Abstract
The present work investigates experimentally the effect of H2O vapor on the removal of NO at elevated temperatures. Breakdown voltage, discharge characteristics and NO removal efficiency were studied under various conditions of water vapor content. The experimental results indicate H2O can greatly enhance the NO removal efficiency from a NO/O2/N2/C2H4/H2O system, but the breakdown voltage increases as the relative humidity of the gas increases. Moreover, the effect of temperature on NO removal at a relative gas humidity of 30 % was analyzed. With an increase in temperature, E/N increased, producing more active species and energetic electrons; electron detachment also became significant at high temperature and the rates of major reactions were promoted, intensifying the conversion of NO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-thermal plasmas associated with electrical discharges, for example, in pulse corona discharge [1, 2], radio frequency (rf) discharge [3], and dielectric barrier discharge (DBD) [4–6], have been extensively studied for their removal of nitrogen oxides (NOx). NOx emitted from diesel engines and thermal power plants has become an urgent problem. The major method for removing NOx from lean exhaust gases is selective catalytic reduction (SCR). The optimal temperature of the SCR method is around 300–350 °C, while the temperature of diesel exhaust is typically 100–200 °C, and the typical temperature of gas emitted from thermal power plants is around 100 °C. SCR systems require additional fuel injection to generate the conditions required to reduce NOx. These constitute a few percent of the total operation cost. Exhaust emitted from diesel engines and thermal power plants also contain different volume fractions of water vapor, depending on the driving conditions.
The main role of the dielectric barrier discharge in NOx removal has been understood to be the oxidation of NO to NO2. It was later found that the presence of hydrocarbons in the gas promotes the oxidation reactions. Shin and Yoon [7] simulated the effects of ethylene on NO removal in a non-thermal plasma, indicating that the ethylene additive strongly enhanced the NO removal efficiency. Yin [8] showed that increasing the water vapor content in an N2/NO/H2O system decreased the NO removal efficiency significantly. Zhu [9] studied experimentally the conversion of NO in NO/N2, NO/O2/N2, NO/C2H4/N2 and NO/C2H4/O2/N2 systems.
For applications to the treatment of NOx from diesel engines and thermal power plants by DBD, it is necessary to determine the discharge characteristics and reaction mechanism in the NO/O2/N2/C2H4/H2O system. Up until now, there have been few specific reports in the literature that considered the effect of water vapor and temperature on NO removal using C2H4 as an additive. The objective of this study was to systematically analyze the effect of water vapor. The experiments were carried out at different water vapor contents and temperatures, investigating the breakdown voltage and NO removal efficiency.
Experimental Section
Experimental Design
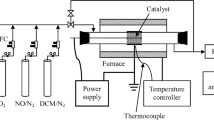
The schematic diagram of the experimental setup is shown in Fig. 1. Flow rates of all gases were controlled by mass flow controllers (MFC). Gases were mixed completely and flowed into the reactor. Exhaust gas was absorbed by alkali. The concentrations of NO and NO2 were measured by a gas analyzer (TESTO Pro.350). The consumed energy in the reactor was obtained using a voltage–charge curve (V–Q Lissajous figure), which was obtained using an oscilloscope (Rigol DS1202CA, 2-Gs/s maximum sampling rate, 200 MHz bandwidth).
The main components of the feed gas to simulate practical exhaust gas contained 500 ppmv (parts per million, volumetric) NO, 1,000 ppmv hydrocarbon C2H4, 6 % O2 and N2 as the balance gas. The total flow rate was 10 L/min.
HV in Fig. 1 is a high voltage transformer, which was connected to the power source. The voltage from the power source could be increased by 1,000 times with the high voltage transformer. The final output voltage to generate the plasmas in the DBD reactor was within 0–30 kV, the frequency was 10 kHz, measure capacitance (Cm) equaled 0.14 μF and the voltage was decreased 1,000 fold by a capacitive divider (that is, C1:C2 = 1,000:1).
The DBD reactor was made of a dielectric layer tube with a thickness of 2 mm. The inner diameter was 24 mm and the length was 530 mm. The material of the dielectric layer tube was quartz. The dielectric layer tube was wrapped in copper mesh with a length of 450 mm. The material of the inner electrode was copper with a diameter of 12 mm.
When taking the effect of H2O into account, an extra device, an ultrasonic atomizer (max ≥3 mL/min, 402 AI), was used to humidify the N2 and O2. The component of H2O was adjusted by controlling the degree of atomization, and the humidity was measured by a psychrometer (±3 % RH, testo 605-H1) located at the entrance to the reactor. When studying the effects of temperature on NO removal by DBD, wet gases including N2, O2 and H2O, flowed into the tube type resistance furnace (SK2-2.5-13TS). And after the relative humidity showing on the psychrometer remained stable, the gases were heated by the resistance furnace, and the temperature was controlled by a temperature regulator (KSYD-6.3-16Z). The experiments proceeded when the thermometer detected a stable temperature of the input gases (Table 1).
Definition of Energy Density
The definition of energy density is given in Eq. 1. The discharge power was obtained from the V–Q curve of the Lissajous figure (see Fig. 2), and the method adopted to measure the discharge power is as follows [10].
In Eq. 1, ε is energy density, J/L; P is discharge power, W; and Q is gas flow, L/s.
Definition of Removal Efficiency
NO and NOx removal efficiencies are defined by Eqs. 2 and 3. The input and output concentrations were obtained using the gas analyzer.
In Eqs. 2 and 3, η NO is NO removal efficiency, \(\eta_{{{\text{NO}}_{\text{x}} }}\) is NOx (NO and NO2) removal efficiency, cin,NO is the input concentration of NO, cout,NO is the output concentration of NO, and \({\text{c}}_{{{\text{out,NO}}_{ 2} }}\)is the output concentration of NO2.
Results and Discussion
Effects of Water Vapor on Breakdown Voltage
To examine the effects of water vapor on NO removal by DBD in the NO/N2/O2/C2H4/H2O system, we added water vapor into the experimental gas, adjusting the relative humidity of the gases to 30, 60 and 90 %, respectively. Figure 3 shows the breakdown voltage under conditions of different relative humidity; the breakdown voltage was 11.7, 13.3 and 15.5 kV as the relative humidity of the gas was increased from 30 to 60 to 90 %, respectively. In the DBD reactor, a neutral molecule of gas in a region with a strong electrical field is ionized by an exogenous environmental event (e.g. the result of a photon interaction), to create a positive ion and a free electron. The electric field then accelerates these charged particles and imparts each of them with kinetic energy. As a result of the energization of the electrons (which have a much higher charge/mass ratio and so are accelerated to a higher velocity), further electron/positive-ion pairs may be created by collision with neutral atoms. These then undergo the same separating process creating an electron avalanche [11]. When more water vapor is added in the gas stream, more negative ions are expected to be produced by dissociative electron attachment to H2O molecules at low temperatures. Negative ions show slow movement in an electric field and a weak capacity for ionization, reducing the effective amounts of electrons joining the electron avalanche [12]. The dependence of the discharge power on applied voltage and relative humidity is shown in Fig. 4. Discharge power increased with increasing applied voltage, and the corresponding relative humidity of the gas. When the relative humidity of the gas was 30, 60 and 90 %, respectively, the discharge power was 53.6, 45.1 and 35.0 W with an applied voltage of 13.8 kV. To sum up, high water vapor content hindered gas discharge in the DBD reactor.
Effects of Water Vapor on NOx Removal
Figure 5 shows the effects of water vapor on NO and NOx removal from the NO/N2/O2/C2H4/H2O system. When the energy density was 339 J/L, NO and NOx removal efficiencies were 44.4 and 30.3 %, respectively, without H2O, but increased to 56.6 and 50.1 %, respectively, with 30 % RH. This result implies that H2O can greatly increase the rate of NO removal. In the presence of H2O, active electrons collide with N2 and H2O:[13–17]
and, compared to O atoms, the generated OH radicals react more easily with hydrocarbons:[18, 19].
The H generated via R1 can react with O2 to form the strongly oxidizing radical HO2:[20–26].
These radicals (C2H4OH and HO2) can enhance the oxidation of NO via several reactions as follows:[27–29].
The increase in the oxidation of NO is obviously attributable to the formation of OH radicals from water vapor, which induces the following reactions:[8, 30–32]
Furthermore, we examined the influence of relative humidity on NO removal efficiency. The results are summarized in Fig. 6. The NO removal efficiencies increased with increasing energy density, showing very high removal efficiencies at high energy densities. Figure 6 also indicates that the higher the water content, the lower the conversion of NO. When the energy density was 564 J/L, with a relative gas humidity of 30, 60 or 90 %, the NO removal efficiency was 76.6, 70.0 and 63.6 %, respectively. That is because H2O is an efficient quencher; it consumes some of the active species. H−, OH− and a few O− anions are produced by dissociative electron attachment to H2O molecules, so that the amounts of active species participating in the NO reduction reaction are reduced. Furthermore, as the relative humidity of the gas increases, the reaction between H2O and N is intensified, generating more HN, which can react with OH to form NO:[8].
Effects of Temperature on NO Removal
Figure 7 shows the experimental results for NO removal by DBD in the NO/O2/N2/C2H4/H2O system with 30 % RH at 25, 90, and 130 °C, respectively. During the experiments, N2 and O2 flow into the ultrasonic atomizer and resistance furnace. The results show very high NO removal efficiencies in the high energy density range; in the low energy density range, high temperature raises the NO removal efficiency. The influence of temperature on NO removal efficiency is negligible in the high energy density range. Temperature can affect NOx removal by DBD in two major respects [33]: E/N (the electric field strength divided by the total gas density) and reaction rate. The gas density N decreases as the temperature is increased, causing E/N to increase, while the gas pressure and electric field E are kept constant. BOLSIG [34, 35] was used to solve the BE for the electron energy distribution function (EEDF); the result is shown in Fig. 8. With an increase in E/N, the proportion of energetic electrons also increases considerably. In a DBD reactor, the electrons and ions gain energy from the alternating electric field. If the electron energy is high enough, ionization and excitation will be promoted in collisions between electrons and heavy particles [36]. The non-elastic collisions of the energetic electrons with H2O and N2 molecules will induce H2O and N2 molecules to be dissociated, excited and ionized to produce active species, producing yet more active species (H, OH and N). At high temperature, electron detachment becomes significant so that radicals of O, OH, and N may play a more important role than their anionic counterparts [37]. At temperatures of 25, 90, and 130 °C, the reaction rate of R4 is kept constant, but that of R5 is 9.47 × 10−11, 1.03 × 10−10 and 1.11 × 10−10 cm3/s, respectively, generating more strongly oxidizing radicals, and therefore strengthening the reactions of strongly oxidizing radicals (C2H4OH and HO2) with NO. For the NO conversion reactions, R7, R8 and R9, the reaction rates also increase with the rising temperature, intensifying the NO removal reactions. The rates of NO formation reactions via R10 and R11 are 5.44 × 10−39 and 8.00 × 10−11 cm3/s at 25 °C, while they change to 2.77 × 10−30 and 8.00 × 10−11 cm3/s at 130 °C. In other words, the rate of R10 obviously increases while that of R11 is kept constant. However, the rate of R10 is so small compared to those of R7–R9 that the effect of the increase may be negligible. To summarize, in the NO/O2/N2/C2H4/H2O system, as the temperature increases, the generation of active species and the rates of NO removal reactions are both promoted. Both contribute considerably to NO removal efficiency.
Conclusions
This study was mainly focused on investigating the effect of water vapor content on NO removal at various temperatures. When the water vapor content is increased, a larger breakdown voltage is required and the discharge power is decreased under the same applied power. H2O can greatly enhance NO removal efficiency in the NO/O2/N2/C2H4/H2O system, but high water content prevents NO conversion. H2O is an efficient quencher, consuming some of the active species and increasing the rate of NO formation. As the temperature rises, electron detachment become significant and E/N also increases considerably, producing more active species, and the rates of the NO removal reaction increase. Therefore, when the temperature increases, the generation of active species and the reaction rates are promoted and both contribute considerably to the overall NO removal efficiency.
References
Tas MA, van Hardeveld R, van Veldhuizen EM (1997) Plasma Chem Plasma Process 17(4):371–391
Shen X, Gao X, Li ZS, Li B, Zheng CH, Sun ZW, Ni MJ, Cen KF, Alden M (2012) Fuel 102:729–736
Tsai CH, Wang YF, Yang HH, Liao CN (2008) J Hazard Mater 150(2):401–407
Schmidt M, Basner R, Brandenburg R (2013) Plasma Chem Plasma Process 33(1):323–335
Wang T, Sun BM, Xiao HP (2013) Plasma Chem Plasma Process 33(1):307–322
Wang T, Sun BM, Xiao HP, Zeng JY, Duan EP, Xin J, Li C (2012) Plasma Chem Plasma Process 32(6):1189–1201
Shin HH, Yoon WS (2003) Plasma Chem Plasma Process 23(4):681–704
Yin SE, Sun BM, Gao XD, Xiao HP (2009) Plasma Chem Plasma Process 29(6):421–431
Zhu AM, Sun Q, Niu JH, Xu Y, Song ZM (2005) Plasma Chem Plasma Process 25(4):371–386
Manley TC (1943) Trans Electrochem Soc 84(1):83–96
Ciesielska IL, Masajtis J (2007) Fibres Text East Eur 15(5–6):179–184
Xu X, Zhu TC (1996) Gas discharge physics. Fudan University Press, Shanghai
Shi HC, Wang WC, Yang DZ, Huo Y, Jia L (2011) Acta Phys Chim Sin 27(8):1979–1984
Aubry O, Cormier JM (2009) Plasma Chem Plasma Process 29(1):13–25
Liu F, Wang W, Zheng W, Wang Y (2006) Eur Phys J D 38(3):515–522
Liu F, Wang WC, Zheng W, Wang YN (2006) Plasma Chem Plasma Process 26(5):469–480
Kim DJ, Kim KS (2003) IEEE Trans Plasma Sci 31(2):227–235
Ravi V, Mok YS, Rajanikanth BS, Kang HC (2003) Fuel Process Technol 81(3):187–199
Mok YS, Ravi V, Kang HC, Rajanikanth BS (2003) IEEE Trans Plasma Sci 31(1):157–165
Sathiamoorthy G, Kalyana S, Finney WC, Clark RJ, Locke BR (1999) Ind Eng Chem Res 38(5):1844–1855
Mizuno A, Shimizu K, Chakrabarti A, Dascalescu L, Furuta S (1995) IEEE Trans Ind Appl 31(5):957–963
Nie Y, Wang JY, Zhong K, Wang LM, Guan ZC (2007) IEEE Trans Plasma Sci 35(3):663–669
Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson RF, Hynes RG, Jenkin ME, Rossi MJ, Troe J (2004) Atmos Chem Phys 4(6):1461–1738
Dorai R, Kushner MJ (2000) J Appl Phys 88(6):3739–3747
Dorai R, Kushner MJ (2002) J Phys D Appl Phys 35(22):2954–2968
Shang KF, Wu Y, Li J, Li GF, Li D (2007) Plasma Sources Sci Technol 16(1):104–109
Tonkyn RG, Barlowa SE, Hoard JW (2003) Appl Catal B 40(3):207–217
Mok YS, Dors M, Mizerazcyk J (2004) IEEE Trans Plasma Sci 32(2):799–807
Lombardi G, Simiand NB, Jorand F, Magne L, Pasquiers S, Postel C, Vacher JR (2007) Plasma Chem Plasma Process 27(4):414–445
Chang MB, Kushner MJ, Rood MJ (1992) Plasma Chem Plasma Process 12(4):565–580
Yan K, Kanazawa S, Ohkubo T, Nomoto Y (1999) Plasma Chem Plasma Process 19(3):421–443
Chalise PR, Wang Y, Mustafa KA, Watanabe M, Hayashi Y, Okino A, Hotta E (2004) IEEE Trans Plasma Sci 32(3):1392–1399
Chang MB, Yang SC (2001) AIChE J 47(5):1226–1233
Donko Z (2011) Plasma Sources Sci Technol 20(2):024001
Hagelaar GJM, Pitchford LC (2005) Plasma Sources Sci Technol 14(4):722–733
Li S, Tang ZC, Gu F (2010) Heat Mass Transf 46(8–9):851–857
Tanthapanichakoon W, Charinpanitkul T, Chaiyo S, Dhattavorn N, Chaichanawong J, Sano N, Tamon H (2004) Chem Eng J 97(2–3):213–223
Acknowledgments
This work was supported by National Natural Science Foundation of China (51206047).
Conflict of interest
There is no potential conflict of interest about this submission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, T., Sun, BM., Xiao, HP. et al. Effect of Water Vapor on NO Removal in a DBD Reactor at Different Temperatures. Plasma Chem Plasma Process 33, 681–690 (2013). https://doi.org/10.1007/s11090-013-9452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-013-9452-x