Abstract
This work investigated the destruction of a halogenated carbon (trichloromethane) using different types of nonthermal plasma reactors. Three reactors, i.e., a surface discharge reactor, a dielectric-packed bed reactor and a barrier discharge reactor with a perforated dielectric tube, were compared with respected to the trichloromethane destruction efficiency. The effect of oxygen content and input power on the trichloromethane destruction was examined, and the byproducts were analyzed to elucidate the destruction pathways. The dielectric-packed bed reactor was found to show better performance in the trichloromethane destruction than the other two reactors. The increase in the oxygen content decreased the destruction efficiency, and the highest destruction efficiency was obtained at oxygen content 0.5%. The calculations for electron-molecule collisions indicated that the most abundant reactive species initiating the destruction of trichloromethane are metastable nitrogen molecules. The major byproducts were CO and Cl2, and the formations of NO2 and N2O were also significant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gaseous organic pollutants including volatile organic compounds (VOCs) and halogenated carbons such as chlorofluorocarbons (CFCs), hydrofluorocarbons (HFCs), and perfluorocarbons (PFCs) are the main causes of photochemical smog, stratospheric ozone depletion, and global warming that are regarded as serious environmental problems. Recently, efforts have been made to abate the organic pollutants using either thermal or nonthermal plasma processes [1–5]. Thermal plasma processes such as DC arc, microwave discharge, and inductively coupled plasma are often used for treating high organic concentration streams, but not suitable for gas streams containing low concentrations of organic compounds because they necessarily consume a lot of electrical energy. In terms of electrical energy consumption, dielectric barrier discharge (DBD), surface discharge, dielectric-packed bed discharge, and pulsed corona discharge processes that are classified as nonthermal plasma can be effective gas treatment methods, particularly useful for low organic concentration gas streams.
The destruction of halogenated carbons using nonthermal plasma has been studied by several researchers including Fitzsimmons et al. [6], Kang [7], Ricketts et al. [8], Futamura and Gurusamy [9], Ogata et al. [10], etc., demonstrating that the halogenated carbons can be efficiently destroyed by this method. The performance of the halogenated carbon destruction by the nonthermal plasma can be improved by adopting catalysts. It has been reported that various catalysts such as MnO2, AlPO4, Al2O3, and TiO2 can be combined with the nonthermal plasma to increase the destruction efficiency [9–11]. The use of gaseous additives such as water vapor, oxygen, and hydrogen or the change in the gas composition by adding plasma-assisting gas like argon can also enhance the decomposition of halogenated carbons, and affect the distribution of decomposition products [10–13]. As well, AC operating frequency, residence time of gas stream, electrode material, and thickness influence the destruction of halogenated carbons [7, 14, 15]. Chen et al. [16] investigated the removal of CF4 and SF6 using DBD and dielectric packed-bed reactors, who found that the dielectric packed-bed reactor achieved higher CF4 removal efficiency and lower SF6 removal efficiency than that of the DBD reactor, indicating that the chemical characteristics of pollutants should be considered in selecting an appropriate nonthermal plasma reactor. Park et al. [17] and Kim et al. [18] also reported that reactor structure significantly affected the decomposition of CF4.
In this work, we investigated the destruction of a halogenated carbon using three different types of nonthermal plasma reactors. The reactors employed were a surface discharge reactor, a dielectric-packed bed reactor and a DBD reactor with a perforated dielectric tube, which were operated by AC high voltage. The target halogenated carbon compound was trichloromethane (CHCl3). The major use of this compound is in the production of the refrigerant chlorodifluoromethane (R-22). It has also widely been used as a solvent because it is relatively unreactive and miscible with most organic liquids. Despite its wide range of industrial uses, studies on the decomposition of this compound are scarce in the literature, [19, 20]. The performances of the three nonthermal plasma reactors mentioned above were evaluated with a simulated exhaust gas composed of nitrogen, oxygen, and trichloromethane. The effect of several parameters such as oxygen content and input power on the destruction of trichloromethane was examined, and the results were discussed. To elucidate the mechanism for trichloromethane destruction, byproducts were identified and analyzed.
Experimental
Apparatus
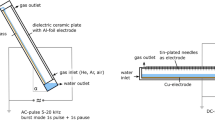
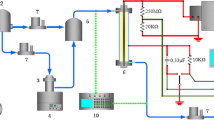
The schematic diagram of the experimental setup is shown in Fig. 1. The three nonthermal plasma reactors as shown in Fig. 2 were individually used for the destruction of trichloromethane. The surface discharge reactor was made up of a quartz tube (outer diameter (OD)/inner diameter (ID): 22/19 mm), a perforated copper tube electrode (OD/ID: 19/18 mm) with holes of 3 mm in diameter, a stainless steel tube electrode (OD/ID: 23/22 mm). The quartz tube, the perforated copper tube and the stainless steel tube were concentrically arranged. The dielectric-packed bed reactor was composed of a quartz tube (OD/ID: 22/19 mm), a stainless steel tube electrode (OD/ID: 23/22 mm), a 6 mm thick coaxial screw, and 3 mm glass beads (Sigmund Lindner). The DBD reactor with perforation was comprised of a quartz tube (OD/ID: 22/19 mm), a stainless steel tube electrode (OD/ID: 23/22 mm), and a 9 mm stainless steel rod covered with a 2 mm thick perforated Teflon (polytetrafluoroethylene) tube in which 140 holes of diameter 1.8 mm were drilled at predetermined intervals. AC high voltage in the range of 8–15 kV (operating frequency 400 Hz) was applied to the perforated copper tube, the 6 mm thick coaxial screw or the a stainless steel tube, which acted as the discharging electrodes. The outer stainless steel tube of each reactor was connected to the ground electrode. The effective length of the three reactors was 150 mm. To remove the heat generated during the operation, the plasma reactors were cooled down by circulating water at a constant temperature of 20°C around them.
Methods
The simulated exhaust gas fed to the plasma reactor was formed with nitrogen, oxygen, and trichloromethane. The flow rates of nitrogen and oxygen were controlled by mass flow controllers (MKS Instruments, Inc.). The concentration of trichloromethane was adjusted by using its vapor pressure (21.2 kPa at 20°C) as follows. A flask containing trichloromethane was immersed in a water bath kept at a constant temperature. A small amount of oxygen whose flow rate was regulated by a mass flow controller (MKS Instruments, Inc.) was saturated with trichloromethane as it was bubbled through the flask. This trichloromethane-saturated oxygen was later mixed with nitrogen and oxygen, thereby diluted to a desired concentration. The flow rate of the simulated exhaust gas was 2 L min−1 for the dielectric-packed bed reactor and the DBD reactor with perforation, and 1 L min−1 for the surface discharge reactor. The concentration of trichloromethane was about 900 ppm. According to the literature [8, 18], the effect of oxygen content in the range of 10–20% on the decomposition of halocarbons is not significant. In this work, the oxygen content was varied from 0.5 to 10% by volume with the flow rate of the simulated exhaust gas unchanged.
The concentrations of trichloromethane and byproducts including CO, CO2, N2O, NO, NO2, COCl2 were analyzed by a Fourier Transform Infrared (FTIR) spectrophotometer (Model 1600, Perkin-Elmer) equipped with a long path gas cell (Pike Technologies, Inc.). The concentrations of chlorine (Cl2) and hydrogen chloride (HCl) formed from trichloromethane were analyzed with gas detector tubes (Gastec Co.). The voltage applied to the plasma reactor was measured with a high voltage probe (P6015, Tektronix) having a DC attenuation ratio of 1000:1 and a digital oscilloscope (TDS 3032, Tektronix). The input power delivered to the plasma reactor was directly measured by a digital power meter (WT 200, Yokogawa).
Results and Discussion
Figure 3 shows the trichloromethane destruction efficiencies as a function of input power for the three plasma reactors mentioned above. The oxygen content in the simulated exhaust gas was 10% by volume in all cases. In the surface discharge reactor, the plasma is produced in the vicinity of the perforated copper tube electrode, leaving a dead zone along the axis. The lower destruction efficiency with the surface discharge reactor may be explained by this dead zone. Kang [7] and Ogata et al. [10] were able to effective destroy halocarbons using surface discharge reactors. But it should be noted that there are some differences in reactor structure and operating condition between their systems and ours. One remarkable difference is the reactor diameter. The surface discharge reactors employed in their studies have smaller diameters than that in this study, resulting in smaller dead zones. In addition, the operating frequency, the gas composition and the residence time (or gas flow rate) of their studies are very different from this work. In the packed bed reactor depicted in Fig. 2, the exhaust gas flows through the void volume provided by the dielectric pellets. As shown in Fig. 3, the destruction efficiency was largely enhanced by using the dielectric-packed bed reactor. This enhancement can be explained by reduced dead zone and high electric field strength near the contact points between the dielectric pellets. The main characteristic of the packed bed reactor is the presence of numerous contact points between the dielectric pellets, leading to high electric field strength near the contact points as compared with the mean value of the whole reactor [16]. The DBD reactor with perforation creates a lot of dense plasma channels starting from the holes. In this case, the discharge plasma is not uniform throughout the reactor volume due to the presence of the holes, but chemical bonds in trichloromethane can easily be cleaved in the dense plasma channels. This type of plasma reactor may be suitable for destroying organic compounds with strong chemical bonds such as carbon tetrafluoride and hexafluoroethane [17]. Compared to the dielectric-packed bed reactor, the destruction efficiency obtained with the DBD reactor was relatively low, but higher than that with the surface discharge reactor. The destruction by the DBD reactor with perforation may be improved by increasing the number of holes where dense plasma channels are formed. The rest of the experiments were carried out with the dielectric-packed bed reactor and the DBD reactor with perforation, since they showed better destruction efficiencies than the surface discharge reactor.
Oxygen that is one of the constituents of the simulated exhaust gas serves as an oxidizing agent to scavenge the intermediate destruction products and form carbon oxides. It also affects the destruction efficiency since it can consume reactive species produced by the discharge plasma. Regarding the effect of the oxygen content on the halocarbon destruction, contrary results were reported in the literature. According to Yu and Chang [12] and Ogata et al. [10], the increase in the oxygen content increased the halocarbon destruction. On the other hand, Fitzsimmons et al. [6] and Kim et al. [18] reported that the destruction of halocarbon was maximum in the presence of small amount of oxygen (500 ppm ~ 2% by volume), and further increase in the oxygen content decreased the destruction. To examine its effect on the trichloromethane destruction, the oxygen content was changed from 0.5 to 10.0% by volume with the flow rate of the simulated exhaust kept at 2 L min−1. Figure 4 (a), (b) show the destruction efficiencies obtained with the dielectric-packed bed reactor and the DBD reactor with perforation, respectively. As can be seen, the decrease in the oxygen content resulted in the increase in the destruction efficiency. The effect of the oxygen content was more significant for the DBD reactor with perforation, but at oxygen contents of 0.5–1%, both reactors showed similar performance around 97% destruction efficiency at input power of 47–51 W. The lower destruction efficiency at higher oxygen content may be because oxygen and oxygen radical consume the reactive species such as excited nitrogen molecules and nitrogen atoms produced by the discharge plasma, otherwise to be used for destroying trichloromethane.
Referring to the reaction mechanism for the destruction of dichlorodifluoromethane [11], dichloromethane [6], and trichloroethylene [21] the reactions for the destruction of trichloromethane can be written as
where N2(A) stands for metastable N2(A3Σu +). Oxygen atom can also destroy trichloromethane by abstracting hydrogen atom as follows:
But it should be noted that the contribution of oxygen atom to the destruction is not significant because the major constituent in the simulated exhaust gas is nitrogen. Figure 5 (a) shows the mean electron energy calculated by the BOLSIG code (Siglo) at oxygen content 10%, and Fig. 5 (b) the rate constants for electron-molecule collision processes that were calculated using the data reported by Kulikovsky [22]. In Fig. 5 (a), Td for reduced electric field is equal to 10−17 V cm2. Although the rate constants for the electron-oxygen collisions are somewhat larger than those for the electron-nitrogen collisions, it is easily understood that the metastable N2(A) is the most abundant reactive species responsible for the destruction of trichloromethane since the oxygen content is much lower than that of nitrogen. In this context, reaction (1) can be regarded as the main reaction to initiate the destruction. When oxygen is present, the reactive species capable of destroying trichloromethane are consumed as below
Reactions (6)–(10) get faster as the oxygen content increases, which in turn decreases the rates of reactions (1)–(4). That is why higher oxygen content resulted in lower destruction efficiency.
The oxygen content also affects the formation of nitrogen oxides, as shown in Fig. 6. Both plasma reactors showed similar behavior in the formation of NO2, indicating that it depends mainly on the input power when the other conditions are maintained constant. In both reactors, NO was not detected at all oxygen contents explored. As well known, NO formed by reactions (8) and (9) is oxidized to NO2 by O and O3 [23–25]. Besides, the intermediates such as CHCl2 and CCl3 formed by reactions (1)–(5) can also take part in the oxidation of NO to NO2 in the presence of oxygen. Oxygen reacts with CHCl2 and CCl3 to form peroxy radicals:
which can then decompose via various reactions or oxidize NO to NO2 [6, 26, 27]
Unlike NO2, the formation of N2O did not largely depended on the oxygen content, and the amount of N2O formed was different when the reactor configuration was different. The reason that the dielectric-packed bed reactor produced more N2O can be explained by the lattice oxygen in the dielectric pellets. Ogata et al. [10, 28] have shown that lattice oxygen atoms can be released from the surface of dielectric pellets under plasma discharge to form N2O and CO, which agrees with our results.
The FTIR spectra of the simulated exhaust gas obtained by changing the input power from 18 to 48 W are presented in Fig. 7. Besides NO2 and N2O discussed above, CO, COCl2, and CO2 were identified from the spectra, implying that the intermediates CHCl2O and CCl3O formed by reactions (14)–(16) further decompose [26, 27]:
Reactions (18)–(25) inform that various byproducts including CO, COCl2, HCl, and Cl2 can be formed in the plasma reactor. Considering the reactions discussed above, COCl2 is a key intermediate compound to produce CO and Cl2 via reactions (22)–(25). Figure 8 compares the concentrations of several byproducts formed in the dielectric-packed bed reactor with those in the DBD reactor with perforation. As expected, CO and Cl2 were found to be the main byproducts in both plasma reactors, and the concentration of COCl2 was relatively low.
Figure 9a, b show the distribution of carbon-containing byproducts as a function of oxygen content, where the amount of trichloromethane removed is also given to account for the carbon balance. In these figures, the white circles indicate the sum of the identified carbons, which was somewhat smaller than the amount of the trichloromethane removed. The missing carbons corresponding to the difference between the sum of the identified carbons and the trichloromethane removed appeared to decrease as the oxygen content increased because the intermediate products were oxidized more rapidly at higher oxygen content. Although it was not discussed in this work, a part of trichloromethane can be converted into other chlorinated carbons like CCl4 [19], which can account for the missing carbons. Unlike the carbon balance, the chlorine balance was not quite good, as shown in Fig. 10. The chlorine-containing byproducts detected in this work are COCl2, Cl2, and HCl, which account for about one-half of the chlorine removed. This result implies that considerable amount of chlorine was converted into other species such as Cl2O, HOCl, CCl4, NCl, etc.
Figure 11 describes the reaction pathways for the destruction of trichloromethane, which are suggested from the byproducts identified in this work. The destruction is initiated by the collisions of trichloromethane molecules with the excited nitrogen molecules. Chlorine atom released from trichloromethane and nitrogen atoms can also participate in the destruction, forming HCl, Cl2, and nitrogen chloride (NCl). NCl formed by reaction (2) decomposes to give NO and COCl2 as [27]
As described above, oxygen reacts with CHCl2 and CCl3 to produce peroxy radicals, which decompose via various reactions or oxidize NO to NO2. Succeeding reactions of CHCl2O and CCl3O produce various byproducts including CO, COCl2, and Cl2. Though the destruction of trichloromethane is mainly initiated by active nitrogen species, oxygen is a necessary constituent to oxidize the intermediate species to carbon oxides. But, the content of oxygen should be maintained as low as possible in terms of the destruction efficiency.
Conclusions
The main conclusion drawn from this work may be summarized as follows. Among the three nonthermal plasma reactors investigated, the dielectric-packed bed reactor evidently showed the best performance in the destruction of trichloromethane. The oxygen content in the simulated exhaust gas largely affected the destruction efficiency, obviously because excited nitrogen molecules and nitrogen atoms responsible for the destruction of trichloromethane were dissipated by oxygen species such as O2, O, and O3. The formation of NO2 was also affected by the oxygen content while the formation of N2O was not largely dependent on it. The major byproducts formed from trichloromethane were found to be CO and Cl2. The byproducts identified suggest that the intermediates such as CHCl2 and CCl3 formed from the dissociation of trichloromethane by metastable nitrogen molecules and nitrogen atoms react with oxygen to produce peroxy radicals, and then decompose to CO, COCl2, and Cl2 via various reactions.
References
Sun JW, Park DW (2003) Korean J Chem Eng 20:476
Sichler P, Büttgenbach S, Baars-Hibbe L, Schrader C, Gericke K-H (2004) Chem Eng J 101:465
Kim HH (2004) Plasma Proc Polym 1:91
Mizeraczyk J, Jasinski M, Zakrzewski Z (2005) Plasma Phys Control Fusion 47:B589
Kuroki T, Mine J, Okubo M, Yamamoto T, Saeki N (2005) IEEE Trans Ind Appl 41:215
Fitzsimmons C, Ismail F, Whitehead JC, Wilman JJ (2000) J Phys Chem A 104:6032
Kang HC (2002) J Ind Eng Chem 8:488
Ricketts CL, Wallis AE, Whitehead JC, Zhang K (2004) J Phys Chem A 108:8341
Futamura S, Gurusamy A (2005) J Electrostat 63:949
Ogata A, Kim HH, Futamura S, Kushiyama S, Mizuno K (2004) Appl Catal B Environ 53:175
Wallis AE, Whitehead JC, Zhang K (2007) Catal Lett 74:29
Yu SJ, Chang MB (2001) Plasma Chem Plasma Proc 21:311
Mok YS (2006) Plasma Sci Technol 8:661
Oda T, Takahashi T, Nakano H, Masuda S (1993) IEEE Trans Ind Appl 29:787
Yamamoto T, Mizuno K, Tamori I, Ogata A, Nifuku M, Michalska M, Prieto G (1996) IEEE Trans Ind Appl 32:100
Chen HL, Lee H-M, Cheng LC, Chang MB, Yu SJ, Li S-N (2008) IEEE Trans Plasma Sci 36:509
Park JY, Jung JG, Kim JS, Rim G-H, Kim K-S (2003) IEEE Trans Plasma Sci 31:1349
Kim Y, Kim K-T, Cha MS, Song Y-H, Kim SJ (2005) IEEE Trans Plasma Sci 33:1041
Föglein KA, Szabó PT, Babievskaya IZ, Szépvölgyi J (2005) Plasma Chem Plasma Proc 25:289
Song HK, Choi J-W, Lee H, Indarto A (2005) Toxicol Environ Chem 87:509
Futamura S, Yamamoto T (1997) IEEE Trans Ind Appl 33:447
Kulikovsky AA (2001) IEEE Trans Plasma Sci 29:313
Mok YS, Kim JH, Ham SW, Nam I (2000) Ind Eng Chem Res 39:3938
Tas MA, van Hardeveld R, van Veldhuizen EM (1997) Plasma Chem Plasma Proc 17:371
Sathiamoorthy G, Kalyana S, Finney WC, Clark RJ, Locke BR (1999) Ind Eng Chem Res 39:1844
Biggs P, Canosa-Mas CE, Percival CJ, Shallcross DE, Wayne RP (1999) Int J Chem Kinet 31:433
National Institute of Standards and Technology Chemical Kinetics Database: Version 2Q98 (1998)
Ogata A, Mizuno K, Kushiyama S, Yamamoto T (1999) Plasma Chem Plasma Proc 18:363
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mok, Y.S., Lee, SB., Oh, JH. et al. Abatement of Trichloromethane by Using Nonthermal Plasma Reactors. Plasma Chem Plasma Process 28, 663–676 (2008). https://doi.org/10.1007/s11090-008-9151-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-008-9151-1