Abstract
Spontaneous periodic up and down transitions are considered to be a significant phenomenon that is characteristic of slow-wave sleep. We studied a neural network model of spontaneous up and down transitions based on our former study of a single-neuron model. We expanded the model by using two types of neurons—excitatory and inhibitory neurons—and redefining the connecting function between two neurons instead of assuming a constant connection, so that the model is closer to the in vivo network. Using this model, we studied the relationship between the transitions and network parameters such as size, structure and the ratio of excitatory to inhibitory neurons. We found that the network parameters have little impact on these spontaneous periodic up and down transitions. However, the intrinsic currents were found to play a leading role in the process. Then, we studied the transitions in the presence of stimulation and found that the addition of stimulation did have an effect on the network transitions. Through the observation and analysis of the findings, we have tried to explain the dynamics of up and down transitions and to lay the foundation for future work on the role of these transitions in cortex activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The phenomenon of spontaneous periodic up and down transitions is considered to be a significant characteristic of slow-wave sleep. This slow oscillation characterized by large delta waves predominates electroencephalograms taken when a subject is asleep. Neural electrophysiology experiments also have shown that membrane potentials make spontaneous transitions between two different levels called up and down states [1] during slow-wave sleep in the primary visual cortex of anesthetized animals [2–4] and during quiet wakefulness in the somatosensory cortex of unanesthetized animals [5]. These two states characterize the bistability of the membrane potentials. The importance of both bistability, up and down transitions, and slow oscillations has been emphasized in different fields of research [6–14], including spatial coherence resonance [15]. Moreover, the findings have also been applied to biochemical [16] and calcium [17] research.
Why these transitions occur and whether this spontaneous activity plays a role in brain functions are still not clear. In fact, we know little about the induction of neuron membrane potentials and interactions among neural networks, especially the relationship between neural coding and cognitive behaviors. In earlier researches, the activation of inward currents by hyperpolarizations was implicated in the pacemaking of both single cell and network rhythmicity [18–20]. Other results indicate that the dynamic interplay between the gating and kinetic properties of \(I_{h}\) and \(I_{\mathrm{NaP}}\) is essential for the generation of rhythmic subthreshold oscillations by the stellate cells [21]. Further more, some studies used coupled nonlinear differential equations and obtained results which showed chaos and limits cycle activity [7, 22–26].
Our former study on up and down transitions of a single neuron [27] and a small network with constant connection [28] tried to explain the dynamic mechanism involved in these transitions at the ionic channel level. At the single-neuron level, cortical neurons switch between two stable states. Moreover, cortical local field potential (LFP) changes over time. LFP is used to describe the state of the whole cortex [10, 29–31]. In this paper, we expanded the model by clarifying the mechanism in two types of neurons, namely, excitatory and inhibitory neurons, and by substituting the constant connection state with a changing connecting function state involving two types of neurons, so that the model reflects the in vivo mechanism better. Using this model, we explored the factors that influence spontaneous periodic up and down transitions and their impact on these transitions.
Studies on the relationship between the transitions and network parameters such as size, structure and the ratio of excitatory neurons to inhibitory neurons showed that the network parameters have little impact on these spontaneous periodic up and down transitions. However, the intrinsic currents play a leading role in the process. There is also some debate about the topological features of neuronal networks. In this sense, it would be a good idea to built a model based on the actual connectivity, observed in neuronal networks, as done in other research [32]. However, it is difficult to elucidate the actual connections that are present in vivo. Fortunately, our models were independent of the network structure and dependent on the channel conditions. We believe that this work will lay the foundation for studying the relationship between neural coding and cognitive behavior.
2 Biophysical model
In this paper, we extended our former network model [28, 33], and modeled two compartment neurons—excitatory neurons (ENs) and inhibitory neurons (INs)—with two kinds of currents, the intrinsic currents and synaptic currents. The main current equations and the intrinsic and synaptic currents are shown below.
2.1 Current equation
The main current equation on which the HH model is based was developed from the single-neuron dynamic model [27, 33] we studied before. For the excitatory neurons,
For the inhibitory neurons,
2.2 Intrinsic current
The intrinsic current [33] consists of the following four ionic currents: an instantaneous, inward current (sodium current), a slow h-like current, and two outward currents (a slow potassium current and a leak current).
where
2.3 Synaptic current
Three types of receptors, namely, AMPA receptors, NMDA receptors and GABA\(_{\mathrm{A}}\) receptors, are significant for the transmission of information between neurons. The AMPA and NMDA receptors are excitatory in nature, while the GABA\(_{\mathrm{A}}\) receptors are inhibitory one. Furthermore, the AMPA receptors mediate the prototypical fast excitatory synaptic currents, while the NMDA receptors mediate synaptic currents that are substantially slower than the AMPA current [34].
ENs excite other connecting neurons, including ENs and INs, via AMPA receptors and NMDA receptors. However, INs only inhibit one type of neurons, the ENs, through the GABA\(_{\mathrm{A}}\) receptors. The three types of currents are represented by the following equations.
AMPA current,
NMDA current,
GABA\(_{\mathrm{A}}\) current,
In the equations, the stationary relationship between the transmitter concentration [T](V) and presynaptic voltage is fit to \([T](V)=T_{\mathrm{max}}/((1+\exp ^{-\frac{V-V_p}{K_p}}))\) [34]. The slow component of the NMDA synaptic receptor is represented by \(f_\mathrm{NMDA}(V)\) \(=1/((1+\exp ^{-\frac{V-T_f}{\sigma _f}}))\) [35]. The variables \(s_\mathrm{AMPA}\), \(s_\mathrm{NMDA}\), and \(s_{\mathrm{GABA}_{\mathrm{A}}}\) define the degree of opening of the protein channels. \(\alpha \) and \(\beta \) represent the forward and backward constants, respectively. The synaptic weights from neuron j to i are defined as w(j, i), the value of which depends on the different network topology.
2.4 Parameters
The values of the constants used in the model are shown in Table 1 [33–35].
2.5 Network topology
The following pattern is true for all the networks described in this paper, regardless of size: One EN excites other connecting neurons including ENs and INs. At the same time, it is also excited by other ENs and inhibited by the INs. However, a IN only inhibits one other type of neurons, ENs, and is excited by the connecting ENs. All the excitatory connections are mediated by a fast AMPA component as well as a slow voltage-dependent NMDA component. The inhibitory connections are modulated by a fast GABA\(_{\mathrm{A}}\) synaptic receptors.
Next, we examine how ENs and INs interact with each other. The exact ratio of ENs to INs is still unclear. Different cortical regions have different characteristics in this regard. However, the EN to IN ratio has been reported to be 4 : 1 [36] in some studies. We tested our models using this ratio as well as other possible ratios.
Finally, we tried to understand the interaction between ENs and INs according to their numbers. Here, we used the full-connection-like (TP_A) link. Every EN was found to be connected to all the other neurons, including ENs and INs, while every IN was linked to all the other ENs but not other INs. This can be described in detail as w(EN, EN or\( IN)=1/(n-1)\), \(w(IN,EN)=1/n_{ENs}\), \(w(IN,IN)=0\). Here, n refers to the total number of neurons and \(n_{ENs}\) refers to the number of ENs.
Here is another possible connection that was found to be popular in most stimulations: five cells in AMPA- and NMDA-mediated EN-EN synapses, one cell in AMPA- and NMDA-mediated EN–IN synapses, and five cells in GABA\(_{\mathrm{A}}\)-mediated IN-EN synapses (TP_B) [36]. In detail, \(w(EN,EN)=1/5\)(choose five ENs randomly), \(w(EN,IN)=1\)(choose one IN randomly), \(w(IN,EN)=1/5\)(choose five ENs randomly), \(w(IN,IN)=0\). This possibility was also tested in this paper.
3 Results
3.1 Influence of network structure on up and down transitions
In this paper, we discussed about four types of network structure elements namely, network size, network topology, the original state of the network, and the ratio of ENs to INs in the network. In order to represent the in vivo mechanisms as far as possible, we used this patten of interactivity consistently: ENs were excited by other ENs and inhibited by INs, and these ENs also excited other neurons including other ENs and INs through fast AMPA- and slow NMDA-mediated synapses. Moreover, INs were excited by ENs and inhibited ENs meanwhile. The inhibitory synapses were mediated via fast GABA\(_{\mathrm{A}}\). This was an important part of the network structure that had to be taken into consideration.
Another feature of our model was the number of the neurons in the links. A full-connection-like (TP_A) link was created since in small neural networks such as the one used here, neurons were likely to be combined in this way. Another structure (TP_B) used in most stimulations was also tested in this study. Furthermore, we hypothesized that this type structure (TP_B) may represent the in vivo structure better, so we used it in most of the simulations.
The precise ratio of ENs to INs is unclear, and it is not clear whether there are more ENs or INs or whether they are present in a balanced manner. We used the most commonly accepted EN/IN ratio (4 : 1 [36]), as well as two other possible ratios, 1 : 1 and 9 : 1.
With regard to size, we tested network sizes of 25, 50, and 100 neurons, while keeping the other conditions constant. The original state of the network, which mainly reflects the average membrane potential of all the neurons, was initially in the down state or up state.
All the situations specified above were tested on the model, and the results are shown in Fig. 1. Every small plot in Fig. 1 represents the average membrane potential of the network under a specific set of different network parameters: sizes \((n=25, 50, 100)\), structure (TP_B, TP_A), and ratio of ENs to INs \((ENs/INs = 1{:}1, 4{:}1, 9{:}1)\). The small plots illustrated similar spontaneous slow subthreshold activities with different network parameters. This also showed the stability of network activity with spontaneous up and down transitions, which provided further evidence that slow-wave sleep can be considered as deep sleep to some extent.
From the above findings on spontaneous up and down transitions, we deduced that the results were independent of network structure. The network parameters including network size, network topology, the original state of the network, and the ratio of ENs to INs in the network had little impact on these spontaneous periodic up and down transitions. This network activity showed stronger stability and robustness without any external stimulation.
3.2 Influence of channel conditions on up and down transitions
In the previous part of the results, we found that spontaneous up and down transitions are independent of network structure. Our next goal was to examine the factors that influence on up and down transitions, and in this regard, we examined the role of intrinsic currents in our model.
In Fig. 2, we have illustrated all the currents, except for the leak current of one EN in the network (top) and three types of intrinsic currents (bottom) that seemed to be involved in up and down transitions.
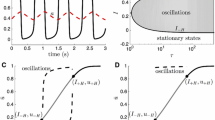
In the top subfigure of Fig. 2, there are six voltage-dependent currents of the EN. All of them displayed voltage-dependent characteristics that were reflected in the spontaneous up and down transitions. Three of them were intrinsic currents, namely the sodium current (\(I_{Na}\)), the potassium current (\(I_K\)), and the h-like current (\(I_h\)). The other three were synaptic currents mediated by the three types of receptors, AMPA receptors, NMDA receptors, and GABA\(_{\mathrm{A}}\) receptors. The synaptic currents were so small compared with the intrinsic currents that they made little contribution to the integrated current. The findings indicated that the network structure hardly had an impact on up and down transitions, as specified in the previous section.
The bottom subfigure of Fig. 2 is a plot of the simulated membrane potential against the amplitude of three intrinsic currents, \(I_{Na}\), \(I_K\), and \(I_h\). The plot corresponding to \(I_{Na}\) is an inverted S-shaped curve with a negative slope without hysteresis. Therefore, during the up transitions or down transitions, there was a relatively instantaneous increase or decrease in the sodium current. On the other hand, the plots corresponding to \(I_K\) and \(I_h\) presented a greater degree of delay. These two closed circuits travelled counterclockwise. From the bottom subfigure, we can see that the maxima and minima of the amplitudes of \(I_K\) and \(I_h\) occurred just after the membrane potential reached its most hyperpolarized and depolarized values. Therefore, in contrast to the sodium current, the amplitudes of the potassium current and h-like current demonstrated a delayed increase or decrease with the transitions happening.
From Fig. 2, it was estimated that the intrinsic currents may be responsible for up and down transitions. We then tried to understand the mechanism underlying the effect of these currents. At the ionic level, the ion channel properties and conditions may play a role. Therefore, we examined different channels under different conductance conditions (as shown in Figs. 3, 4). For this, we used TP_B structure, with 4 : 1 as the EN/IN ratio and 25 as the network size.
All the plots in Fig. 3 represent the average membrane potentials of the network at various levels of conductance. The left column represents different potassium conductance values; the center column, different sodium conductance values; and the right column, the conductance values of the h-like channel. If the potassium channel conductance was within the appropriate range, the average membrane potential demonstrated spontaneous up and down transitions with bistability. The higher the potassium conductance, the fewer were the transitions and the shorter was the duration of the up state. Furthermore, if the potassium conductance was excessive, the membrane potential would always be in the down state. Conversely, when it was insufficiently activated, the membrane potential was in the up state. This was in agreement with the results of our previous work [27, 28]. On the other hand, the findings for the sodium channel and h channel showed an opposite trend: The higher the conductance, the more were the transitions and the longer was duration of the up state.
The results of the quantitative statistic analysis are illustrated in Fig. 4. In every column of Fig. 3, we listed the membrane potential results under three different parameter values of iconic conductance. In Fig. 4, we tried more parameter values and calculated up state duration (see top three subfigures) and cycle of up state transition (including one up state duration and one down state duration, see middle three subfigures) of average membrane potential. Then, divide the up state duration by the cycle of up state transition, and the results were noted as up state ratio in the cycle (see bottom three subfigures). So we studied the average up state duration of the membrane potential (top row), the average cycle of the transitions (middle row), and the average proportion of the duration of up state in the cycle (bottom row) of the three types of channels—potassium channel (left column), sodium channel (center column), and h channel (right column) in Fig. 4.
In most observations, the up state duration increased with decrease in the potassium channel conductance and increase in sodium channel conductance and h channel conductance, during appropriate ranges in which the membrane potential showed up and down transitions. However, the cycle of transitions did not change much under most conditions, except for cases in which the membrane potential was close to but did not reach that of a permanent down states. The up state proportion in the cycles showed a similar tendency to the up state duration.
The mechanism of up and down transitions in a single neuron was examined in our previous work [27]. Briefly, depolarization activates the sodium channel, letting the sodium ions move into cells, after which the potassium channel is activated slowly, making the potassium ions move out of the cells; this leads to the resultant hyperpolarization, which then activates the h channel. The h-like current is a slow inward current with a reversal potential between \(-43\) and 0 mV, but which requires hyperpolarization to become active. A mixture of sodium and potassium ions [37] is involved. At the network level, the ionic movement mechanism is the same. This is depicted in Figs. 3 and 4. The higher the potassium channel conductance, the higher the number of potassium ions leaving the cells, which leads to a shorter up state duration. On the other hand, the lower the sodium channel conductance, the higher the number of sodium ions moving into the cells, which results in a longer up state duration. With regard to the conductance of the h channel, the results were similar to that for sodium conductance. Therefore, we think that the sodium ions largely contribute to the h-like current.
From the above discussion on how the channel conditions influence spontaneous up and down transitions, we think that the degree of opening of the ionic channels had an influence on the up and down transitions, especially the up state duration. The higher the degree of opening of the potassium channel, the shorter the up state duration. Extremely, the much higher potassium channel conductance led to lasting down state, and the much lower one resulted in lasting up state. However, the sodium and h channels showed opposite trends, as a higher degree of opening of the channels resulted in a longer up state duration. Moreover, the much higher conductances resulted in lasting up state and the much lower ones resulted in lasting down state. This similarity between the sodium and h channels indicated that sodium ions largely contribute to the h-like current, although this current involves a mixture of sodium and potassium ions.
3.3 Influence of stimulations on up and down transitions
The above two sections described spontaneous transitions without any stimulation. Next, we will discuss transitions in response to stimulation, in order to determine whether stimulations are related with up and down transitions. If this is found to be true, we will try to determine the number of neurons that need to be excited to stimulate transitions in the whole network. Furthermore, we will also examine whether stimulation of only one neuron can trigger the whole network. We have attempted to answer these questions in Figs. 5 and 6.
Figure 5 shows some clear findings with regard to up and down transitions of the whole network, as a results of excitation of a number of neurons via channel stimulations. (A) Sodium channel stimulation of 5 of 25 (20 %) neurons with by increasing the sodium conductance to 1.2 \(\hbox {ms/cm}^2\), lasting 5ms every 2s. (B) Potassium channel stimulation of 15 of 25 (60 %) neurons by increasing the conductance from 0.15 to 20 \(\hbox {ms/cm}^2\), lasting 5ms every 1.5s. (C) h channel stimulation of 17 of 25 (68 %) neurons by increasing the conductance of h channel from 0.23 to 23 \(\hbox {ms/cm}^2\), lasting 5 \(\hbox {ms}\) every 2 s. The other parameters are presented in Table 1.
The global network showed up and down transitions at points where the stimulations were added. Based on the number of the neurons that needed to be excited, we think that the sodium channel may play a more important role in up and down transitions than the other two channels.
Unfortunately, if too few neurons were excited than those shown in Fig. 5, the average membrane potential varied between the up and down transition potentials irregularly rather than showing clear up and down transition potentials. Therefore, it was difficult to determine whether stimulating only one neuron can trigger global network transitions based on the model in this paper. However, we did show that stimulating only one neuron can trigger the local network transitions in Fig. 6.
To study the local conditions of the network, we fixed the connection weights between every two neurons and slightly modified the topology as follows: Four cells for AMPA- and NMDA-mediated EN–EN synapses, one cell for AMPA- and NMDA-mediated EN–IN synapses, and four cells for GABA\(_{\mathrm{A}}\)-mediated IN–EN synapses. Therefore, based on the results of Fig. 6, one EN (Type A) has four direct connecting ENs (Type B) and one direct connecting IN. These direct connecting neurons (Type B) also have their own direct connecting neurons (Type C). The other neurons (Type D) are not connected to the first neuron (Type A) within two steps of connection. The definition of the concept of “local region” (E) over here is the average membrane potential of the target neuron (Type A) and the directly connected neurons (Type B).
Comparison of the charts in Fig. 6 showed that one stimulated neuron did induce transitions in the local region where the input stimulation (22 mA, lasting 10 ms every 1.5 s) showed a little delay. This result confirms that switching of the membrane potential of the stimulated neuron from slow up and down oscillations to a persistent-up state or vice versa is associated with concurrent changes in the temporal pattern of cortical LFP recorded several millimeters away [38]. In Fig. 6, the stimulated neuron also had little effect on the transitions of indirectly connected neurons via changes in the tempo of the original transitions, which were not strong enough to cause immediate transitions via two-layer connections. In fact, each of these neurons is likely to project to several thousand cortical neurons, and a small percentage of these connections may be strong enough to trigger postsynaptic spiking and activate a local network [37].
4 Conclusion
This paper mainly discusses about the factors that influence the spontaneous periodic up and down transitions by using a dynamic network model that shows spontaneous transitions. The main conclusions were as follows:
-
The network parameters including network size, network topology, the original state of the network, and the ratio of ENs and INs in the network had little impact on spontaneous periodic up and down transitions. Moreover, network activity was more stable and robust without any external stimulation.
-
The degree of opening of the ion channels had an influence on the up and down transitions, especially the duration of the up state. The greater the degree of opening of potassium channel, the shorter the up state duration. Extremely, the much higher potassium channel conductance led to lasting down state, and the much lower one resulted in lasting up state. However, the sodium and h channels showed opposite trends, as a higher degree of opening of the channels resulted in a longer up state duration. Moreover, the much higher conductances resulted in lasting up state and the much lower ones resulted in lasting down state. This similarity between the sodium and h channels indicated that sodium ions largely contribute to the h-like current, although this current involves a mixture of sodium and potassium ions.
-
Up and down transitions of the global network can be induced by adding channel stimulations to a number of neurons. Moreover, the sodium channel may play a more important role in the up and down transitions than the other two channels. In the model used in this paper, stimulation of only one neuron triggered local network transitions but failed at the level of the global network, which will be investigated in the future.
We believe that these results shed light on the dynamics of up and down transitions and the role of these transitions in network behaviors, and also lay the foundation for understanding the dynamic neural mechanism of cortex activities.
References
Parga, N., Abbott, L.F.: Network model of spontaneous activity exhibiting synchronous transitions between up and down states. Front. Neurosci. 1(1), 57 (2007)
Anderson, J., Lampl, I., Reichova, I., Carandini, M., Ferster, D.: Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nat. Neurosci. 3(6), 617–621 (2000)
Lampl, I., Reichova, I., Ferster, D.: Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron 22(2), 361–374 (1999)
Steriade, M., Nuñez, A., Amzica, F.: Intracellular analysis of relations between the slow (1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J. Neurosci. 13(8), 3266–3283 (1993)
Petersen, C.C., Hahn, T.T., Mehta, M., Grinvald, A., Sakmann, B.: Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc. Natl. Acad. Sci. 100(23), 13638–13643 (2003)
Araki, O.: Computer simulations of synchrony and oscillations evoked by two coherent inputs. Cogn. Neurodyn. 7(2), 133–141 (2013)
Ma, J., Hu, B., Wang, C., Jin, W.: Simulating the formation of spiral wave in the neuronal system. Nonlinear Dyn. 73(1), 73–83 (2013)
Wang, G., Jin, W., Wang, A.: Synchronous firing patterns and transitions in small-world neuronal network. Nonlinear Dyn. 81(3), 1453–1458 (2015)
Zhou, J., Wu, Q., Xiang, L.: Impulsive pinning complex dynamical networks and applications to firing neuronal synchronization. Nonlinear Dyn. 69(3), 1393–1403 (2012)
Liu, Y., Wang, R., Zhang, Z., Jiao, X.: Analysis of stability of neural network with inhibitory neurons. Cogn. Neurodyn. 4(1), 61–68 (2010)
Gu, H., Pan, B., Li, Y.: The dependence of synchronization transition processes of coupled neurons with coexisting spiking and bursting on the control parameter, initial value, and attraction domain. Nonlinear Dyn. 82(3), 1191–1210 (2015)
Njap, F., Claussen, J.C., Moser, A., Hofmann, U.G.: Modeling effect of GABAergic current in a basal ganglia computational model. Cogn. Neurodyn. 6(4), 333–341 (2012)
Zheng, C., Zhang, T.: Alteration of phase-phase coupling between theta and gamma rhythms in a depression-model of rats. Cogn. Neurodyn. 7(2), 167–172 (2013)
Wang, Q., Zheng, Y., Ma, J.: Cooperative dynamics in neuronal networks. Chaos Solions Fractals 56(7), 19–27 (2013)
Perc, M.: Spatial coherence resonance in excitable media. Phys. Rev. E 72(1), 016207 (2005)
Gosak, M., Marhl, M., Perc, M.: Spatial coherence resonance in excitable biochemical media induced by internal noise. Biophys. Chem. 128(2), 210–214 (2007)
Perc, M., Gosak, M., Marhl, M.: Periodic calcium waves in coupled cells induced by internal noise. Chem. Phys. Lett. 437(1), 143–147 (2007)
DiFrancesco, D.: Pacemaker mechanisms in cardiac tissue. Annu. Rev. Physiol. 55(1), 455–472 (1993)
Lüthi, A., McCormick, D.A.: H-current: properties of a neuronal and network pacemaker. Neuron 21(1), 9–12 (1998)
Pape, H.C.: Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu. Rev. Physiol. 58(1), 299–327 (1996)
Dickson, C.T., Magistretti, J., Shalinsky, M.H., Fransén, E., Hasselmo, M.E., Alonso, A.: Properties and role of Ih in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J. Neurophysiol. 83(5), 2562–2579 (2000)
Wilson, A., Cowan, J.D.: Excitatory and inhibitory interactions in localized populations of model neurons. Biophys. J. 12(1), 1–24 (1972)
Compte, A., Sanchez-Vives, M.V., McCormick, D.A., Wang, X.J.: Cellular and network mechanisms of slow oscillatory activity (1 Hz) and wave propagations in a cortical network model. J. Neurophysiol. 89, 2707–2725 (2003)
Holcman, D., Tsodyks, M.: The emergence of up and down states in cortical networks. PLOS Comput. Biol. 2, 174–181 (2006)
Yang, Z., Wang, Q., Danca, M.F., Zhang, J.: Complex dynamics of compound bursting with burst episode composed of different bursts. Nonlinear Dyn. 70(3), 2003–2013 (2012)
Ma, J., Tang, J.: A review for dynamics of collective behaviors of network of neurons. Sci. China Technol. Sci. 58, 2038–2045 (2015)
Xu, X., Wang, R.: Neurodynamics of up and down transitions in a single neuron. Cogn. Neurodyn. 8(6), 509–515 (2014)
Xu, X., Wang, R.: Neurodynamics of up and down transitions in network model. Abstr. Appl. Anal. 2013, 9 (2013). doi:10.1155/2013/486178
Wang, R., Zhang, Z., Chen, G.: Energy coding and energy functions for local activities of the brain. Neurocomputing 73(1), 139–150 (2009)
Wang, R., Zhang, Z., Chen, G.: Energy function and energy evolution on neuronal populations. Neural Netw. IEEE Trans. 19(3), 535–538 (2008)
Wang, R., Zhang, Z.: Energy coding in biological neural networks. Cogn. Neurodyn. 1(3), 203–212 (2007)
Wang, Q.Y., Murks, A., Perc, M., Lu, Q.S.: Taming desynchronized bursting with delays in the Macaque cortical network. Chin. Phys. B 20(4), 040504 (2011)
Loewenstein, Y., Mahon, S., Chadderton, P., Kitamura, K., Sompolinsky, H., Yarom, Y., Häusser, M.: Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat. Neurosci. 8(2), 202–211 (2005)
Koch, C., Segev, I.: Methods in neuronal modeling: from ions to networks. MIT Press, Cambridge (1998)
Destexhe, A., Mainen, Z.F., Sejnowski, T.J.: Synthesis of models for excitable membranes, synaptic transmission and neuromodulation using a common kinetic formalism. J. Comput. Neurosci. 1(3), 195–230 (1994)
Bazhenov, M., Timofeev, I., Steriade, M., Sejnowski, T.J.: Potassium model for slow (2–3 Hz) in vivo neocortical paroxysmal oscillations. J. Neurophysiol. 92(2), 1116 (2004)
Ermentrout, T.J., Terman, D.H.: Mathematical foundations of neuroscience. Springer, Berlin (2010)
Li, C.Y., Poo, M.M., Dan, Y.: Burst spiking of a single cortical neuron modifies global brain state. Science 324(5927), 643–646 (2009)
Acknowledgments
This work is supported by the National Natural Science Foundation of China (No. 11232005) and The Ministry of Education Doctoral Foundation (No. 20120074110020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, X., Ni, L. & Wang, R. A neural network model of spontaneous up and down transitions. Nonlinear Dyn 84, 1541–1551 (2016). https://doi.org/10.1007/s11071-015-2587-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-015-2587-y