge reduction with increasing RH.
Conclusions
The distinct trends of charging of aerosols from Pulmicort® and Bricanyl® Turbuhalers® was explained by differences in hygroscopicity and other physicochemical factors between the two drugs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Dry powder inhalers (DPIs) are popular for delivering pharmaceutical aerosols to the lungs. Although the aerosol characteristics of DPIs such as particle size and mass output have been evaluated extensively for many years, their electrostatic properties are poorly studied. Electrostatic deposition is one of the five major mechanisms of aerosol deposition in the lungs (1). Computer modelling and limited in vivo inhalation studies conducted on human subjects indicate that electrostatic charges may affect total and regional deposition in the lungs (2–7). Electrostatic charges enhance deposition by increasing attractive forces to airway surfaces via the space charge force and the image charge force. The space charge force is the repulsion between charged particles in an aerosol cloud (8). The image charge force is the attraction between a charged particle and its image charge on a surface. Although human airways are normally electrically neutral, image charges with equal magnitude and opposite polarity to the charged particles may be induced on the surfaces, especially inside small airways in the peripheral lung (4,7). Thus knowledge of pharmaceutical aerosol charging is important in understanding particle deposition and drug delivery to the lungs.
Aerosol dispersion from DPIs is a mechanical process in which particles inevitably undergo electrification through contact with, and friction against, solid surfaces (9). This charging process is termed triboelectrification. However, the fundamental charging mechanism of insulators, such as pharmaceutical solids, is not well understood (10,11). On the other hand, contact charging between metals is the most thorough model for solid electrification and often serves as the basis for understanding charging of other materials (10,12). Each metal has a unique work function, defined as the energy required to remove a valence electron from an atom (10,12). When two different metals establish contact, electrons flow from the metal with the lower work function to that with the higher. A potential difference, called contact potential, is thus generated across the metal surfaces. Oppositely charged metals are obtained when the two parts are separated in an insulated system (12). Although the metal–metal charging model is not directly applicable to insulators, the concept of work function facilitates interpretation of charging phenomena in non-metals. However, surface characteristics and electron energies of insulators are ill-defined thus their charging is complex and difficult to control (10). Triboelectrification is sensitive to ambient relative humidity, temperature, surface impurities, surface roughness, area of contact, and other physicochemical factors (9,12,13). In addition, frictional charging also generates heat, which further reduces the predictability of the outcome (13).
Electrostatic charges generated from metered dose inhalers have been previously measured using an aerosol electrometer apparatus that was built in-house (14–17). The instrument is essentially a two-stage impactor with a Faraday pail serving as the second stage for collecting fine particles <5.8 μm at an airflow of 45 l/min. The net charge on the fine particle mass was measured with an electrometer. The setup is limited as the bipolar charges among different size fractions could not be discerned as only the net charge is detected. The drawback has been overcome with a modified setup of the electrical low pressure impactor (ELPI), the details of which were described elsewhere (18–20). Essentially, particles are sized by aerodynamic impaction into 13 size fractions. Twelve of these stages are connected to individual electrometers that measure the net charge of each size fraction. The ELPI provides a high resolution of size classification and can detect variation of net charge within the various size fractions. Both the size and charge are measured simultaneously. The current ELPI marketed by Dekati, Finland only operates at 10 or 30 l/min, which are too low for common pharmaceutical DPIs. In the present study the ELPI setup was further modified to aerosolise at 60 l/min.
In previous papers the ELPI had been applied to the measurement of charges from metered dose inhalers (19,20). The present study focuses on the Pulmicort® and Bricanyl® Turbuhalers®, both of which are drug-only DPIs with no carriers or excipients. They represent the simpler formulations for understanding charging behaviour, as compared with binary systems with carriers. Furthermore, they are two of the few drug-only DPI products on the market. Lastly, the two products have the same inhaler design and share the same dispersion mechanism for the powders. This eliminates one variable and facilitates data interpretation when comparing the charging of the two drugs.
The Turbuhaler® is a breath-actuated, multi-dose DPI with a powder reservoir. To protect the powder from moisture, the inhaler has an internal dessicant compartment and an external tight-fitting screw cap covering the whole device (21). The drug powder and dessicant are contained in separate compartments. The moisture-proof design of the inhaler had been shown to be efficient in vitro (22,23) and in vivo (23–25). Thus patients are instructed by the manufacturer to keep the cap tightly closed when the inhaler is not in use. The primary particles of the powder are 2–4 μm in size (26). These are co-spheronised by tumbling to form free-flowing agglomerates (27). Metered doses are individually dispensed from the reservoir with an internal scraper and dosing unit prior to each inhalation (21). The resistance to airflow of Turbuhaler® is ranked as medium (28,29), thus 60 l/min is a suitable flow rate for the in vitro dispersions as the flow is readily achievable by patients (27,30) using this inhaler.
Relative humidity (RH) has long been recognised to affect triboelectrification. Moisture reduces surface contact, changes the conductivity or other bulk properties of the particles, and facilitates charge neutralisation (31). The improvement of surface conductivity of the particles through moisture adsorption is the major factor, but changes to bulk conductivity also occurs via water absorption at high humidities (32). Similarly, the time constant for charge relaxation decreases with increasing RH and amount of adsorbed water on the powder (33). Recently, it was found that RH had negligible effects on the charging of solids with low hygroscopicity, such as α-lactose monohydrate (34). On the contrary, the charging of sodium starch glycolate, a hygroscopic compound, is inversely related to RH (34). These indicate that water is the determinant of charge reduction. Many humidity studies have been conducted on the charging of bulk powders (31–34) but few on aerosols. Since the particle size and dose output from DPIs can be affected by RH (35–37), it is important to investigate the electrostatic properties. The ELPI has only recently been used for measuring charges of micronised albuterol with lactose carriers generated from Rotahalers® and Inhalators® under ambient conditions (38). However, there is no published data at present examining the effect of relative humidity on the electrostatic charges of dry powder inhaler aerosols.
The aim of this study was to investigate the charging of particles from Pulmicort® and Bricanyl® Turbuhalers® dispersed at different RHs.
MATERIALS AND METHODS
Dry Powder Inhalers and Aerosol Electrostatic Charge Measurement
The two types of DPIs examined in the study were Pulmicort® (400 μg budesonide; AstraZeneca, Australia; Batch 1420061C00) and Bricanyl® (500 μg terbutaline sulfate; AstraZeneca, Australia; Batch 3520210B00) Turbuhalers®. Twelve inhalers of each type were used before expiry. All were kept in their original packaging under ambient conditions until experimentation.
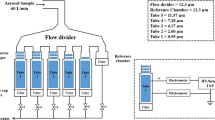
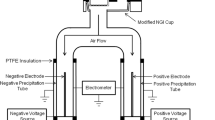
The ELPI (Dekati, Finland) connected to a vacuum pump normally operates at an airflow of 30 l/min because the jet plate of Stage 1 is the flow-limiting critical orifice (39). The setup was modified to disperse the DPIs at 60 l/min in this study. The corona charger frame was removed from the ELPI and was replaced with a stainless steel straight tube, a Y-piece, and a USP throat (Fig. 1). A plastic unit dose sampler coupled to another vacuum pump was also connected to the Y-piece to provide the additional airflow (Fig. 1). A 47-mm glass fibre type A/E filter (Pall, USA) was placed inside the unit dose sampler to capture drug particles. Before each experiment, the flow rate through a blank Turbuhaler at the throat was checked and adjusted to 60 l/min with both pumps operating at 30 l/min. A silicone rubber adaptor ring was fitted onto the DPI mouthpieces for airtight sealing during aerosol sampling. Particle bounce and re-entrainment were minimised by the application of 90 μl of a 1 g/15 ml solution of silicone fluid (200 Fluid/60000 cSt; Dow Corning, Australia) in cyclohexane (Mallinckrodt, USA). The solvent was allowed to evaporate before assembling the impactor stages. The aerosol charges were measured and recorded using the Dekati ELPIVI 4.0 software with the current range set at 400,000 fA.
The ELPI was enclosed in a polycarbonate box with a controller (Vaisala HMD60Y temperature and RH transmitter coupled to two Shimaden SR71 controllers; custom made by Active Instrument Services, Australia) to establish and maintain the relative humidity (RH) at the ambient temperature (24 ± 2°C). Four RHs were chosen in this study for the dispersions (15, 40, 65, and 90%), maintained at ±5% RH. The ELPI was kept at 90% RH only for several hours a day to avoid damage of the internal electronic components at prolonged exposures. Electric detection is not affected at high RHs unless significant condensation is formed on the Teflon rings separating the ELPI stages, in which case the inter-stage insulation would be lost (Isherwood, personal communication, 2006). This did not occur in the present study and the ELPI functioned normally at all RHs.
Three new inhalers of each DPI product were randomly assigned for each RH and four doses were individually sampled from each inhaler for both charge and mass determinations. Prior to dispersion, each inhaler was put inside the box upright with the cap closed to condition for one hour at the experimental RH before the first aerosol sampling.
For dispersion, the ELPI electrometers were zeroed without flushing. The flushing function is normally used to pump clean air through the ELPI during the zeroing when the corona charger is switched on. This prevents disturbances in the baseline by highly charged particles. No flushing was used in this study because there was no corona charging. The inhaler cap was removed, the rubber adaptor fitted onto the mouthpiece, and both vacuum pumps were switched on. When a stable zero baseline was obtained the inhaler was inserted into the throat and kept there until all aerosol charges from the dose were measured. It was observed that the background electrical signals were always temporarily disturbed upon the insertion of the inhaler to the throat. These disturbances were relatively low in magnitude and lasted only for a few seconds, after which the signals returned to a stable zero baseline. Immediately after dose sampling the inhaler was reinserted into and removed from the throat three times to generate the blank signals, the mean of which was calculated and subtracted from the aerosol measurements to account for these effects. The rubber adaptor was removed from the mouthpiece for chemical assay after the dispersion and the cap was replaced tightly. The length of time in which the inhaler was uncapped was less than a minute. The ELPI stages were then disassembled and the drug deposits on which were extracted with solvents for quantification.
Mass Assays of Drug Deposits
Drug deposits were quantified chemically using high performance liquid chromatography (HPLC), the conditions for which are summarised in Table I. Fresh standard solutions were prepared to generate standard curves before each HPLC run. New Pulmicort® and Bricanyl® inhalers of the same batches as the tested ones were sacrificed to obtain the drugs for making the standard solutions.
Pulmicort®
The adaptor, USP throat, Y-piece, straight tube, and unit dose sampler were each washed exhaustively with 5 ml of a 20:80 v/v water-in-methanol mixture. The impactor stages were sonicated for 5 min in beakers containing 5 ml of the same solvent. The unit dose sampler washing was centrifuged at 12045×g for 15 min to separate out the filter fibres before loading into HPLC vials.
Bricanyl®
The drug extraction method was similar to that for Pulmicort® except that 5 ml of water was used instead of water-in-methanol for the adaptor, USP throat, Y-piece, straight tube, and unit dose sampler. The stages were sonicated in 5 ml of water and 5 ml of cyclohexane, which was used to dissolve the silicone fluid to allow the extraction of the drug into the mobile phase. The liquid mixtures were centrifuged at 3080×g for 15 min to separate the two phases. Samples were taken from the aqueous phase for assay.
Data Analysis
The charge on each impactor stage was derived from the electric current data by calculating the area under the curve in the current-versus-time plot for that particular stage. The net charge was calculated from the sum of charges on all stages. The dose splitting by the modified ELPI setup was assessed by considering drug deposition downstream from the Y-piece. The percentage of the total amount deposited in the ELPI and that in the unit dose sampler to the sum of these two (which denotes the post Y-piece dose) were calculated. The ideal dose split ratio is 50:50 between the ELPI and the unit dose sampler. The emitted dose is the total drug mass recovered from all the components assayed. The fine particle dose (FPD) is the sum of drug mass deposits on Stage 11 (cutoff size = 4.04 μm) and below divided by the fraction of the post Y-piece dose in the ELPI to account for the fine particles deposited in the unit dose sampler. The fine particle fraction (FPF) is the percentage of the FPD in the emitted dose. The specific charge, or charge-to-mass ratio (q/m), for a given size fraction is the quotient of the charge and drug mass in that size fraction. The FPD net specific charge was derived by dividing the net charge (i.e. arithmetic sum of charges) from Stage 11 and below by the FPD. The FPD absolute specific charge was obtained in a similar manner except that the absolute magnitude of charges from Stage 11 and below was used instead. This parameter reveals the level of real charges without charge neutralisation by opposite polarities, which occurs for the FPD net specific charge. The number of elementary charges per drug particle (n) in a particular size fraction was estimated by the following equation:
where ρ is the true density of the particles, V the volume of a particle, and e the elementary charge (1.602 × 10−19 C). The particles were assumed to be non-agglomerated, spherical, non-porous, and have an average true density of 1.5 g/cm3 because pharmaceutical powders generally have true densities between 1.0 and 2.0 g/cm3 (40). For a given ELPI stage, V was calculated from the physical particle diameter, which was derived using the following equation: \( d_{p} = d_{a} {\left( \rho \right)}^{{ - 0.5}} \), where d p is the physical particle diameter, d a the aerodynamic cutoff diameter of the ELPI stage, and ρ the true density (1).
The data were analysed statistically using one-way analysis of variance (ANOVA), followed by the Tukey multiple comparisons post hoc test (α = 0.05) to determine differences between the means unless specified otherwise. All statistical procedures were performed with Minitab™ Statistical Software 13.31.
Dynamic Water Vapour Sorption
The hygroscopicity of powders from Pulmicort® and Bricanyl® were tested using a dynamic vapour sorption (DVS) analyser (Surface Measurement Systems, UK) equipped with a Cahn D-200 microbalance (ATI Instruments, USA). All experiments were conducted at 25°C. Samples (30–40 mg) of powder tapped out from the drug reservoir of Pulmicort® and Bricanyl® inhalers were loaded directly into the DVS sample pan. The powders were subjected to two sorption–desorption cycles from 0 to 90% RH in 10% RH steps. The step-change criterion was the rate of weight change (dm/dt) ≤ 0.0002 % of the initial weight per minute over five minutes. A separate powder sample from Brianyl® was subjected to another water vapour sorption scheme to further examine its moisture uptake. After equilibrating at 0% RH, the RH was changed to 50% and then sequentially increased to 90% RH at 10% RH steps. Each step was maintained for eight hours. No desorption was applied in this scheme. The data were analysed with the DVS Analysis Suite Version 3.6 (Advanced) (Surface Measurement Systems, UK).
RESULTS
The charge and mass profiles of Pulmicort® and Bricanyl® are shown in Fig. 2. Both products generated bipolar charges, with the larger particles charging negatively and the smaller ones positively. The particle size at which the polarity changed over is different for the two DPIs, despite the similarity in their mass distributions. Particles larger than 2 and 0.9 μm were negatively charged for Pulmicort® and Bricanyl®, respectively. Interestingly, high positive charges from Bricanyl® were measured below 0.6 μm, where negligible drug mass was detected. Since the DPI contains no excipients, these charges must be due to the presence of a very small amount of highly charged ultrafine terbutaline sulfate particles in the aerosol. The Pulmicort® charge profiles remained the same at different RHs, whereas those of Bricanyl® reduced in magnitude with increasing RH. The mass profiles for both inhalers remained unchanged from 15 to 65% RH, only slightly lowered at 90% RH.
The dose split ratios between the ELPI and the unit dose sampler for most of the dispersions were better than 60:40 in either direction. Thus the aerosols can be considered as evenly divided by the Y-piece. Fig. 3 shows the emitted doses, FPDs, FPFs, and FPD absolute specific charges across the different RHs. Except for the FPD of Pulmicort®, the mass output of both DPIs were not significantly affected by the RH.
Mean emitted dose, FPD, FPF, and absolute FPD specific charge of Pulmicort® (white bars) and Bricanyl® (grey bars). Error bars represent standard errors (n = 12). The columns are designated with a letter in the middle of a column where applicable. Asterisk letters above a column indicate the corresponding columns that are statistically different to the one concerned (p ≤ 0.05). All statistical differences were determined using ANOVA followed by the Tukey multiple comparisons test (α = 0.05) except that the Fisher test (α = 0.05) was used on the Pulmicort® FPD plot.
The effect of RH on the FPD absolute specific charges was distinctly different for the two inhalers (Fig. 3). The Pulmicort® FPD absolute specific charge showed a trough at 40% RH but increased at lower and higher RHs. In contrast, the trend for Bricanyl® was simpler, the FPD absolute specific charge decreased with increasing RH.
The specific charges and the estimated number of elementary charges per drug particle in the size fractions with detectable drug mass are presented in Tables II and III. The FPD net specific charges of Pulmicort® were all positive and those of Bricanyl® were low negative, due to the relative amount of positive and negative charges in their charge profiles (Fig. 2). A substantial amount of charges from both polarities were cancelled out when the net charges of Bricanyl® were calculated. For both inhalers, the specific charges and the number of elementary chargers per drug particle in each size fraction (Tables II and III) show the same order of magnitude ranking with respect to RH as that of the FPD absolute specific charges (Fig. 3). This indicates that the RH affected particle charging in all size fractions.
The sorption isotherms and weight change profiles for budesonide and terbutaline sulfate powder samples from Turbuhalers® are shown in Figs. 4 and 5. The maximum RH of 90% was not high enough for deliquescence to occur. Moisture uptake was 0.17 and 0.25% for budesonide and terbutaline sulphate, respectively (Fig. 4). After the first sorption–desorption cycle, budesonide showed a very slight gross weight loss (<0.01%). On the other hand, terbutaline sulfate showed comparatively higher gross weight loss (0.1%) with a large hysteresis loop, which are typical signs of recrystallisation of amorphous regions (41,42). This indicates that terbutaline sulfate underwent more recrystallisation when exposed to moisture, as also evident from the sequential weight losses at each RH step from 50 to 90% RH (Fig. 5). The sequential weight loss was reproduced over this RH range on another terbutaline sulphate sample when each RH step was maintained for eight hours (Fig. 6).
DISCUSSION
The aim of this study was to investigate the effect of RH on aerosol charging as there is no published data on this. In actual patient use the aerosols will always be exposed to the humid air in the respiratory tract, with the relative humidity ranging from about 40% RH in the mouth, 60% in the pharynx, to almost 100% RH in the deep airways (43). Since the effects of moisture on charging during aerosol generation were initially unknown, a wide range of RHs were chosen for a broader coverage, from 15 to 90%.
Pulmicort® and Bricanyl® generated aerosols with bipolar charges but their charging behaviour differed: the absolute specific charges of fine particles from Bricanyl® decreased monotonically with increasing RH, whereas the fine particles from Pulmicort® carried the highest specific charges at 15 and 90% RH but lowest at 40% RH.
In the present study, the inhalers were kept under the specified RH with the cap closed to mimic normal patient use in the same environment. Dispersion at 60 l/min was successful with the modified ELPI setup using a Y-piece and an extra vacuum pump. The 50:50 split ratio was aimed at to obtain a representative sample with the ELPI. The experimental results show that the dose splitting was acceptable. Moreover, the emitted dose and FPF yielded were comparable to those from other studies using conventional impaction methods on the Turbuhaler® at 60 l/min (28,44). Thus the modified setup was suitable for measuring charges of DPI aerosols dispersed at a higher flow rate.
Both Pulmicort® and Bricanyl® generated bipolar charges but the profiles were different. Since the two products employ the same inhaler, the differences must be due to drug-specific physicochemical properties, such as hygroscopicity, amorphous content, and resistivity (see below). In both formulations the larger size fractions were negatively charged and the smaller ones positively charged. In a theoretical study by Gallo and Lama (45), work function decreases with increasing particle size. This implies that charges can be transferred between particles of different sizes, even if both are of the same material (45). In particular, electrons are transferred from the material with the lower work function to that with the higher (10,12). This implies that larger particles would be the electron donor due to their lower work function and should become positively charged. However, the charge profiles from the present study showed the opposite trend. It must be noted that the work function model only provides a simplified analysis of triboelectrification from an energetic perspective. The model also assumes that the only interaction that occurs is the contact between two spherical particles under idealised conditions. As mentioned in the Introduction, many physicochemical factors contribute to and influence the actual charging process. During aerosol dispersion, the deaggregation of drug agglomerates and the subsequent movement of particles in the turbulent airstream create numerous physical interactions inside the inhaler. Drug particles come into contact with each other, as well as with the interior surfaces of the device, from the powder reservoir through to the mouthpiece channels. Indeed, powders became charged immediately after metering from the reservoir, before aerosolisation (46). Both Bricanyl® and Pulmicort® Turbuhalers® employ the same device design, but the specific details of the inhaler composition materials are not available as they are proprietary information of the manufacturer. It is not known whether the device composition materials differ between the two products. However, from visual inspection, the Turbuhaler® clearly consists of many components made from different plastics and pigments, which add even more physicochemical variables to the triboelectrification. The aerosol charging process is therefore very complex and the results do not necessarily conform to the predictions of the work function model. Fundamental studies, such as those examining single impact charging of a particle (47,48), would be needed to determine the elementary factors that are likely to be involved.
The number of elementary charges per drug particle was estimated from the specific charge in the present study. Since true density is used in Eq. 1, the physical particle diameter, rather than the aerodynamic diameter, is more appropriate for calculating the particle volume. However, aerosols generated from DPIs commonly consist of both primary particles and agglomerates. Due to the presence of void space, the apparent density of an agglomerate is lower than the true density of the primary particles. For instance, the void fraction of the closet packing arrangement for congruent spheres is 0.2595 (49), thus the apparent density is 25.95% lower than the true density. Since n ∝ ρ in Eq. 1, the number of elementary charges per drug particle will also be reduced by the same proportion. The apparent densities of loosely packed agglomerates would be even lower. Hence the values in Table III may be over-estimates.
Comparing the number of elementary charges per drug particle with those obtained from MDIs in a previous study (19) reveal that for particles in a given size fraction, those from DPIs generally carry lower charges. For instance, at 65% RH, the estimated number of elementary charges for Stage 10 of Pulmicort® and Briancyl® were −16 and −24, respectively. On the other hand, the corresponding figures for MDI particles in the same size fraction measured under ambient conditions ranged from −339 to +317 (values recalculated with the physical particle diameters), depending on the product (19). The 13 to 20-fold difference between DPI and MDI charges cannot be sufficiently accounted for by any error in the assumed true density of 1.5 g/cm3 used in the calculation as the true density range of pharmaceuticals is relatively narrow (1.0 and 2.0 g/cm3) (40). The higher charges from MDIs may be due to the high shear stress (force per unit area) experienced by the particles blasting out from the confined spaces in the metering valve, valve stem, and the actuator block. The dimensions of the inhalation and mouthpiece channels in the Turbuhaler® are comparatively much larger so the shear stress should be lower. Consequently triboelectrification would be less intense in the DPI.
Charges produced from Pulmicort® 100 μg and Bricanyl® 500 μg Turbuhalers® had been measured by Byron et al (46) using the aerosol electrometer apparatus. The fine particles dispersed from Pulmicort® under ambient temperature and RH were positively charged (46). Despite the difference in the dose of budesonide used, the polarity agrees with that measured in the present study where most of the fine particle size fractions were highly positively charged. Byron et al (46) reported that Bricanyl® displayed high inter- and intra-inhaler variations in charge polarity. Electrostatic measurements in the present study were more reproducible, as indicated by the short error bars in Fig. 2. However, the ELPI also provided more detailed information, owing to the finer divisions of size ranges for charge measurement. Substantial levels of positive and negative charges <5.8 μm were measured by the ELPI (Fig. 2). It is likely that Byron et al (46) reported low specific charges of terbutaline sulfate because the bipolar nature of the aerosol could not be discerned by their setup, hence the net charge of fine particles would be low in magnitude due to neutralisation by opposite polarities, as evident from the low FPD net specific charges derived from ELPI data in the present study. In addition, the FPD specific charges and the number of elementary charges per particle of budesonide determined by the aerosol electrometer apparatus were approximately 4 μC/g and 200, respectively (46). These are in the same order of magnitude as the corresponding values obtained in the present study (Fig. 3 and Table III).
The drawback of describing bipolar charging in terms of net specific charges is overcome by expressing the data as absolute charges. From the plots of the FPD absolute specific charge, Pulmicort® and Bricanyl® showed distinctly different fine particle charging behaviours with increasing RH. Triboelectrification generally decreases with increasing RH (29) but Pulmicort® did not follow the expected pattern. The statistical differences indicated for the values in Tables II and III showed that Bricanyl® was more sensitive to RH than Pulmicort® in particle charging. An explanation of this apparent anomaly is attempted below.
The dielectric constant of air increases with RH, but the effect is negligible. Under ambient temperature (22°C) and pressure (760 mmHg), the dielectric constant of air only increases by 0.017% if the RH increases from 15 to 90% (50). Rather, moisture adsorption on a powder is thought to be a major factor as it reduces interparticulate contacts and increases the particle surface conductivity, thus facilitating charge dissipation (29,31–33). Moisture layers form on solid surfaces when the RH is above about 50% (51). Changes to the bulk conductivity also occur via water absorption at high RHs (32). Conversely, RH has negligible effects on the charging of solids with low hygroscopicity (34).
It should be noted that the charges detected by the ELPI resulted from a combination of two dynamic and concomitant mechanisms: charge generation from particle deaggregation/dispersion and charge dissipation through exposure to moisture in the entrained air. Since these processes are difficult to separate, it cannot be ascertained whether either one, or both, of them were affected by RH in the present study. Although moisture is known to enhance charge dissipation, as discussed above, its possible influence on charge generation should not be discarded.
In the present study, the Turbuhalers® have been kept dry by being tightly capped (27). Thus interactions between the drug and the moisture in the air were possible only during the cap-off period. Exposure of the device and powder surfaces to the specified RH only occurred shortly before and during the dispersion while the cap was removed, which lasted for less than a minute, before the cap was replaced. It is well known that aerosolisation performance can worsen if a powder was stored for hours under high RH (27,37,52). However, during short exposures, humidity equilibrium was unlikely to have been reached, and thus capillary forces or interparticulate liquid bridging would not have fully developed (53). The relatively stable mass profiles of Pulmicort® and Bricanyl® (Fig. 2) suggest that particle deaggregation was not adversely affected by capillary forces. Nevertheless, moisture adsorption on particulate and inhaler surfaces would have begun after the cap was removed. Indeed, a brief 3-min exposure of powders prior to their dispersion at various RHs was shown to reduce the aerosolisation performance (35,36) and the degree of influence of RH was dependent on the salt form of the drug (35). Furthermore, the work functions of budesonide and terbutaline sulfate may have different sensitivities to RH, as has been reported on other materials (54). These observations suggest that the interactions between the humidified air and the drug during the cap-off period are likely to have contributed to the aerosol triboelectrification.
The absolute FPD charging profile of budesonide particles from Pulmicort® may be the result of two concomitant processes with increasing RH: (1) enhanced charge dissipation rate due to the facilitated movement of charges through the adsorbed moisture layer on particle surfaces; and (2) increased cohesion of particles in the agglomerates. At 15% RH, the charge was high due to the low charge dissipation rate so the particles retained the charges generated from aerosolisation. At 40%, the higher moisture adsorption increased charge dissipation, hence the lower specific charge. When the RH reached 65% and above, the high moisture level rendered the agglomerates progressively more difficult to deaggregate. Consequently, the particles that were successfully dislodged from the agglomerates and became dispersed would be those carrying higher charges to counteract the cohesive forces, particularly at the high RH of 90%. The similar FPDs between 40 and 65% RH were associated with comparable FPD absolute specific charges at these RHs. This supports the premise that the amount of fine particles dispersed is related to the level of charges they carry. The charge dissipation rate would still increase from 65% RH onwards, but budesonide may be sufficiently insulating to retain enough charges to be measured after dispersion. This charging model may also be applicable to terbutaline sulfate from Bricanyl® but this compound is more hygroscopic (Figs. 4 and 5) and more electrically conductive because it is a salt. Thus the charges generated on the dispersed fine particles would dissipate quicker than those on budesonide, hence only low charges were measured at 65 and 90% RH.
The DVS data indicated that the terbutaline sulfate particles contain amorphous regions and most of them had recrystallised in the first sorption–desorption cycle since there was no further gross weight loss in the second cycle (Fig. 4). Initially it was thought that recrystallisation of the powder was slow at 50% RH, thus another sample was subjected to moisture exposure for prolonged periods in an attempt to drive the process to completion under one RH. However, the sequential recrystallisation at progressively increasing RH was reproduced to each RH level at prolonged periods (Fig. 6). This suggests that the amorphous regions processed various surface energy levels such that different regions interacted with the adsorbed water to different extents (Figs. 5 and 6). In contrast, budesonide was relatively non-hygroscopic so amorphicity and recrystallisation may not be obvious due to reduced interaction with water. The slight gross weight loss after the first cycle (Fig. 4) suggests that certain amorphous regions may exist on the particles. However, no weight reduction was detected at each step of the sorption cycles (Fig. 5) to support this is the case. Since amorphous surfaces have higher surface energy than crystallised ones (55), they may have different electrostatic properties. This would affect electron transfer with other surfaces during contact and the resultant charges developed by triboelectrification. Discontinuous amorphous domains distributing over particle surfaces have been reported (56). This implies that an individual particle may be inherently heterogeneous in charge, depending on the nature of the surface region concerned.
This study has generated a few hypotheses on aerosol charging in DPI involving various physicochemical properties (e.g. hygroscopicity, amorphous content, and resistivity). Subsequent studies would focus on the fundamental roles of these parameters in triboelectrification and verify the hypotheses. For example, resistivities of the powders may be monitored at different RHs or particle surfaces may be characterised by atomic force microscopy. Other pertinent factors on charging may also be uncovered in the future.
In conclusion, a modified ELPI setup was successfully used to disperse Pulmicort® and Bricanyl® DPI aerosols at 60 l/min and measure the size and charge distributions. The DPIs showed drug-specific responses to particle charging at different RHs. Bricanyl® was more susceptible to RH, showing decreased charges with increasing RH. In contrast, particle charges from Pulmicort® decreased at 40% RH but increased at higher RHs. A dual mechanistic charging model was proposed to explain the charging behaviours.
References
W. C. Hinds. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, Wiley, New York, NY, 1999, pp. 191–192.
C. Melandri, V. Prodi, G. Tarroni, M. Formignani, T. De Zaiacomo, G. F. Bompane, G. Maestri, and G. Maltoni. On the deposition of unipolarly charged particles in the human respiratory tract. Inhaled Part. 4 Pt 1:193–201. (1975).
C. Melandri, G. Tarroni, V. Prodi, T. De Zaiacomo, M. Formignani, and C. C. Lombardi. Deposition of charged particles in the human airways. J. Aerosol Sci. 14:657–669 (1983).
W. Balachandran, W. Machowski, E. Gaura, and C. Hudson. Control of drug aerosol in human airways using electrostatic forces. J. Electrost. 40 & 41:579–584 (1997).
A. G. Bailey. The inhalation and deposition of charged particles within the human lung. J. Electrost. 42:25–32 (1997).
A. H. Hashish, A. G. Bailey, and T. J. Williams. Selective deposition of pulsed charged aerosols in the human lung. J. Aerosol Med. 7:167–171 (1994).
A. G. Bailey, A. H. Hashish, and T. J. Williams. Drug delivery by inhalation of charged particles. J. Electrost. 44:3–10 (1998).
D. Koolpiruck, S. Prakoonwit, and W. Balachandran. Numerical modeling of inhaled charged aerosol deposition in human airways. IEEE Trans. Ind. Appl. 40:1239–1248 (2004).
A. G. Bailey. Electrostatic phenomena during powder handling. Powder Technol. 37:71–85 (1984).
C. D. Hendricks. Charging macroscopic particles. In A. D. Moore (ed.), Electrostatics and its Applications Wiley, New York, NY, 1973, pp. 57–85.
S. Matsusaka and H. Masuda. Electrostatics of particles. Adv. Powder Technol. 14:143–166 (2003).
F. A. Vick. Theory of contact electrification. Br. J. Appl. Phys., Suppl. 2:S1–S5 (1953).
A. G. Bailey. Charging of solids and powders. J. Electrost. 30:167–180 (1993).
P. Kulphaisal, J. Peart, and P. R. Byron. Influence of water on electrical properties in hydrofluoroalkane based metered dose inhalers. In R. N. Dalby, P. R. Byron, J. Peart, and S. J. Farr (eds.), Respiratory Drug Delivery VIII Davis Horwood International, Raleigh, NC, 2002, pp. 783–785.
J. Peart, P. Kulphaisal, and J. C. Orban. Relevance of electrostatics in respiratory drug delivery. Business Briefing: Pharmagenerics 2003 84–87 (2003).
J. Peart, C. Magyar, and P. R. Byron. Aerosol electrostatics—metered dose inhalers (MDIs): reformulation and device design issues. In R. N. Dalby, P. R. Byron, and S. J. Farr (eds.), Respiratory Drug Delivery VI Interpharm Press, Buffalo Grove, IL, 1998, pp. 227–233.
J. Peart, J. C. Orban, P. McGlynn, M. P. Redmon, C. M. Sargeant, and P. R. Byron. MDI electrostatics: valve and formulation interactions that really make a difference. In R. N. Dalby, P. R. Byron, J. Peart, and S. J. Farr (eds.), Respiratory Drug Delivery VIII, Davis Horwood International, Raleigh, NC, 2002, pp. 223–230.
W. Glover and H.-K. Chan. Electrostatic charge characterization of pharmaceutical aerosols using electrical low-pressure impaction (ELPI). J. Aerosol Sci. 35:755–764 (2004).
P. C. L. Kwok, W. Glover, and H.-K. Chan. Electrostatic charge characteristics of aerosols produced from metered dose inhalers. J. Pharm. Sci. 94:2789–2799 (2005).
P. C. L. Kwok, R. Collins, and H.-K. Chan. Effect of spacers on the electrostatic charge properties of metered dose inhaler aerosols. J. Aerosol Sci. 37:1671–1682 (2006).
K. Wetterlin. Turbuhaler: a new powder inhaler for administration of drugs to the airways. Pharm. Res. 5:506–508 (1988).
B. J. Meakin, J. Cainey, and P. M. Woodcock. Effect of exposure to humidity on terbutaline delivery from turbuhaler dry powder inhalation devices. Eur. Respir. J. 6:760–761 (1993).
L. Borgström, L. Asking, and P. Lipniunas. An in vivo and in vitro comparison of two powder inhalers following storage at hot / humid conditions. J. Aerosol Med. 18:304–310 (2005).
A. Nana, P. Youngchaiyud, N. Maranetra, J. Boe, C.-G. Löfdahl, O. Selroos, and E. Ståhl. β2-Agonists administered by a dry powder inhaler can be used in acute asthma. Respir. Med. 92:167–172 (1998).
D. A. Lindsay, N. L. Russell, J. E. Thompson, T. H. Warnock, I. D. Shellshear, and P. R. Buchanan. A multicentre comparison of the efficacy of terbutaline Turbuhaler™ and salbutamol pressurized metered dose inhaler in hot, humid regions. Eur. Respir. J. 7:342–345 (1994).
L. Borgström, L. Asking, and L. Thorsson. Idealhalers or realhalers? A comparison of diskus and turbuhaler. Int. J. Clin. Pract. 59:1488–1495 (2005).
L. Borgström, H. Bisgaard, C. O’Callaghan, and S. Pedersen. Dry-powder inhalers. In H. Bisgaard, C. O’Callaghan, and G. C. Smaldone (eds.), Drug Delivery to the Lung, Marcel Dekker, New York, NY, 2002, pp. 421–448.
M. Hindle and P. R. Byron. Dose emissions from marketed dry powder inhalers. Int. J. Pharm. 116:169–177 (1995).
X. M. Zeng, G. P. Martin, and C. Marriott. Particulate Interactions in Dry Powder Formulations for Inhalation, Taylor & Francis, London, 2001, p. 24 and 79.
L. Borgström. On the use of dry powder inhalers in situations perceived as constrained. J. Aerosol Med. 14:281–287 (2001).
J. Eilbeck, G. Rowley, P. A. Carter, and E. J. Fletcher. Effect of contamination of pharmaceutical equipment on powder triboelectrification. Int. J. Pharm. 195:7–11 (2000).
M. P. Grosvenor and J. N. Staniforth. The influence of water on electrostatic charge retention and dissipation in pharmaceutical compacts for powder coating. Pharm. Res. 13:1725–1729 (1996).
T. Nomura, T. Satoh, and H. Masuda. The environment humidity effect on the tribo-charge of powder. Powder Technol. 135–136:43–49 (2003).
G. Rowley and L. A. Mackin. The effect of moisture sorption on electrostatic charging of selected pharmaceutical excipient powders. Powder Technol. 135–136:50–58 (2003).
R. N. Jashnani and P. R. Byron. Dry powder aerosol generation in different environments: performance comparisons of albuterol, albuterol sulfate, albuterol adipate and albuterol stearate. Int. J. Pharm. 130:13–24 (1996).
R. N. Jashnani, P. R. Byron, and R. N. Dalby. Testing of dry powder aerosol formulations in different environmental conditions. Int. J. Pharm. 113:123–130 (1995).
P. M. Young, R. Price, M. J. Tobyn, M. Buttrum, and F. Dey. Effect of humidity on aerosolization of micronized drugs. Drug Dev. Ind. Pharm. 29:959–966 (2003).
M. J. Telko, J. Kujanpää, and A. J. Hickey. Investigation of Triboelectric Charging in Dry Powder Inhalers using Electrical Low Pressure Impactor (ELPI™). Int. J. Pharm. 336:352–360 (2007).
M. Marjamäki, J. Keskinen, D.-R. Chen, and D. Y. H. Pui. Performance evaluation of the electrical low-pressure impactor (ELPI). J. Aerosol Sci. 31:249–261 (2000).
R. C. Rowe, P. J. Sheskey, and P. J. Weller. Handbook of Pharmaceutical Excipients, Pharmaceutical, London (2003).
A. Mangel. Investigating a range of solid samples by automatic water sorption. J. Therm. Anal. Calorim. 55:581–599 (1999).
G. Buckton and P. Darcy. Assessment of disorder in crystalline powders. A review of analytical techniques and their application. Int. J. Pharm. 179:141–158 (1999).
E. Daviskas, I. Gonda, and S. D. Anderson. Mathematical modeling of heat and water transport in human respiratory tract. J. Appl. Physiol. 69:362–372. (1990).
H. Steckel and B. W. Müller. In vitro evaluation of dry powder inhalers I: drug deposition of commonly used devices. Int. J. Pharm. 154:19–29 (1997).
C. F. Gallo and W. L. Lama. Some charge exchange phenomena explained by a classical model of the work function. J. Electrost. 2:145–150 (1976).
P. R. Byron, J. Peart, and J. N. Staniforth. Aerosol electrostatics. I: properties of fine powders before and after aerosolization by dry powder inhalers. Pharm. Res. 14:698–705. (1997).
T. Matsuyama and H. Yamamoto. Impact charging of particulate materials. Chem. Eng. Sci. 61:2230–2238 (2006).
H. Watanabe, M. Ghadiri, T. Matsuyama, Y. L. Ding, K. G. Pitt, H. Maruyama, S. Matsusaka, and H. Masuda. Triboelectrification of pharmaceutical powders by particle impact. Int. J. Pharm. 334:149–155.
M. Suzuki. Description of particulate assemblies. In K. Gotoh, H. Masuda, and K. Higashitani (eds.), Powder Technology Handbook, Marcel Dekker, New York, NY, 1997, pp. 305–320.
I. Koga. Dielectric constant of moist air. Electrotech. J. 4:108–110 (1940).
B. Vonnegut. Atmospheric electrostatics. In A. D. Moore (ed.), Electrostatics and its Applications, Wiley, New York, NY, 1973.
P. M. Young, M. J. Tobyn, R. Price, M. Buttrum, and F. Dey. The use of colloid probe microscopy to predict aerosolization performance in dry powder inhalers: AFM and in vitro correlation. J. Pharm. Sci. 95:1800–1809 (2006).
A. D. Zimon. Adhesion of Dust and Powder, Plenum, New York, NY, 1969.
J. H. Anderson. Humidity dependence of tribocharging of electrophotographic carriers coated with poly(vinylidene fluoride)-poly(methyl methacrylate) blends. J. Electrost. 63:59–67 (2005).
J. Zhang, S. Ebbens, X. Chen, Z. Jin, S. Luk, C. Madden, N. Patel, and C. J. Roberts. Determination of the surface free energy of crystalline and amorphous lactose by atomic force microscopy adhesion measurement. Pharm. Res. 23:401–407 (2006).
P. Begat, P. M. Young, S. Edge, J. Sebastian Kaerger, and R. Price. The effect of mechanical processing on surface stability of pharmaceutical powders: visualisation by atomic force microscopy. J. Pharm. Sci. 92:611–620 (2003).
Acknowledgements
This work was supported financially by the Australian Research Council (ARC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwok, P.C.L., Chan, HK. Effect of Relative Humidity on the Electrostatic Charge Properties of Dry Powder Inhaler Aerosols. Pharm Res 25, 277–288 (2008). https://doi.org/10.1007/s11095-007-9377-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9377-2