Abstract
In this present research work, we report the preparation of successfully synthesized ZnO films as pristine and doped with Al(Al-ZnO) on glass via facile, eco-friendly, and controllable SILAR method. A systematic evolution of structural, surface morphology, composition, photoluminescence and ammonia gas sensing behaviour of the system was investigated with a variation of Al dopant. XRD examination disclosed polycrystalline nature with the hexagonal system of all films and crystallite size was noticed between 37 and 51 nm. EDX study approves the presence of Al doping in ZnO. Surface morphological tests through SEM presented the formation of nanoparticles and nanorods with a variation of Al content. The photoluminescence study revealed that due to Al doping the PL intensity was quenched which signifies the reduction of defects in the films. It was shown that the estimated values of the energy gap are enlarged to 3.12 from 3.01 on rising the Al content till 3wt.% and finally decreased for 5wt.% Al content. The gas sensing analysis showed that Al doping content was made to drastically increase the gas sensing response. Compared with other dopant levels, the 3wt.% Al-ZnO nanorods unveiled the uppermost retort when tested to 100 ppm ammonia (NH3) gas concentration at room temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Environmental pollution and various hazards gases released from various chemical factories initiate the scientists to advance chemical sensors able to precisely monitor and alert for industrial and technological related systems in real-time. These developed sensors also can be properly utilized to prevent fire or explosion. Currently, nanostructured metal oxides have drawn significant attention in the field of nanotechnology for making active sensors to solve environmental issues. Semiconducting metal oxide nanostructures (SMONs) are currently focused extensively and plays a key role in enhanced gas sensing. These nanostructures are conventional for resistance type gas sensors (Li et al. 2019). When exposed to hazardous gases Nanostructured materials exhibit higher sensitivity and rabid response under relatively low operating temperatures. This fast response might be owed to a large surface/size ratio of nanomaterials particularly in nanoscale, that enhance response kinetics of surface through growing the no. of adsorptions centers on the surface of the sensor coating (Dilova et al. 2020). Reducing the grain size to nanoscale may lead to induce new effects such as bandgap widening, room temperature (RT) photoluminescence, etc. due to the quantum confinement (Viter and Iatsunskyi 2019). It is worthy to note that compared to bulk counterparts these nanostructured materials have extraordinary unique and enhanced chemical, physical, and mechanical properties (Nunes et al. 2019). Different morphological structures and shapes also have attained significant interest in employing novel type gas sensor (Aslani 2011). Various patterns like nanoparticles, nanoarrays, nanowires, nanosheets, nanorods, nanotubes, tower-like structures, nanobelts, nanosprings, nanocombs, nanorings and nanofilms, are the latest progress for developing or designing advanced chemical gas sensor (Zhang and Gao 2019; Schmidt-Mende and MacManus-Driscoll 2007). However, for real usages, the key task is focused on discovering low cost, high performance, the sensitive and scalable fabrication process for the manufacture of metal oxide gas sensors.
ZnO is a talented, supreme, and trustworthy material for the above-mentioned purpose. In addition ZnO has prospective application in many fields such as piezoelectric devices (Qin et al. 2016), spintronics (Shaaban et al. 2019), acoustic waveguides (Fu et al. 2010), light emitting diode (Kwon et al. 2018), Photodetector (Liu et al. 2009) photocatalyst (Binjing et al. 2019), solar cells (Linhua et al. 2018), nonlinear characteristics (Sofiani et al. 2007; Abed et al. 2015), etc. Recently ZnO got immense consideration in the gas sensitivity field because of its explicit chattels, like finest conductivity/low toxicity/cost/chem-thermosolidity (Dilova et al. 2020). It also possesses higher mobility, lifetime, and intrinsic impurities. To enhance gas sensitivity Vo, Zni, and Oi are possible defects for ZnO material (Schmidt-Mende and MacManus-Driscoll 2007). Nanostructures point of view also it can be easily grown with a variety of nanostructures. ZnO nanostructures have been extensively utilized for proving their excellency in detecting plenty of gases, like H2/CO/NO2/NH3/H2S/ethanol/acetone etc. (Dilova et al. 2020). The aforementioned gases ammonia is mostly considered as one of the highly toxic gas because of long duration NH3 inhalation primes to demise (Liu et al. 2019).
Further, based on ZnO-gas sensing devices possess some drawbacks as well like operation at high temperature/limited detection content/selectivity, etc. (Eisele et al. 2001; Stegmeier et al. 2011). Hence, such disadvantages of such sensors limit their use in commercially. To overcome this issue various techniques like doping has been implemented to operate at room temperature in order to surge the efficacy/choosiness and sensitivity of ZnO based sensors. It is worth mentioning that thin film gas sensors that are operated at room temperature (RT) have many advantages like less power consumption, a long lifetime, and catching fire is not possible (Imad and Kadhim 2016). Generally, group III dopants (transition metals) as Al, Ga, In, Mn, Co, Cr, Ni, Fe, and Cu have been suitably added to improve gas sensing properties. These dopants can boost the oxygen type for sensing and expand gas molecules adsorption (Sankar Ganesh et al. 2017e). Recently, Al dopant is an important candidate, which plays a key role in making ZnO-based gas sensors. This is because Al dopant offers improvement in the gas detection properties of ZnO by changing the energy-band structure, surface morphology, bandgap, transparency, resistivity or conductivity. This alternation is related to small ionic radii of Al3+ ion related to Zn2+ion (Aydın and Yakuphanoglu 2019), hence it is called as fast diffusion dopant.
In the past few efforts have been taken by numerous research workers to sense the leakage of various gases by appropriate doping in ZnO. Zhang et al. prepared Al-doped narcissus-like ZnO for NO2 gas by hydrothermal method. The narcissus-like structure showed a fast response and recovery for the lower operating temperature of 240 °C (Zhang et al. 2019). Ghosh et al. tuned the visible photoluminescence in Al-doped ZnO thin film for detecting the glucose on the basis of quenching in fluorescence. Advanced sensitivity was attained by structure tuning (2018). Sheeba et al. (2017) studied sensing of LPG by a clear Al:ZnO films obtained by the thermo-evaporation process, an appreciable response time of 40 s was achieved for 3 wt.% Al-doped ZnO. Benelmadjat et al. (2013) fabricated highly oriented Al and Al/Sb:ZnO films for H2 gas on Si wafer by sol–gel method. Rabid responds to the low H2 concentrations were noted. Gas detecting action of Al-ZnO film for H2S recognition was systematically studied by Kolhe et al. (2018) the Al dopant increased the oxygen vacancy related defects, which enhances the gas response at a minimum operating temperature of 200 °C. Madhumita Sinha et al. synthesized quick response/sensing at a low temperature of acetone/ethanol by Al:ZnO, they achieved acetone detection at RT and ethanol detection at a low temperature of 150 °C (Sinha et al. 2020). Aydınk et al. (2019) studied the ammonia sensing through Al doping into ZnO for different ppm level in which 5 wt.% Al-doped films operated at room temperature shows the fast response for 660 ppm. Ravichandran et al. (2019) prepared W:ZnO films for sensing of ammonia gas by spray technique, in which 1% tungsten shows a fast response towards 100 ppm ammonia level operated under room temperature.
Up to our knowledge, only a few scientific reports are available for Al-ZnO films which could be operated at RT to detect NH3 gas. Moreover, fast response and recovery time is necessary to detect a low-level concentration of such harmful gas. So, to achieve these two objectives, we synthesized Al-ZnO films for the NH3 sensor by the SILAR method. Compared with several other techniques SILAR technique is preferred most because this promising route has the following advantages such as simplicity, non-hazardous, inexpensive, less time consuming, does not require sophisticated instruments and high-quality substrates, easy route for the introduction of different dopants, suitable for large areas deposition and also to build functional structures (Deshmukh et al. 2018; Raut et al. 2017; Omran et al. 2019). Si, current work is focuses to fabricate Al-ZnO films via SILAR process and investigate their characteristics towards ammonia gas sensing.
2 Investigational procedures
2.1 Preparation on Al-ZnO thin films
Here SILAR technique was adopted for the deposition of Al-ZnO thin films on glass slides. Zinc sulfate [ZnSO4], NaOH, and Hexahydrate aluminium chloride (AlCl3 0.6H2O) were collected from locally available commercial markets in India and they were used without further purified. In order to remove the visible dirt, all the glass slides were initially cleaned with soapy water. Then they were successively washed with chromic acid, well rinsed with deionized water and then dried.
To deposit the films the cleaned substrates were vertically dipped into the sodium zincate solution, which can be prepared by mixing 0.1 M ZnSO4and 0.2 M NaOH. Before dipping the temperature of this solution was maintained at 90 °C. The pH of sodium zincate solution bath was maintained at 9. After immersing the substrate for 10 s in a sodium zincate bath, it was immersed in hot water (90 °C) for 10 s. This course was repetitive for 80 times to get pristine ZnO films. Aluminium doping was done by adding hexahydrate aluminium chloride at different proportions of Al, such as 0, 1, 3, and 5wt.%. After the completion of the dipping cycles, the substrates drying was done in the air and then annealed at 400 °C for about 2 h.
The equations for the formation of ZnO thin films are as follows:
2.2 Characterization
The prepared high-quality ZnO thin films were analyzed and recorded to recognize their phase, morphology, composition, and most importantly ammonia gas sensing behaviors affected by Al content. The structure information of undoped and Al-ZnO films was obtained by XRD (Bruker D8 Advance Eco XRD). A scanning electron microscope (EVO18 CARL ZEISS) was employed to inveterate the evolution of the morphology of samples. The room temperature Photoluminescence (PL) data were collected using a Varian Carry Eclipse PL Spectrometer. The optical properties were accessed through Ocean Optics HR 2000 optical fiber-based spectrophotometer. Stylus profile meter (Mitutoyo SJ-301) was employed to determine the thickness of prepared films and are noted to ∽ 920, 980, 1020, and 880 nm for 0, 1, 3, and 5 wt.% of Al dopings, respectively.
3 Result and conversation
3.1 XRD studies
Figure 1 depicted detailed XRD profiles of pristine and Al-ZnO films. From Fig. 1 we noted that the nature of all films is polycrystalline and exhibited multiple peaks with hexagonal structure. Moreover, it is strongly oriented along the (002) direction. This strongest peak is usually observed in ZnO thin films suggesting that free energy of the surface of (002) plane is the lowermost in ZnO films (Fidal and Chandra 2018; Taabouche et al. 2016). The other diffraction peaks correspond to (100), (101), (102), (112), (103) (112) and (201) orientations also appeared. All the diffraction peaks of ZnO which matched with the JCPDS card no. 005–0664 (Linhua et al. 2014). All appeared diffraction peaks are sharp and well distinct which suggests the formation of high-quality crystalline phase of ZnO.No additional secondary phases related to Al or Al2O3 species were occurred not even for the highest proportion. Hence, we assumed that Al3+ substituted into the Zn2+ sites/incorporated interstitially in host ZnO lattice with unchanged hexagonal structure crystal structure (Humayan Kabir et al. 2018). This may indicate that the precursors have been fully converted into ZnO phase (Sinha and Mahapatra 2020). The intensity of (002) and (101) planes increases with increasing the Al concentration up to 3% doping concentration confirms the incorporation of Al atoms into ZnO lattice and its crystallite size was increased. Furthermore, for 5wt.% Al concentration the peak intensity is considerably decreased due to Al atoms placed in interstitial position or surface of the grains causes structural disorder in the crystal structure. A decrease in the crystallite size was an indication of deterioration of the crystallinity of the films. A similar type of observation was reported by Kamal Rudra et al. for Mn doping of nanostructure ZnO (Kamal Rudra and Prajapati 2020). Goktas et al. also reported the same type of observation for various transition metal dopants like Mn, Co, Fe into ZnO film (Goktasa et al. 2016).
The structural constraints like size of crystallites (D), micro strain (ε), number of dislocations (δ) and quantity of crystallites (N) were evaluated by (Deva Arun Kumar et al. 2018)
Here all codes are well-recognised.
It has been noticed that the crystallite size became bigger when the concentration of Al reached 3wt.% then leads to a decrease for 5wt.% Al level. The undoped ZnO possesses the smallest crystalline size. The FWHM of XRD peaks decreases firstly then rises on rising of Al impurity content, which supports the variation of crystallite size. The calculated crystallite size is comparable with previously reported values by Zaiour et al. (2019). The calculated microstrain and dislocation density values are low for 3wt.% Al content which indicates the formation of fewer defects. The N values initially declined and finally enlarged with Al impurity. Table 1 shows the calculated structural parameter values.
The cell volume ‘V’ and lattice constants ‘a’ and ‘c’ are evaluated for (002) peak by Vijayprasath et al. (2014).
here (hkl) and d have their usual meanings. The calculated lattice constants ‘a’, ‘c’ and ‘V’ for Al- ZnO films were depicted in Fig. 2b revealed that the cell volume has been decreased with Al doping and the highest value was noticed for undoped ZnO. It is noticed that a rise in Al content roots the decrement in both a and c parameters. This is due to the small ionic radius of Al3+ (0.68 Å) to the ionic radius of Zn2+ (0.74 Å). The change in the lattice parameter suggesting the formation of uniform strain (Hong and Choi 2018). The estimated lattice parameter values comparable with earlier data reported by Osali et. al. (a = 3.25 Å and c = 5.207 Å) (Osali and Karami 2018).
3.2 Morphological studies
Figure 2a–d provided the surface morphological images of as-deposited Al-ZnO films. Some notable changes between the samples were observed as the doping percentage of Al content increases. It can be observed that the surface of the un-doped film has been covered with nano. From Fig. 2b, it is observed that film contains small size nanowire like morphology. However, with 3wt% Al doping, the film morphology is completely nanowire shape [see Fig. 2c] is very clear, which may be due to higher concentration doping of Al. The 5% doped film exhibits an entire wire-like nanostructure and its size is higher than previous samples. It is worthy to note the increment in the length of the nanowire has been observed. Moreover, a small difference was observed in all the upper parts of the fabricated films. The highly packed particles are observed on the surface of pure ZnO film along with few particles on top leads to agglomerate. These suggest that Al impurity is deposited preferably on the upper parts of ZnO which leads to the formation of a nano wire-like structure. Yuanyuan Lv et al. (2018) fabricated ZnO Nanowire by influencing Al dopant into the host lattice of ZnO. Antaryami Mohanta et al. (2019) also prepared ZnO nanowires through Al addition. F:ZnO NWs achieved by plasma action was reported by Wang et al. (2018). Kim et al. (2019) formed Pd on ZnO NWs for improved sensitivity/selectivity towards H2 gas. Room temperature gas nanosensors based Au-modified ZnO nanowires synthesized by Lupan et al. (2019). Mohd Arif et al. (2019) applied Sol–gel spin coating to fabricate the pure and different concentrations of aluminum-doped ZnO nanostructure. Sankar Ganesh et al. (2017e) tune the response of NH3 sensing through Cu-in ZnO. Hence it is worth to our nano wire-like morphology results also resembles these results.
The elemental analysis of Al-doped thin films was achieved by EDX is projected in Fig. 3. The EDX spectrum obtained for the SILAR prepared ZnO thin film deposited with 3wt.% Al which confirmed that Zn, O, and Al elements have emerged within the structure. The appearance of additional peaks may be owing to the glass substrate.
3.3 Photoluminescence studies
PL spectroscopy is an inevitable tool to analyze Photoluminescence behavior because it furnishes more information about sensing behaviors, point defects, and band edge emissions. Figure 4 displays RT PL spectra of Al doped ZnO films, which signify that luminescence character dependency on Al content. On increasing Al content emission intensity reduced to 3 wt.%, however, increased for 5 wt.%. Two intense PLE bands exist in all films from which one is for NBE occurs owing to recombining the excitons among CB and VB. Figure 4 contains 4 sharp peaks at 407, 420, 486, and 517 nm and two small peaks at 444, and 459 nm total of six defects were presented. Generally, six kinds of intrinsic imperfections are likely names as VO, Zni, Oi, ZnV, and Zn/O antisite (Nkosi et al. 2020). Moreover, it is worthy to note that the presence of defects and vacancies are beneficial for gas sensing applications (Patil et al. 2019). The PLE band at ~ 406 nm is for NBE, the other PLE bands at 458 and 484 nm are ascribed to Zni and at 540 nm is for Vo. The low-intensity blue PLE at 445 nm is for Zni, an intense PLE band at 518 nm (green) is for Vo (Anita et al. 2016; Renitta and Vijayalakshmi 2017). Normally higher the ZnO crystal quality possesses stronger UV emission (Ahmed et al. 2019). It can be known from XRD patterns that 3 wt. %Al doped ZnO shows the strongest diffraction peak; e.g. some ZnO with better crystallinity like NRs, NWs possess an exceptional UV emission act (Linhua et al. 2019).
3.4 Optical studies
A detailed study on optical transmittance can be used to analyze the effect (optical energy bandgap, optical transitions) of Aluminium dopant on the optical nature of ZnO film. Figure 5a demonstrates the optical transparency for Al-ZnO films prepared with various Al concentrations. All samples exhibit average transmittance values between 30 and 82%, depending on the dopant concentrations in the visible wavelength region. While increasing Al in ZnO the transparency is enhanced significantly in VIS-region. The 3 wt.% Al-ZnO film exhibits the highest transmission, whereas the lowest one around 30% is shown by the undoped film. This maximum optical transmittance spectrum specified that the Al-ZnO films can have good structural homogeneity (Hou and Jayatissa 2017). Compared to 5% Al-ZnO films, the film doped with 3 wt.% shows slightly increasing transmittance values shows a maximum optical transmission which indicates the degradation in the crystal quality for higher doping. It is matched with XRD results. These transmittance findings are perfectly matched with the workers Musaab Khudhr et al. (xxxx), Zhai et al. (2016), and Mkawi et al. (2015). Further, a drop in T is observed in all the prepared films at around 400 nm. This drop may be due to the fundamental absorption by excitonic transitions.
The Eg values were estimated via Tauc’s rule (Firdous et al. 2013)
Here, α is the coefficient of absorption, Eg is the direct energy gap, and B is a constant. The inset in Fig. 5b displayed the attained Eg of all Al-ZnO films. As can be seen from Fig. 5b the Eg value of undoped ZnO film was found to be equal to 3.01 eV. When the Al concentration is increased up to 3wt.% the Eg value is enlarged from 3.01 to 3.12 eV then finally decreased for a higher doping level. This result is in accordance with the report of Rajendra Prasad et al. (Rajendra Prasad and Haris 2018).
3.5 Gas sensing studies
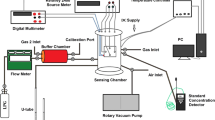
An indigenously designed set-up (Keithley) was employed to investigate the sensing act of undoped and Al-ZnO films. A High-quality Ag paste was used to make contacts on the surface. The sensing element was kept in the sensor testing chamber. Baseline resistance was noted at RT for the Al-ZnO sensor by keeping an open chamber to allow atmosphere air. Then ammonia is injected by syringe to the tube which is connected by a heater. Ammonia vapour is produced by applying the required heat to the tube that relates to film and so lessening resistance. All sensor-related tests are done at RT by keeping the sensing area constant. For sensing of gas, the applied voltage was fixed at 5 V. Sensitivity is defined by Sivalingam et al. (2012): S = Ra/Rg, where Ra and Rg are strengths of air and gas (Table 2)
.
Figure 6 reveals the response/recovery of Al–ZnO (0, 1, 3, and 5wt. %) films for 100 ppm of NH3 gas. The observed timings of responses are 52, 47, 34, and 39 s, and recoveries are 11, 10, 6 and 9 s for Al–ZnO (0, 1, 3, and 5wt. %) samples, respectively. The observed response time initially increased on rising Al doping content in ZnO and maximum response time was observed for 3 wt% Al-doped film due to high crystalline and nanowire structure. The sensitivity of the samples is 547, 6470, 10,700, and 12,300 for Al (0, 1, 3, and 5wt. %) doped ZnO film. The excellent sensitivity observed for Al 3wt.% film rather than other doped films due to its high crystalline quality and nanowire structure is suitable for diffusion and interaction of more ammonia vapours. Figure 7 elaborates sensing features of 3wt.% Al-ZnO film for diverse contents of NH3 at RT operating condition. Sensitivity increases as increasing ammonia vapour ppm from 100 to 200. It is clear from the observations that the amount of vapour interact on the surface of Al-ZnO films is a vital constraint governing the response. It is also confirmed from the figure that Al-ZnO display an enhancement in the sensitivity and high selectivity for ammonia.
The observed value of response and recovery time of present work is compared with other works. Sankar Ganesh et al. (2017d) synthesized enriched of NH3 gas sensor as Ag/ZnO by hydrothermal method. They showed that the addition of Ag exhibited quick response/recovery timings of 13/20 s for operating temperature 150 °C. On the other hand, Fairose et al. (2019) lessened the response/recovery timing to 8/37 s through a nanostructured ZnO sensor by the SILAR method but the operating temperature of the sample was 200 °C which is higher than our work. Ravichandran et al. (2019) studied room temperature ammonia vapour sensing ability for tungsten doped ZnO. The loaded tungsten showed a response and recovery time of 293 s and 13 s for the 100 ppm level. This achievement was due to a greater number of Vo and Zi these are the key reasons for the enrichment of sensing capability. But Kulandaisamy et al. (2016) reduced the response and recovery time up to 34 s and 28 s by introducing Mg into ZnO host lattice. This room-temperature sensing nature might be because of lesser grain size. Ganesh Kumar Mani et al. (2014) attained much better performance than those already discussed so far with a quicker response/recovery of 30/14 s for room temperature ammonia sensor for 50 ppm. Smaller grain size reflected faster response/recovery time. Thus, compared to the above-mentioned reports the Al-ZnO film is showing a best response/recovery time of 34/9 s for 100 ppm. This fast response is might be because of the generation of nanowire like morphology formed by Al content. Mariappan et al. (2014) reported Na-ZnO NRs films by NSP and their HN3 sensing activity. The uniformly distributed nanorods show the sensing response of 1.7 for 500 ppm operated at 400 °C. Sankar Ganesh et al. (2017a) increased the sensitivity of 15.1 for the operating temperature of 400 °C when exposed to 100 ppm. It was due to enhanced catalytic activity. On the other hand Pt nanoparticles (NPs) are deposited on a sputtered aluminum-doped zinc oxide exhibited a sensing response of 2183 once exposed to 1000 ppm but the working temperature was 300 °C by Chen et al. (2018). Mani et al. (2015) presents a very selective co-doped ZnO RT NH3 sensor. Its sensing response was 225. Sankar Ganesh et al. (2017b) investigated the influence of Al doping on RT ammonia sensing by ZnO nanorods. It displayed a fast sensing response of 350 owed to the existence of frequent Vo, which indorses oxygen adsorption. In current, we accomplished a high response of 12,300 towards RT NH3 sensing due to the nanostructure of nanowire-like structure. Furthermore, we have listed some work that was reported and published already.
3.5.1 Sensing mechanism
Figure 8 presents the mechanism for NH3 sensing towards pure and 3wt. % Al-ZnO films. The sensing of NH3 behavior is primarily built on chemical reactions amongst test gas and targeted specimen. This process might be discussed in 2 main steps. Initially, when the undoped ZnO thin film was bare to ambient air, oxygen molecules were captured by electrons apprehending from CB of ZnO. As a result, the width of the potential barrier changed which leads to an increase of resistance. It is expressed through Eq. (8), once plummeting gas like NH3 is exposed to ZnO films surface, NH3 vapours interact its surface adsorbed oxygen, and electrons gets freed to surface, thus reduces the width of the potential barrier. Consequently, the resistance decreases and gas sensing response increases. This express able by Eq. (9) (Rana and Rajasree 2016; Kanaparthi and Singh 2020).
Similarly, 3wt.%Al-doped ZnO film contains more nanowires causes, more oxygen species can interact than undoped ZnO film thus the depletion layer width is reduced. Hence, owing to its thin depletion layer, additional electrons are injected when NH3 reacts with oxygen species.
4 Conclusions
In this present work, we report the sensor-based Al-doped ZnO films using the SILAR route. Aluminium ions were successfully incorporated into the ZnO structure which was confirmed from the XRD and further confirmation was carried out by EDX. The morphological analysis approves the development of nanowire like structure formed by dopant impurity which is favorable for sensing. The evaluated Eg varied from 3.01 to 3.12 eV on rising the Al doping content. RT ammonia sensing analysis showed an excellent response towards ammonia. In addition, the film doped with 3wt.% displays improvement in fast response and recovery characteristics. The outcomes signify that the grown Al-doped ZnO films are of great importance for gas sensing.
References
Abed, S., Bougharraf, H., Bouchouit, K., Sofiani, Z., Derkowska-Zielinska, B., Aida, M.S., Sahraoui, B.: Influence of Bi doping on the electrical and optical properties of ZnO thin films. Superlattices Microstruct. 85, 370–378 (2015). https://doi.org/10.1016/j.spmi.2015.06.008
Adel, H., Alkhayatt, O., Jaafer, M.D., Al Alak, H.H.A., Ali, A.H.: Characterization of CuO/n–Si pn junction synthesized by successive ionic layer adsorption and reaction method. Opt. Quantum Electron. 51(7), 233 (2019). https://doi.org/10.1007/s11082-019-1951-4
Ahmed, M.A.M., Meyer, W.E., Nel, J.M.: Effect of (Ce, Al) co-doped ZnO thin films on the Schottky diode properties fabricated using the sol-gel spin coating. Mater. Sci. Semicond. Process. 103, 104612 (2019). https://doi.org/10.1016/j.mssp.2019.104612
Anita, H., Kohli, N., Singh, R.C.: Temperature dependent selective and sensitive terbium doped ZnO nanostructures. Sens. Actuators B 231, 110–119 (2016). https://doi.org/10.1016/j.snb.2016.03.001
Antaryami Mohanta, J.G., Jr, Shen, S.G., Kim, S.M., Kung, P., Everitt, H.O.: Al doping in ZnO nanowires enhances ultraviolet emission and suppresses broad defect emission. J. Lumin. 211, 264–270 (2019). https://doi.org/10.1016/j.jlumin.2019.03.049
Aslani, A.: VahidOroojpour, CO gas sensing of CuO nanostructures, synthesized by an assisted solvothermal wet chemical route. Phys. B 406, 144–149 (2011). https://doi.org/10.1016/j.physb.2010.09.038
Aydın, H., Yakuphanoglu, F., Aydın, C.: Al-doped ZnO as a multifunctional nanomaterial: Structural, morphological, optical and low-temperature gas sensing properties. J. Alloys Comp. 773, 802–811 (2019). https://doi.org/10.1016/j.jallcom.2018.09.327
Benelmadjat, H., Boudine, B., Keffous, A., Gabouze, N.: Photo response and H2 gas sensing properties of highly oriented Al and Al/Sb doped ZnO thin films. Mater. Int. 23(6), 519–523 (2013). https://doi.org/10.1016/j.pnsc.2013.11.001.
Binjing, Hu., Sun, Q., Zuo, C., Pei, Y., Yang, S., Zheng, H., Liu, F.: A highly efficient porous rod-like Ce-doped ZnO photocatalyst for the degradation of dye contaminants in water. Beilstein J. Nanotechnol. 10, 1157–1165 (2019). https://doi.org/10.3762/bjnano.10.115
Chen, H.-I., Chi, C.-Y., Chen, W.-C., Liu, I.-P., Chang, C.-H., Chou, T.-C., Liu, W.-C.: Sens. Actuators B: Chem. 267, 145–154 (2018). https://doi.org/10.1016/j.snb.2018.04.019
Deshmukh, S.G., Kheraj, V., Panchal, A.K.: Preparation of nanocrystalline CdS thin film by successive ionic layer adsorption and reaction (SILAR) method. Mater. Today: Proc. 5 21322–21327 (2018). https://doi.org/10.1016/j.matpr.2018.06.535
Deva Arun Kumar, K., Ganesh, V., Valanarasu, S., Shkir, M., Kulandaisamy, I., Kathalingam, A., AlFaify, S.: Effect of solvent on the key properties of Al doped ZnO films prepared by nebulized spray pyrolysis technique. Mater. Chem. Phys. 212, 167–174 (2018). https://doi.org/10.1016/j.matchemphys.2018.03.035
Dilova, T., Atanasova, G., Dikovska, A. Og., Avdeev, G., Machida, M., Terakawa, M., Stefanov, P., Nedyalkov, N.N.: Effect of Pd-decoration on the sensing properties of ZnO nanostructures. Thin Solid Films. 693, 137693 (2020). https://doi.org/10.1016/j.tsf.2019.137693.
Eisele, I., Doll, T., Burgmair, M.: Low power gas detection with FET sensors. Sens. Actuators B 78, 19–25 (2001)
Fairose, S., Ernest, S.: Nanostructured ZnO sensor fabricated by successive ionic layer adsorption and reaction method for ammonia sensing application. Phys. B: Condensed Matter 557 63–73 (2019). https://doi.org/10.1016/j.physb.2018.12.041.
Fidal, V.T., Chandra, T.S.: Effect of neutral red incorporation on Al-doped ZnO thin films and its bio-electrochemical interaction with NAD+/NADP+ dependent enzymes. Enzyme Microbial Technol. 116, 57–63 (2018). https://doi.org/10.1016/j.enzmictec.2018.05.008
Firdous, A., Singh, D., Ahmad, M.M.: Electrical and optical studies of pure and Ni-doped CdS quantum dots. Appl Nanosci. 3 13–18 (2013). https://doi.org/10.1007/s13204-012-0065-0.
Fu, Y., Luo, J., Du, X., Flewitt, A., Li, Y., Markx, G., Walton, A., Milne, W.: Recent developments on ZnO films for acoustic wave based bio-sensing and microfluidic applications: a review. Sensors Actuators B Chem. 143, 606–619 (2010). https://doi.org/10.1016/j.snb.2009.10.010
Ganesh Kumar Mani, John Bosco Balaguru, R.: Influence of copper doping on structural, optical and sensing properties of spray deposited zinc oxide thin films. J. Alloy. Compd. 582, 414–419 (2014). https://doi.org/10.1016/j.jallcom.2013.07.146
Ganesh Kumar Mani, John Bosco Balaguru Rayappan: A highly selective and wide range ammonia sensor—nanostructured ZnO: Co thin film. Mater. Sci. Eng. B 191, 41–50 (2015). https://doi.org/10.1016/j.mseb.2014.10.007
Ghosh, J., Ghosh, R., Giri, P.K.: Tuning the visible photoluminescence in Al doped ZnO thin film andits application in label-free glucose detection. Sens. Actuators B 254, 681–689 (2018). https://doi.org/10.1016/j.snb.2017.07.110.
Goktasa, A., Aslan, F., Tumbul, A., Gunduz, S.H.: Tuning of structural, optical and dielectric constants by various transition metal doping in ZnO:TM (TM=Mn Co, Fe) nanostructured thin films: A comparative study. Ceramics Int. 43, 704–713 (2016). https://doi.org/10.1016/j.ceramint.2016.09.217
Gopala Krishnan, V., Elango, P.: Influence of Ba doping concentration on the physical properties and gas sensing performance of ZnO nanocrystalline films: aautomated nebulizer spray pyrolysis (ANSP) method. Optik Int. J. Light Electron Optics 141, 83–89 (2017) .https://doi.org/10.1016/j.ijleo.2017.05.045.
Hong, M.-H., Choi, H., Shim Ii, D., Cho, H.H, Park, H.-H.: Study of the effect of stress/strain of mesoporous Al-doped ZnO thin films on thermoelectric properties. Solid State Sci. 82, 84–91 (2018). https://doi.org/10.1016/j.solidstatesciences.2018.05.010
Hou, Y., Jayatissa, A.H.: Influence of laser doping on nanocrystalline ZnO thin films gas sensors. Progr. Natural Sci. Mater. Int. 27, 435–442 (2017). https://doi.org/10.1016/j.pnsc.2017.07.002
Humayan Kabir M., Al Amin, M., Rahman, M.S., Khan, M.K.R.: Infuence of Al doping on microstructure, morphology, optical and photoluminescence properties of pyrolytic ZnO thin Þlms prepared in an ambient atmosphere. Chin. J. Phys. 56, 2275–2284 (2018). https://doi.org/10.1016/j.cjph.2018.07.004
Kadhim, I.H., Abu Hassan, H.: Room temperature hydrogen gas sensor based on nanocrystalline SnO2 thin film using sol–gel spin coating technique. J Mater Sci: Mater Electron 27(5), 4356–4362 (2016). https://doi.org/10.1007/s10854-016-4304-0
Kanaparthi, S., Singh, S.G.: Highly sensitive and ultra-fast responsive ammonia gas sensor based on 2D ZnO nanoflakes. Mater. Sci. Energy Technol. 3, 91–96 (2020). https://doi.org/10.1016/j.mset.2019.10.010
Kim, J.-H., Mirzaei, A., Kim, H.W., Kim, S.S.: Pd functionalization on ZnO nanowires for enhanced sensitivity and selectivity to hydrogen gas. Sens. Actuators: B. Chem. 297, 126693 (2019). https://doi.org/10.1016/j.snb.2019.126693
Kolhe, P.S., Shinde, A.B., Kulkarni, S.G., Maiti, N., Koinkar, P.M., Sonawane, K.M.: Gas sensing performance of Al doped ZnO thin film for H2S detection. J. Alloys Comp. 748, 6–11 (2018).https://doi.org/10.1016/j.jallcom.2018.03.123.
Kulandaisamy, A.J., Reddy, J.R., Srinivasan, P., Jayanth, Babu K., Mani, G.K., Shankar, P., Rayappan, J.B.B.: Room temperature ammonia sensing properties of ZnO thin films grown by spray pyrolysis: Effect of Mg doping. J. Alloys Compounds 688, 422–429 (2016). https://doi.org/10.1016/j.jallcom.2016.07.050
Kwon, D.-K., Porte, Y., Myoung, J.-M.: Fabrication of ZnO nanorods p–n homojunction light-emitting diodes using Ag film as self-doping source for p-type ZnO nanorods. J. Phys. Chem. C 122, 11993–12001 (2018). https://doi.org/10.1021/acs.jpcc.8b02330
Linhua Xu, Shaorong Xiao, Chengyi Zhang, Gaige Zheng, Jing Su, Lilong Zhao, Junfeng Wang.: Optical and structural properties of Sr-doped ZnO thin films. Mater. Chem. Phys. 148 720e726 (2014). https://doi.org/10.1016/j.matchemphys.2014.08.040.
Linhua, Xu., Miao, J., Chen, Y., Jing, Su., Yang, M., Zhang, L., Zhao, L., Ding, S.: Characterization of Ag-doped ZnO thin film for its potential applications in optoelectronic devices. Optik 170, 484–491 (2018). https://doi.org/10.1016/j.ijleo.2018.06.016
Linhua, X., Wang, X., Xian, F., Jing, S.: Doping-induced transition from polycrystals to single crystals in ZnO thin films. Mater. Res. Bull. 123, 110724 (2019). https://doi.org/10.1016/j.materresbull.2019.110724
Liu, C.Y., Zhang, B.P., Lu, Z.W., Binh, N.T., Wakatsuki, K., Segawa, Y., Mu, R.: Fabrication and characterization of ZnO film based UV photodetector. J Mater Sci: Mater Electron 20, 197–201 (2009). https://doi.org/10.1007/s10854-008-9698-x
Liu, I.-P., Chang, C.-H., Chieh Chou, T., Lin, K.-W.: Ammonia sensing performance of a platinum nanoparticle-decorated tungsten trioxide gas sensor. Sens Actuators: B. Chem. 291, 148–154 (2019). https://doi.org/10.1016/j.snb.2019.04.046
Lupan, O., Vasile Postica, Thierry Pauporté, Bruno Viana, Maik-Ivo Terasa, Rainer Adelung.: Room temperature gas nanosensors based on individual and multiple networked Au-modified ZnO nanowires. Sens. Actuators: B. Chem. 299, 126977 (2019). https://doi.org/10.1016/j.snb.2019.126977
Madhumita Sinha, Rajat Mahapatra, Manas Kumar Mondal, Satheesh Krishnamurthy, Ranajit Ghosh.: Fast response and low temperature sensing of acetone and ethanol using Al-doped ZnO microrods. Phys. E: Low-Dimensional Syst. Nanostruct. 118, 113868 (2020). https://doi.org/10.1016/j.physe.2019.113868.
Mariappan, R., Ponnuswamy, V., Suresh, R., Suresh, P., Chandra Bose, A., Ragavendar, M.: Role of substrate temperature on the properties of Na-doped ZnO thin film nanorods and performance of ammonia gas sensors using nebulizer spray pyrolysis technique. JALCOM 582, 387–391 (2014). https://doi.org/10.1016/j.jallcom.2013.08.048
Mkawi, E.M., Ibrahim, K., Ali, M.K.M., Farrukh, M.A., Mohamed, A.S.: The effect of dopant concentration on properties of transparent conducting Al-doped ZnO thin films for efficient Cu2ZnSnS4 thin-film solar cells prepared by electrodeposition method. Appl Nanosci. 5, 993–1001 (2015). https://doi.org/10.1007/s13204-015-0400-3
Mhlongo, G.H., Motaung, D.E., Swart, H.C.: Pd2+ doped ZnO nanostructures: structural, luminescence and gas sensing properties. Mater. Lett. 160, 200–205 (2015). https://doi.org/10.1016/j.matlet.2015.07.139
Mohd Arif, Mohd Shkir , Salem AlFaify, Vanga Ganesh, Amit Sanger, Hamed Algarni, Paula M. Vilarinho, Arun Singh.: A structural, morphological, linear, and nonlinear optical spectroscopic studies of nanostructured Al-doped ZnO thin films: An effect of Al concentrations. J. Mater. Res. 34, 1309-1317 (2019). https://doi.org/10.1557/jmr.2018.506.
Musaab Khudhr, M., Khalid Haneen A..: Effect of Al-doping on the optical properties of ZnO thin film prepared by thermal evaporation technique. Int. J. Eng. Technol. 7, 25–31 (2016) https://www.scipress.com/IJET.7.25
Nanto, H., Minami, T., Takata, S.: Zinc oxide thin film ammonia gas sensors with high sensitivity and excellent selectivity. J. Appl. Phys. 60, 482 (1986). https://doi.org/10.1063/1.337435
Nkosi, S.S., Kortidis, I., Motaung, D.E., Kroon, R.E., Leshabane, N., Tshilongo, J., Ndwandwe, O.M.: The effect of stabilized ZnO nanostructures green luminescence towards LPG sensing capabilities. Mater. Chem. Phys. 242, 122452 (2020). https://doi.org/10.1016/j.matchemphys.2019.122452
Nunes, D., Pimentel, A., Gonçalves, A., Pereira, S., Branquinho, R., Barquinha, P., Fortunato, E., Martins, R.: Metal oxide nanostructures for sensor applications. Semiconductor Sci. Technol. 34, 043001 (2019). https://doi.org/10.1088/1361-6641/ab011e
Osali, S., Esfahani, H., Karami, H.R.: Effect of Al doping on crystallography and electro-optical properties of ZnO semiconductor thin films prepared by electrospinning. Solid State Sci. 83, 90–98 (2018). https://doi.org/10.1016/j.solidstatesciences.2018.05.016
Patil, V.L., Vanalakar, S.A., Tarwal, N.L., Patil, A.P., Dongale, T.D., Kim, J.H., Patil, P.S.: Construction of Cu doped ZnO nanorods by chemical method for low temperature detection of NO2gas. Sens. Actuators A 299, 111611 (2019). https://doi.org/10.1016/j.sna.2019.111611
Qin, W., Li, T., Li, Y., Qiu, J., Ma, X., Chen, X., Xuefeng, Hu., Zhang, W.: A high power ZnO thin film piezoelectric generator. Appl. Surf. Sci. 364, 670–675 (2016). https://doi.org/10.1016/j.apsusc.2015.12.178
Rajendra Prasad, M., Haris, M., Sridharan, M.: NH3 sensing properties of surface modified Ce-doped nanostructured ZnO thin films prepared by spray pyrolysis method. Sens. Actuators A 269 435–443 (2018). https://doi.org/10.1016/j.sna.2017.11.045.
Rana, A.K., Rajasree, D., Kumar, Y., Sen, S., Shirag, P.M.: Growth of transparent Zn1−xSrxO (0.0≤x≤0.08) films by facile wet chemical method: Effect of Sr doping on the structural, optical and sensing properties. APSUSC 379, 23–32 (2016). https://doi.org/10.1016/j.apsusc.2016.04.066
Raut, S.S., Bisen, O., Sankapal, B.R.: Synthesis of interconnected needle-like Bi2O3 using successive ionic layer adsorption and reaction towards super capacitor application. Ionics 23(7), 1831–1837 (2017). https://doi.org/10.1007/s11581-017-1994-0
Ravichandran, K., Manivasaham, A., Subha, K., Chandrabose, A., Mariappan, R.: Cost-effective nebulizer sprayed ZnO thin films for enhanced ammonia gas sensing—effect of deposition temperature. Surfaces Interfaces 1–3, 13–20 (2016). https://doi.org/10.1016/j.surfin.2016.06.004
Ravichandran, R., JansiSanthosam, A., Sridharan, M.: Effect of tungsten doping on the ammonia vapour sensing ability of ZnO thin films prepared by a cost effective simplified spray technique. Surfaces Interfaces 18, 100412 (2019). https://doi.org/10.1016/j.surfin.2019.100412
Renitta, A., Vijayalakshmi, K.: High performance hydrogen sensor based on Mn implanted ZnO nanowires array fabricated on ITO substrate. Mater. Sci. Eng. C 77, 245–256 (2017). https://doi.org/10.1016/j.msec.2017.03.234
Rudra, K., Prajapati, Y.K.: Effect of Mn doping on defect-related photoluminescence and nanostructure of dense 3-D nano-root network of ZnO. Ceramics Int. 46, 10135–10141 (2020). https://doi.org/10.1016/j.ceramint.2020.01.004
Sankar Ganesh, R., Durgadevi, E., Navaneethan, M., Patil, V.L., Ponnusamy, S., Muthamizhchelvan, C., Kawasaki, S., Patil, P.S., Hayakawa, Y.: Low temperature ammonia gas sensor based on Mn-doped ZnO nanoparticle decorated microspheres. J. Alloy. Comp. 721 182–190 (2017a). https://doi.org/10.1016/j.jallcom.2017.05.315.
Sankar Ganesh, R., Navaneethan, M., Mani, Ganesh Kumar, Ponnusamy, S., Tsuchiya, K., Muthamizhchelvan, C., Kawasaki, S., Hayakawa, Y.: Influence of Al doping on the structural, morphological, optical, and gas sensing properties of ZnO nanorods. J. Alloys Compounds 698, 555–564 (2017b). https://doi.org/10.1016/j.jallcom.2016.12.187
Sankar Ganesh, R., Durgadevi, E., Navaneethan, M., Patil, V.L., Ponnusamy, S., Muthamizhchelvan, C., Kawasaki, S., Patil, P.S., Hayakawa, Y.: Chem. Phys. Lett. 689, 92–99 (2017c). https://doi.org/10.1016/j.cplett.2017.09.057
Sankar Ganesh, R., Navaneethan, M., Patil, V.L., Ponnusamy, S., Muthamizhchelvan, C., Kawasaki, S., Patil, P.S., Hayakawa, Y.: Sensitivity enhancement of ammonia gas sensor based on Ag/ZnO flower and nanoellipsoids at low temperature. Sens. Actuators B: Chem. 255, 672–683 (2017d). https://doi.org/10.1016/j.snb.2017.08.015
Sankar Ganesh, R., Durgadevi, E., Navaneethan, M., Patil, V.L., Ponnusamy, S., Muthamizhchelvan, C., Kawasaki, S., Patil, P.S., Hayakawa, Y.: Tuning the selectivity of NH3 gas sensing response using Cu-doped ZnO nanostructures. Sens. Actuators A 269, 331–341 (2017e). https://doi.org/10.1016/j.sna.2017.11.042
Sberveglieri, G., Groppelli, S., Nelli, P., Tintinelli, A, Giunta, G.: A novel method for the preparation of NH3 sensors based on ZnO-In thin films. Chem. Sens. Actuators B 24–25 588–590 (1995).
Schmidt-Mende, L., MacManus-Driscoll, J.L.: ZnO—nanostructures, defects, and devices. Mater. Today, 10, 40–48 (2007).
Shaaban, E. R., Abbady, G.H., El Sayed Yousef, Ali, G.A.M., Mahmoud, S.A.., Afify, N.: Structural, optical and magnetic properties of Gd-doped ZnO thin films for spintronics applications. Optoelectronics Adv. Materi. Rapid Commun. 13, 235–242 (2019)
Sheeba, N.H., Vattappalam, S.C., Okram, G.S., Sharma, V., Sreenivasan, P.V., Mathew, S., Reena Philip, R.: Mater. Res. Bull. 93, 130–137 (2017). https://doi.org/10.1016/j.materresbull.2017.04.021
Sinha, M., Mahapatra, R., Mondal, M.K., Krishnamurthy, S., Ghosh, R.: Fast response and low temperature sensing of acetone and ethanol using Al-doped ZnO microrods. Physica E (2020). https://doi.org/10.1016/j.physe.2019.113868
Sivalingam, D., Jeyaprakash, B.G., John Bosco Balaguru, R.: Structural, morphological, electrical and vapour sensing properties of Mn doped nanostructured ZnO thin films. Sens. Actuators B: Chem. 166–167 624–631 (2012)
Stegmeier, S., Fleischer, M., Tawil, A., Hauptmann, P., Egly, K., Rose, K.: Sensing mechanism of room temperature CO2 sensors based on primary amino groups. Sens. Actuators B 154, 270–276 (2011). https://doi.org/10.1016/j.snb.2010.01.022
Taabouche, A., Bouabellou, A., Kermiche, F., Hanini, F., Sedrati, C., Bouachiba, Y., Benazzouz, C.: Preparation and characterization of Al-doped ZnO piezoelectric thin films grown by pulsed laser deposition, Ceramics International (2016). https://doi.org/10.1016/j.ceramint.2016.01.033.
Vijayprasath, G., Murugan, R., Ravi, G., Mahalingam, T., Hayakawa, Y.: Characterization of dilute magnetic semiconducting transition metal doped ZnO thin films by sol-gel spin coating method. Appl. Surf. Sci. 313, 870–876 (2014)
Vinoth, E., Gopalakrishnan, N.: Ammonia sensing characteristics of Yttrium doped ZnO thin films by RF magnetron sputtering. Mater. Res. Express (in press). https://doi.org/10.1088/2053-1591/aac99a.
Viter, R., Iatsunskyi, I.: Metal oxide nanostructures in sensing, nanomaterials design for sensing applications. Micro Nano Technol., pp 41–91. https://doi.org/10.1016/B978-0-12-814505-0.00002-3.
Wagh, M.S., Jain, G.H., Patil, D.R., Patil, S.A., Patil, L.A.: Modified zinc oxide thick film resistors as NH3 gas sensor. Sens. Actuators B 115, 128–133 (2006). https://doi.org/10.1016/j.snb.2005.08.030
Wang, Y., Chen, Y., Song, X., Zhang, Z., She, J., Deng, S., Ningsheng, Xu., Chen, J.: Electrical properties of fluorine-doped ZnO nanowires formed by biased plasma treatment. Phys. E (2018). https://doi.org/10.1016/j.physe.2018.01.028
Yuanyuan Lv, Zhiyong Zhang, Junfeng Yan, Wu Zhao, Chunxue Zhai.: Al doping influences on fabricating ZnO nanowire arrays enhanced field emission property. Ceramics Int. https://doi.org/10.1016/j.ceramint.2018.01.118.
Zaiour, A., Benhaya, A., Bentrcia, T.: Impact of deposition methods and doping on structural, optical and electrical properties of ZnO-Al thin films. Optik Int. J. Light Electron Optics 186, 293–299 (2019). https://doi.org/10.1016/j.ijleo.2019.04.132
Zhai, C.-H., Zhang, R.-J., Chen, X., Zheng, Y.-X., Wang, S.-Y., Liu, J., Dai, N., Chen, L.-Y.: Effects of Al doping on the properties of ZnO thin films deposited by atomic layer deposition. Nanoscale Res. Lett. (2016). https://doi.org/10.1186/s11671-016-1625-0
Zhang, B., Gao, P.-X.: Metal oxide nanoarrays for chemical sensing: a review of fabrication methods, sensing modes, and their inter-correlations. Front. Mater. (2019). https://doi.org/10.3389/fmats.2019.00055
Zhang, Y.-H., Li, Y.-L., Gong, F.-L., Xie, K.-F., Liu, M., Zhang, H.-L., Fang, S.-M.: Al doped narcissus-like ZnO for enhanced NO2 sensing performance: an experimental and DFT investigation. Sens. Actuators: B. Chem. (2019). https://doi.org/10.1016/j.snb.2019.127489
Zhijie Li, Hao Li, Zhonglin Wu, Mingkui Wang, Jingting Luo, Hamdi Torun, PingAn Hu, Chang Yang, Marius Grundmann, Xiaoteng Liu, YongQing Fu,: Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz., 6, 470–506 (2019). https://doi.org/10.1039/c8mh01365a.
Zouhair Sofiani, Bouchta Sahraoui, Mohammed Addou, Rahma Adhiri, Mehdi Alaoui Lamrani, Leila Dghoughi, Nabil Fellahi, Beata Derkowska, Waclaw Bala.: Third harmonic generation in undoped and X doped ZnO films (X: Ce, F, Er, Al, Sn) deposited by spray pyrolysis. J. Appl. Phys. 101(6), 063104–063104-5 (2007). https://doi.org/10.1063/1.2711143
Acknowledgements
The authors would like to express their gratitude to Deanship of Scientific Research at King Khalid University for funding this work through Research Groups Program under Grant No. R.G.P2/93/41.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devi, K.R., Selvan, G., Karunakaran, M. et al. Enhanced room temperature ammonia gas sensing properties of Al-doped ZnO nanostructured thin films. Opt Quant Electron 52, 501 (2020). https://doi.org/10.1007/s11082-020-02621-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-020-02621-0