Abstract
Purpose
To investigate the applicability of Bipolar Charge Analyzer (BOLAR), a new commercial instrument developed by Dekati Ltd., in simultaneously characterizing the bipolar electrostatic charge profile and measuring the size distribution of commercial metered dose inhalers (MDIs).
Methods
Intal Forte® (sodium cromoglycate), Tilade® (nedocromil sodium), Ventolin® (salbutamol sulphate), and QVAR® (beclomethasone dipropionate) were used as model MDIs in this study. Three individual actuations of each MDI were introduced into the BOLAR at an air flow rate of 60 l/min. Charge and mass profiles for each actuation were determined.
Results
The BOLAR was found to have better performance in collecting valid charge data (≥80%) than valid mass data (≥50%). In all tested products, both positively and negatively charged particles were found in five defined size fractions between zero and 11.6 μm, with the charge magnitude decreased with increasing particle size. The net charge profiles obtained from the BOLAR qualitatively agreed with the results reported previously. In all suspension type MDIs, negligible masses were detected in the smallest size fraction (<0.95 μm), for which the charge was most likely caused by the propellant and excipients. QVAR was the only solution MDI tested and the charge and mass profiles were significantly different from the suspension-type MDIs. Its mass profile was found to follow closely with the charge profile.
Conclusions
Positively and negatively charged MDI particles of different size fractions and their corresponding charge-to-mass profiles were successfully characterized by the BOLAR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metered dose inhalers (MDIs) remain one of the mainstays in the treatment of asthma and other respiratory related diseases (1,2). They contain a drug, either suspended or solubilized, in a hydrofluoroalkane (HFA) propellant and may contain co-solvents/excipients. Upon actuation, the pressurized HFA liquid is exposed to atmospheric pressure and rapidly expands to its gaseous state to aerosolize the medicaments as small particles or droplets for inhalation. Triboelectrification can readily take place to generate charged particles during the atomization process, as activities including flash boiling, cavitation and evaporation are involved bringing the solid, liquid and gas components into contact. Formation of electrostatic charges on the drug particles is dependent on the formulation and/or the material of the various components of the canister i.e., valve stem and body (3,4).
There are two general mechanisms controlling the deposition of these charged particles in the respiratory tract, namely the space (the natural repulsive force creating by the charged particles) and image (induction of a transient charge of opposite polarity at the airway wall as charge particles pass through) charges (5). The potential influence of electrostatic charge on total and regional deposition of drug particles in the lungs has been demonstrated in a number of experimental (6–8) and theoretical (9–12) studies. However, the relationship between the charge level and the deposition of inhaled particles has not yet been established. An important step to achieve it would require the accurate measurement of the pharmaceutical aerosol particles charges.
The polarity, level of electrostatic charge, and charge and mass distributions of aerosols generated by commercially available MDIs have previously been characterized using a 13-stage electrical low pressure impactor (ELPI) (13–16) and an electrical Next Generation Impactor (eNGI) (17,18). The resulting data has informed our understanding of the potential influence of electrostatic charge on particle deposition in the lungs (5,19). However, the ELPI and eNGI only measure the net charge of particles deposited on each impactor stage, and do not provide any information on the bipolar characteristics of the aerosols. Additionally, it has been reported the electric charges carried by individual particles, instead of the net charge, plays an important role in the behavior and fate of inhaled pharmaceutical aerosols (20).
While electrical single particle aerodynamic relaxation time (E-SPART) (21) and a custom designed bipolar charge measurement system used in conjunction with an ELPI (22) have been used to characterize bipolar properties, the determinations of charge-to-mass ratio for different particle sizes were indirect. A novel instrument, the Bipolar Charge Analyzer (BOLAR™, Dekati, Finland) was designed to overcome these limitations and is capable of separating, and detecting positively and negatively charged aerosol particles within the same size fraction. Its design details and applicability in measuring the bipolar charge profile of lactose powder were recently documented by Yli-Ojanperä et al. (23). We have reported preliminary results on the charge profiles of commercial DPIs and MDIs, and aerosols generated by a jet nebulizer using BOLAR, and showed aerosols at a given size fraction consisted of both positively and negatively charged particles/droplets (24–26). However, mass assays were not conducted in these studies to reveal the specific charge of the aerosols. The primary objective of this work was to demonstrate the capability of the BOLAR to study the bipolar charging properties of MDIs. Comparisons of net charge data between the BOLAR and ELPI measurements were also made to confirm the reliability of results obtained from the BOLAR.
Materials and Methods
Metered Dose Inhalers
Four commercial MDI products were used in this study, Intal® Forte (5 mg sodium cromoglycate, Aventis Pharma, UK), Tilade® (2 mg nedocromil sodium, Aventis Pharma, UK), QVAR® (100 μg beclomethasone dipropionate, 3 M Health Care, UK) and Ventolin® (100 μg Salbutamol sulphate, Allen & Hanburys, Australia). Details of these products are shown in Table I. All inhalers were used before the expiry date and primed according to instructions described on the consumer medicine information leaflet and they were washed after each measurement. Three individual actuations of each MDI were introduced into the BOLAR. Charge and mass profiles were ascertained for each actuation.
BOLAR
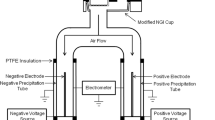
Figure 1 shows a schematic diagram of the BOLAR, using a standard USP induction port to introduce aerosols into the instrument. The design details were documented in Yli-Ojanperä et al. (23). Briefly, it consists of a flow divider to divide the flow evenly to five electrical detection tubes (bipolar detectors) and a reference chamber (an electrical filter to verify successful flow division). Each detection tube is coupled with a pre-separator impactor set (one or several impactors) with a specific particle size cutoff diameter to fractionate particles of a defined size range for charge measurement. Detector tube 5 has only one impactor stage, but other tubes use two or three impactor stages in cascade to minimize particle bouncing and overloading from a single plate. The bipolar detectors consist of two concentric metal cylinders, with the inner cylinder maintained at high positive potential and the outer cylinder grounded, to create an electric field between the cylinders. Therefore, positively and negatively charged particles deposit on the outer and inner cylinders, respectively. Each cylinder is connected to an individual electrometer to measure the current carried by the charged particles for a specific measurement time period. The electric current signals are subsequently integrated over the measurement period to calculate the charge values. The reference chamber measures the net charge of the aerosol, which is used as a reference value for the other bipolar detector tubes. The measured bipolar data is verified by confirming the total charge for tubes 1–5 (detector + impactor) being the same as the reference value.
Particles smaller than the specific cutoff diameter of an impactor are collected within a detector tube (i.e., Tubes 2, 3, 4 and 5 measure charge/mass of particles smaller than 2.60, 4.17, 7.29 and 11.57 μm, respectively). Therefore, the charge/mass of particles within a defined size fraction is calculated by subtraction of the measured charge in two consecutive tubes (for instance, Tube 2 = 6(Qtube2 –Qtube 1) / 6(mtube2 –mtube 1)), as shown in Table II. As the aerosol cloud divides to six fractions equally at the flow divider, the magnitude of both charge and mass for each size fraction equals to the measured values times six . Instead of the aerodynamic cutoff diameter of each detection tube, the mid-point diameter was recommended by the manufacturer as the characteristic diameter for graphical presentation so that the positive, negative and total values are more distinguishable when they are plotted on the same graph.
Charge Measurement
A vacuum pump (model SV25, Sogevac®, Leybold, France) was used to provide air flow at 60 l/min through the BOLAR (10 l/min per detector tube). Following the manufacturer’s suggestion, a self-check test was performed before each measurement to ensure six criteria were met: 1) the vacuum line/pump was working properly; 2) there were no leaks in the system; 3) flow equality was achieved among the six branches; 4) the noise level of the electrometer current was minimum; 5) all electrometers were working properly; and 6) the BOLAR’s electrical control system was working properly when a measurement sequence was executed. For each measurement, a single actuation was discharged into the induction port. The environmental conditions were monitored and kept at a relative humidity of 50 ± 5% and a temperature of 23 ± 2°C.
Mass Quantification of Drug Compounds
After the charge measurement, the detector tubes were carefully disassembled. The drug particles deposited on each part were exhaustively rinsed using specific amounts of the collecting solvent: the mouthpiece (5 ml), adaptor (5 ml), USP throat (5 ml), flow divider inlet (5 ml), flow divider (30 ml), parts within each detector tubes including the impactor (5 ml), centralizer (5 ml), outer detector tube (20 ml), inner detector tube (30 ml), the back-up filter (10 ml), and the reference filter (10 ml). The drug content was assayed chemically by high performance liquid chromatography (HPLC), using the methods described in Table III.
Criteria for Valid Measurement
As shown in Table II, the charge and mass of each detector tube was determined on the basis of the difference between two sequential tubes. Therefore, a balanced flow division between the six branches of the flow dividers (i.e., the particle stream is evenly separated into the five detector tubes and the reference tube) is essential for a valid measurement. There are two criteria, namely the symmetry and cumulative criteria, needed to be fulfilled to ensure the validity of the measured charge and mass data. According to the manufacturer calculation sheet, all tubes must have a deviation smaller than 10% of the average value of the total charge or mass (impactor + positive + negative + filter) among the six tubes to pass symmetry criteria. In some cases, the net charge can be nearly neutral regardless of high positive and negative charge values noted. The symmetry criteria for charge measurements, therefore, can be relaxed to be the sum of 10% deviation and 20 pC. As mentioned above, each detector tube collects particles smaller than the specific cutoff diameter of the coupled pre-separator impactor set. Therefore, the amount of charged (positive and negative) and neutral particles of subsequent tube must be higher than the previous one. However, in cases where there are no particles or a small amount of particles and/or charge presence in some size fractions, the flow adjustment accuracy and the measurement accuracy can result in no increment or reduction in the cumulative values. In these cases, the measurement is still accepted if the cumulative values are inside the cumulative criteria, which the charge and mass values of subsequent tube must be at least 5% and 1% from the previous tube, respectively. The determined values for size ranges that get wrong sign of polarity is calculated as zero. According to the manufacturer, a slightly higher tolerance is used for the charge measurement because a small difference was usually noted between detector tubes 4 and 5 during the product development when lactose powders actuated by a dry powder inhaler were tested (23).
Data Analysis
The fine particle dose (FPD) was defined as the amount of particles ≤ 4.2 μm in the present study, which equals to the total amount of drug detected in detector tube 3 (cutoff diameter 4.2 μm) multiplied by the number of sampling ports (six).
The net charge for a given size fraction was determined as the sum of positive and negative charges of that size fraction. The specific charge or charge-to-mass ratio (q/m), of each polarity was calculated by dividing the charge by the mass. To enable direct comparison with the published net charge data obtained from ELPI, the charge-to-mass ratio of the net charge was also calculated as dividing the net charge (positive + negative) by the total mass collected in the positive electrode, negative electrode, and the filter in each detector tube.
Results and Discussion
Charge Profiles
The successful rate of collecting valid charge data (both the symmetric and cumulative criteria were fulfilled) was higher than 80% for most products. Figure 2 shows the bipolar charge profiles of aerosols generated by the four MDIs. Due to the similarity of the inhaler and formulation designs, the charge profiles of Intal Forte and Tilade suspension MDIs shared similar charge behavior (Fig. 2a and b). Both positively and negatively charged particles were detected for all size fractions. While the charge magnitudes of the negatively charged particles remained at a similar level for all particles sizes, particles within the smallest size fraction (≤0.95 μm) were found to be highly positively charged and its magnitude significantly reduced as the particle size increased. As a result, a positive net charge was detected for the smallest fraction, and the net charge for larger particles (>0.95 μm) became negative, as depicted in Fig. 3. The net charge profiles are in qualitative agreement with those reported using the ELPI, that the polarity change from positive to negative at stage 8 with a cutoff diameter of 0.96 μm (14).
Though the charge magnitudes of both positively and negatively charged particles generated by Ventolin was considerably lower than Intal Forte and Tilade, all these suspension based MDIs demonstrated a decreasing trend in charge magnitude with increasing particles size (Fig. 2c). The lower charge magnitudes observed for Ventolin could be accounted by a combination of 1) the low nominal drug dose, 2) different propellant used (HFA-134a for Ventolin, and HFA-227 for Intal Forte and Tilade), and/or 3) different excipient conditions (no excipient was used in the Ventolin MDI, while Povidone, Macrogol 600 was used in both Intal Forte and Tilade). As seen in Fig. 3, the net charge for Ventolin was negative throughout all size fractions. Unipolar net charges were also reported in Glover and Chan (13) using an ELPI. However, Kwok et al. (14) reported the particles were positively charged in the size range 0.960–4.04 μm when Ventolin was actuated continuously, but unipolar negative charges were observed in the discrete puffs. They attributed the puff-dependent charging of Ventolin to the charge relaxation of the MDI materials – counter-charges resided in the actuator, valve components, and/or drug deposits in these locations after each actuation and may take time to decay. Their presence may affect charging of the particles in the subsequent actuation. Since the mouthpiece was washed after each measurement in the present study, it is unlikely the charge profile was affected by the residual charges from the previous puffs.
The charge profiles generated by the QVAR solution MDI were substantially different from other products. The finest particles (<0.95 μm) emitted from QVAR were highly charged (>4000 pC) with almost balanced positive and negative charges to give a slightly positive net charge (Fig. 2d). QVAR was the only solution MDI product and has a metal valve stem, while all other products are suspension type and have plastic valve stems. In addition, ethanol which may interact with the formulation and valve components is used in QVAR to increase the solubility of the beclomethasone dipropionate. These dissimilarities are likely the sources causing the different charge profile from other MDI products. Unipolar net positive charges were observed for QVAR and the charge magnitude reduced with increasing particle sizes (Fig. 3). The results were consistent with the results reported in Kwok et al. (14).
Mass Profiles
After the charge measurement, the BOLAR was disassembled and the drug masses deposited at each part of the instrument were collected for chemical assay using HPLC. In some cases, the mass deviations were found to be much higher than the charge deviations, leading to valid charge data and invalid mass data. Results reported here passed both the symmetric and cumulative criteria, which was ~ 80% of the experimental runs for suspension MDIs (Intal Forte, Tilade and Ventolin) and ~50% for the solution MDI (QVAR). This can be a limitation of the BOLAR in characterizing products with small amounts of drug per actuation. In Yli-Ojanperä et al. (23), multiple actuations were used to increase the accuracy of the mass assay of lactose using HPLC which may have reduced the deviation among the six branches of BOLAR. However, any charge and mass variation between actuations would have been masked using their multiple actuation method. The charge to mass ratio determined in this manner may not be precise. Other more sensitive drug detection methods, such as liquid chromatography – mass spectrometry (LC-MS) may increase the successful collection of valid mass data.
Figure 4 shows the mass profiles of the four tested MDIs. There were no drug masses detected in detector Tube 1 for Intal Forte, Tilade or Ventolin, whereas a significant fraction of charged drug particles was deposited in detector Tube 1 for QVAR. The results are consistent with previous ELPI (14) and eNGI (17) measurements, in which negligible masses were detected for particles smaller than 0.96 μm in suspension type MDIs (Intal Forte, Tilade and Ventolin), while more than 40% of drug particles were reported to be less than 0.96 μm in QVAR. In fact, the results were not unexpected because micronized particles ranging from 1 to 5 μm are generally used in suspension-type MDIs (27). In contrast, QVAR was the only solution MDI tested, for which drug particles were formed as the propellant and ethanol evaporated. The mass median aerodynamic diameter (MMAD) for QVAR was reported to be in the range of 0.8–1.2 μm (28).
The fine particle dose (FPD) was defined as the amount of particles ≤ 4.2 μm in the present study, which is slightly different from that defined in Kwok et al. (14) as the sum of the mass ≤ 6.06 μm. Nonetheless, the FPDs of Intal Forte (485 μg), Tilade (345 μg) and Ventolin (59 μg) were found to be comparable (Table IV). A greater difference in the FPD was noted for QVAR, which could be due to the much higher emitted dose obtained in this study (116%) compared with Kwok et al. (14) (74%). Similar ratios of FPD to ex-actuator dose were obtained between the BOLAR (~69%) and the ELPI (~67%) measurements reported in Kwok et al. (14). The reason for the higher emitted dose and FPD achieved in the present study compared with those reported in Kwok et al. (14) is unclear, but could be due to batch to batch variations. Overall, these results suggested the BOLAR is suitable for particle sizing of aerosols emitted from MDIs.
It is important to reiterate the charge magnitudes of all products decreased with increasing particle size, despite differences in the formulation and valve components are used. Compared with the finest particles (<0.95 μm), larger particles are negligibly charged (Figs. 2 and 3). Kwok et al. (14) proposed two possible explanations for the suspension formulations: 1) the decreased specific surface area for larger particles reduces the extent of charging, and 2) the high charge measured for smaller particles may be contributed by both the propellant/excipients and the drug. From Fig. 4, while the drug mass detected in detector tube 1 (<0.95 μm) for the suspension type MDIs was negligible (Fig. 4a–c), a significant amount of drug was detected in the finest size fraction for the solution MDI QVAR (Fig. 4d). Therefore, the high charge levels in the finest size fraction observed for Intal Forte, Tilade and Ventolin were more likely attributed to the propellent/excipients. Since the mass ratio of the drug to excipient should remain constant for droplets of all sizes for the solution MDI, the high charge value observed for the finest fraction of QVAR is probably due to the increased mass and specific surface area.
Charge-to-Mass Ratios
No clear trends were observed between aerosol electrostatics and aerosol depositions as most inhalation products are poly-dispersed and dominated by bipolar charges (4). Previous studies have demonstrated mono-dispersed particles of a threshold elementary charge could increase deposition within the respiratory tract (8,12). Therefore, the elementary charge of each particle can be important in understanding the involvement of charges in particle behavior. To investigate this aspect, charge-to-mass ratios for both polarities and the net charge for each size fraction were calculated as dividing the charge by the mass obtained for each polarity and the total mass, respectively (Fig. 5).
Comparing the charge and mass profiles of Intal Forte and Tilade (Figs. 2 and 4), it was noted that significant charge magnitudes (+5300 pC and −400 pC for Intal Forte, and +6500 pC and −1200 pC for Tilade) were detected for the smallest size fraction (≤0.95 μm), but almost no drug masses were recovered for this size fraction. As shown in Fig. 4, Ventolin also had below detection threshold amounts of drug mass smaller than 0.95 μm, though it showed a relatively lower charge level compared with the other two suspension type MDIs (Fig. 2). This led to an impossibly/invalidly high charge-to-mass ratios in the submicron size fractions for these suspension MDIs (data not shown). Kwok et al. (16) reported the droplet sizes of the propellants HFA 134A and 227 were in the submicron range at 5% and 50% RH. Therefore, the high charges measured for the smallest size fraction (≤0.95 μm) in the suspension type MDIs could be attributed to the propellant/excipients and ingressed water (29), rather than the drug particles. However, Kwok et al. (16) also reported the drug-free MDIs containing HFA 134A and 227 propellants were only slightly charged, in the range of +30 pC and −100 pC depending on the moisture content and the relative humidity, which are considerably lower than the studied commercial products. They suggested the difference was caused by the charge transfer between the micronized drug particles and the propellant in the suspension formulations. Recently, Chen et al. (3) demonstrated the level of charge of HFA 134A only MDIs could range between −700 and −1800 pC using different actuator materials and nozzle designs. They also showed the addition of a small amount of ethanol and drug could alter not only the charge level but also the polarity of the formulations. Therefore, the different charge levels observed in the smallest size fraction (accounted by the propellant/excipients) in Intal Forte, Tilade and Ventolin were due to their different formulation and MDI designs. Whereas Povidone, Macrogol 600 and HFA-227 are used in Intal Forte and Tilade, only HFA-134a as the propellant is present in Ventolin without any excipient.
Due to the similar formulation designs of Intal forte and Tilade, they shared similar trends in their charge (Fig. 2) and mass profiles (Fig. 4), resulting in similar charge-to-mass profiles (Fig. 5). The highest charge-to-mass ratio was noted for the fraction 0.95–2.60 μm and reached a plateau for larger particles. The standard deviation for Tilade was noticeably higher, which could be due to the relative lower drug dose emitted per actuation (Table I) thus lowering its detectability. The net charge-to-mass ratios of Intal Forte and Tilade were between −3 and −1 pC/μg and between −15 and −2 pC/μg, respectively, consistent with those reported in Kwok et al. (14). The charge-to-mass ratio of Ventolin varied between 0 and +42 pC/μg and between −26 and −8 pC/μg for positively and negatively charged particles, respectively. Ventolin also showed a high standard deviation in some size fractions, which was very likely due to the low drug dose (100 μg salbutamol sulphate). The net charge-to-mass ratio of Ventolin was negative throughout all particle size fractions (between −15 and −5 pC/μg).
Unlike the suspension-type MDIs, the charge profile of QVAR followed the mass profile closely (Figs. 2 and 4), resulting in a similar charge-to-mass ratio profile (Fig. 5). QVAR is a solution MDI with beclomethasone dipropionate being the only non-volatile component within the formulation. After the evaporation process of the propellant and ethanol, the droplets would give rise to pure drug particles (30). Additionally, the amount of dissolved drug, propellant, and excipients contained in each droplet actuated from the inhaler was expected to be directly proportional to its size, hence their relative charge contributions. The smallest size fraction (≤0.95 μm) had large values of charge-to-mass ratio for both positively (+187 pC/μg) and negatively (−207 pC/μg) charged particles. This is likely due to the combination of the high specific surface area combined with the high charge magnitude generated within the smaller drug particles. A large standard deviation was observed for the largest (7.29–11.57 μm) size fraction. This could be ascribed to the low mass detected in the fifth detector tube. Chen et al. (3) highlighted the addition of 15% w/w ethanol in MDI formulations could reduced the evaporation rate of the propellant, leading to large non-evaporated droplets depositing on the upper ELPI stages. This could also be a reason for the higher degree of variation in the charge-to-mass ratio observed here.
Limitation of BOLAR
While the BOLAR system was capable of characterizing the bipolar charging properties of aerosols generated from MDIs, we recognized the system has several limitations. The validity of collected data profoundly depends on the effectiveness of flow splitting into six even streams entering the five detector tubes and the reference chamber using a flow divider. It was found that a valid charge measurement does not guarantee a valid mass measurement. This would be a major downside of the system for routine measurements, as the mass assay is a very time consuming step. To thoroughly rinse the parts of a detector tube, significant amounts of collecting solvent are needed, resulting in very low drug concentrations for low dose products. Commonly used analytical techniques, such as HPLC, may not be sensitive enough to detect any drug contents. In such case, more sensitive LCMS technique is required for drug quantification. Though multiple actuations are suggested by the manufacturer for improved accuracy of the mass assay (23), mass and charge-to-mass ratio variations between actuations, which are important information, could be masked.
Conclusions
This study demonstrated the capability of the BOLAR system in simultaneously determining the bipolar charge characteristics and particle size distribution of commercial MDI products. Overall, the BOLAR system performed better in quantifying the charge measurements compared with mass measurements, possibly due to the small amounts of drug collected from a single actuation of the MDI products, especially for Ventolin and QVAR. More sensitive chemical assay methods such as LCMS are suggested to increase the successful rate of collecting valid mass data. Net charge and charge-to-mass measurements were found to qualitatively agree with the ELPI results reported previously. The simultaneous measurement of the electrical properties of aerosols and mass analysis provide a greater understanding of electrostatic charging on MDI therapeutics and its influence on deposition within the lungs.
Abbreviations
- BOLAR:
-
Bipolar Charge Analyzer
- ELPI:
-
Electrical low pressure impactor
- eNGI:
-
Electrical Next Generation Impactor
- E-SPART:
-
Electrical single particle aerodynamic relaxation time
- FPD:
-
Fine particle dose
- HFA:
-
Hydro-fluoro-alkane
- HPLC:
-
High performance liquid chromatography
- LCMS:
-
Liquid chromatography-mass spectrometry
- MDI:
-
Metered Dose Inhaler
- MMAD:
-
Mass median aerodynamic diameter
- USP:
-
United States Pharmacopeia
- d:
-
Diameter (μm)
- m:
-
Mass (μg)
- Q:
-
Charge (pC)
- m:
-
Mid-point
- p:
-
Particle
- tube n:
-
Detector tube n where n = 1,2 …5
References
Myrdal PB, Sheth P, Stein SW. Advances in metered dose inhaler technology: formulation development. AAPS PharmSciTech. 2014;15(2):434–55.
Stein SW, Sheth P, Hodson PD, Myrdal PB. Advances in metered dose inhaler technology: hardware development. AAPS PharmSciTech. 2014;15(2):326–38.
Chen Y, Young PM, Fletcher DF, Chan HK, Long E, Lewis D, et al. The influence of actuator materials and nozzle designs on electrostatic charge of pressurised metered dose inhaler (pMDI) formulations. Pharm Res. 2014;31(5):1325–37.
Chen Y, Traini D, Fletcher DF, Chan H-K, Lewis D, Church T, et al. The effect of active pharmaceutical ingredients on aerosol electrostatic charges from pressurized metered dose inhalers. Pharm Res. 2015. doi:10.1007/s11095-015-1674-6.
Kwok PCL, Chan H-K. Electrostatics of pharmaceutical inhalation aerosols. J Pharm Pharmacol. 2009;61(12):1587–99.
Chan TL, Yu CP. Charge effects on particle deposition in the human tracheobronchial tree. Ann Occup Hyg. 1982;26(1–4):65–75.
Tarroni G, Melandri C, Prodi V, Dezaiacomo T, Formignani M, Bassi P. An indication on the biological variability of aerosol total deposition in humans. Am Ind Hyg Assoc J. 1980;41(11):826–31.
Melandri C, Prodi V, Tarroni G, Formignani M, De Zaiacomo T, Bompane GF, et al. On the deposition of unipolarly charged particles in the human respiratory tract. Inhaled Part. 1975;4(Pt 1):193–201.
Balachandran W, Machowski W, Gaura E, Hudson C. Control of drug aerosol in human airways using electrostatic forces. J Electrost. 1997;40–1:579–84.
Bailey AG. The inhalation and deposition of charged particles within the human lung. J Electrost. 1997;42(1–2):25–32.
Bailey AG, Hashish AH, Williams TJ. Drug delivery by inhalation of charged particles. J Electrost. 1998;44(1–2):3–10.
Yu CP, Chandra K. Precipitation of submicron charged-particles in human lung airways. Bull Math Biol. 1977;39(4):471–8.
Glover W, Chan HK. Electrostatic charge characterization of pharmaceutical aerosols using electrical low-pressure impaction (ELPI). J Aerosol Sci. 2004;35(6):755–64.
Kwok PCL, Glover W, Chan HK. Electrostatic charge characteristics of aerosols produced from metered dose inhalers. J Pharm Sci. 2005;94(12):2789–99.
Kwok PCL, Collins R, Chan H-K. Effect of spacers on the electrostatic charge properties of metered dose inhaler aerosols. J Aerosol Sci. 2006;37(12):1671–82.
Kwok PCL, Noakes T, Chan H-K. Effect of moisture on the electrostatic charge properties of metered dose inhaler aerosols. J Aerosol Sci. 2008;39(3):211–26.
Hoe S, Young PM, Chan H-K, Traini D. Introduction of the electrical Next Generation Impactor (eNGI) and investigation of its capabilities for the study of pressurized metered dose inhalers. Pharm Res. 2009;26(2):431–7.
Hoe S, Traini D, Chan H-K, Young PM. The influence of flow rate on the aerosol deposition profile and electrostatic charge of single and combination metered dose inhalers. Pharm Res. 2009;26(12):2639–46.
Wong J, Chan H-K, Kwok PCL. Electrostatics in pharmaceutical aerosols for inhalation. Ther Deliv. 2013;4(8):981–1002.
Balachandran W, Kulon J, Koolpiruck D, Dawson M, Burnel P. Bipolar charge measurement of pharmaceutical powders. Powder Technol. 2003;135:156–63.
Ali M, Mazumder MK, Martonen TB. Measurements of electrodynamic effects on the deposition of MDI and DPI aerosols in a replica cast of human oral-pharyngeal-laryngeal airways. J Aerosol Med Pulm Drug Deliv. 2009;22(1):35–44.
O’Leary M, Balachandran W, Chambers F. Electrical mobility profiling used to illustrate the bipolar nature of pharmaceutical aerosol charge. In: Dalby RN, Byron PR, Peart J, Suman JD, Young PM, Traini D, editors. Respiratory drug delivery 2008, vol. 2. River Grove: DHI Publishing; 2008. p. 445–50.
Yli-Ojanpera J, Ukkonen A, Jarvinen A, Layzell S, Niemela V, Keskinen J. Bipolar charge analyzer (BOLAR): a new aerosol instrument for bipolar charge measurements. J Aerosol Sci. 2014;77:16–30.
Leung SSY, Yang MY, Wong J, Chan H-K. Bipolar electrostatic charge measurements of metered dose inhalers. In: Dalby RN, Byron PR, Peart J, Suman JD, Young PM, Traini D, editors. Respiratory drug delivery 2014. 3rd ed. River Grove: DHI Publishing; 2014. p. 721–6.
Wong J, Yang MY, Kwok PCL, Leung SSY, Chan H-K. Measuring bipolar charge from commercial DPIs using the BOLARTM. In: Dalby RN, Byron PR, Peart J, Suman JD, Young PM, Traini D, editors. Respiratory drug delivery 2014, vol. 3. River Grove: DHI Publishing; 2014. p. 727–30.
Yang MY, Leung SSY, Wong J, Chan H-K. Bipolar electrostatic charge nebulized aerosol. In: Dalby RN, Byron PR, Peart J, Suman JD, Young PM, Traini D, editors. Respiratory drug delivery 2014, vol. 3. River Grove: DHI Publishing; 2014. p. 731–4.
Purewal TS. Metered dose inhaler technology - Introduction. In: Purewal TS, Grant DJW, editors. Metered Dose Inhaler Technology. Interpharm Press: Buffalo Grove, IL, 1998. 1–8.
QVAR™ AUTOHALER™ and QVAR™ INHALER datasheet. http://www.medsafe.govt.nz/profs/Datasheet/q/qvarinhaler.pdf. [cited 2015 April 09].
Miller NC. The effects of water in inhalation suspension aerosol formulations. In: Dalby RN, Byron PR, Peart J, Suman JD, Young PM, Traini D, editors. Respiratory Drug Delivery 1990, Volume 1. River Grove, IL: DHI Publishing. p. 249–258.
Zhu B, Traini D, Chan H-K, Young PM. The effect of ethanol on the formation and physico-chemical properties of particles generated from budesonide solution-based pressurized metered-dose inhalers. Drug Dev Ind Pharm. 2013;39(11):1625–37.
ACKNOWLEDGMENTS AND DISCLOSURES
This research was supported under Australian Research Council’s Discovery Projects funding scheme (project numbers DP110105161 & DP120102778). Sharon Leung is a research fellow supported by the University of Sydney. Authors are thankful for Kevin Samnick for his helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leung, S.S.Y., Chiow, A.C.M., Ukkonen, A. et al. Applicability of Bipolar Charge Analyzer (BOLAR) in Characterizing the Bipolar Electrostatic Charge Profile of Commercial Metered Dose Inhalers (MDIs). Pharm Res 33, 283–291 (2016). https://doi.org/10.1007/s11095-015-1786-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1786-z