Abstract
Epilepsy is characterized by recurrent unprovoked seizures and some seizures can cause neuronal apoptosis, which is possible to make contributions to the epilepsy phenotype, impairments in cognitive function or even epileptogenesis. Moreover, many studies have indicated that microRNA-34a (miRNA-34a) is involved in apoptosis through regulating Notch signaling. However, whether miRNA-34a participates in neuronal apoptosis after seizures remain unclear. Therefore, we aimed to explore the expression of miRNA-34a and its effects on the epileptiform discharge in spontaneous recurrent epileptiform discharges (SREDs) rat hippocampal neuronal pattern. Mg2+-free medium was used to induce SREDs, quantitative reverse-transcription polymerase chain reaction was used to detect the expression of miRNA-34a, western blot was used to determine the expression of Notch pathway and apoptosis-related proteins, and whole cell current clamp recordings was used to observe the alteration of epileptiform discharge. We found obvious apoptosis, increased expression of miRNA-34a and decreased expression of Notch signaling in Mg2+-free-treated neurons. Treatment with miRNA-34a inhibitor decreased the frequency of action potentials, activated Notch signaling and prevented neuronal apoptosis in Mg2+-free-treated neurons. However, treatment with miRNA-34a mimics increased the frequency of action potentials, down-regulated Notch signaling and promoted neuronal apoptosis in Mg2+-free-treated neurons. Furthermore, γ-secretase inhibitor N-[N-(3,5-di-uorophenacetyl)-1-alanyl]-S-phenylglycine t-butylester (DAPT), an inhibitor of Notch signaling, could weaken anti-apoptosis effect of miRNA-34a inhibitor. These results suggest that inhibition of miRNA-34a could suppress epileptiform discharges through regulating Notch signaling and apoptosis in the rat hippocampal neuronal model of SREDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy, one of the most common neurological disorders, is characterized by a long lasting and unpredictable tendency to produce epileptic seizures caused by excessive and synchronized firing of neurons that last for a short time, which affects approximately 1–2% of the population worldwide [1]. About 30% of patients who have epilepsy have still been ineffective to the current antiepileptic drugs (AEDs) and develop to refractory epilepsy [2]. Therefore, insight into the underlying mechanisms and finding novel targets for effective pharmacologic prevention of this disease are still needed.
Recently, micro ribonucleic acids (microRNA or miRNA) are discovered to be altered within brain tissue from experimental patterns and epilepsy patients by the regulation of ion channels, synaptic plasticity, inflammation, neuronal death as well as neuronal microstructure. Targeting key miRNA is presented for altering the excitability of the brain and exacerbating or suppressing seizures, which indicates potential for therapeutics based on miRNA in epilepsy [3, 4]. More than 100 diversified miRNA levels had been discovered to either reduce or increase within the hippocampus, of which over 20 had been identified in two or more researches, which included higher levels of miR-146a, miR-132, miR-34a and miR-23a [5]. MiRNA-34a is a member of the miRNA-34 family, and a large number of researchers have found that miRNA-34a could mediate cell cycle, differentiation, and apoptosis [6, 7]. Hu et al. found an increased expression of miRNA-34a in post-status epilepticus rats, and indicated that miR-34a inhibition was able to inhibit protein expression of activated caspase-3, which is possible to make contributions to the decreased apoptosis and the increased neuronal survival [8]. Besides, proofs have appeared that the activity of apoptotic pathways continues well beyond the period of major cell death after the initial precipitating injury (e.g. status epilepticus) into the time of epileptogenesis [9]. Those data suggested that upregulated miRNA-34a might contribute to precipitating injury-induced neuronal apoptosis, which is closely related with secondary injury, such as cognition impairment, following epilepsy.
MiRNA-34a, a direct target of p53 tumor suppressor gene, promote cell apoptosis in p53-dependent or independent manner through regulating various target proteins, such as Notch1 and Notch2 [6]. Notch signaling is considered to be a significant “switch” controlling cell fate decisions (death, proliferation or differentiation) during development in numerous tissues [6]. Nevertheless, the probable relation of Notch signaling and miRNA-34a in epilepsy keeps to be elucidated. According to it, we wanted to explore miRNA-34a’s impact on spontaneous recurrent epileptiform discharges (SREDs). Though the neuronal cultures don’t have real anatomical links and don’t show clinical seizures, such in vitro pattern is adopted routinely for characterizing electrophysiological, biochemical, as well as molecular mechanisms which underlie acquired epilepsy [10].
Materials and Methods
Primary Cultures of Hippocampal Neurons
Primary cultures of hippocampal neurons were performed according to previous study with slight modifications [10, 11]. Briefly, hippocampi had been digested with trypsin for 20 min and prepared from 2-day postnatal Sprague–Dawley rats. Hippocampal neurons had been plated at a density of 2 × 105 cells/cm2 onto 35-mm cell culture dishes (Corning, America) originally coated with poly-l-lysine (0.05 mg/ml, Sigma, America). Neurons had been kept within Neurobasal medium (Invitrogen, Carlsbad, CA, America) including 100 U/mL streptomycin, 100 U/mL penicillin, 0.5 mM glutamine as well as 2% B27 and kept within a 5% CO2/95% air atmosphere at room temperature. Immunofluorescence had been conducted on the 7th day of culture with mouse protein-2 associated with anti-microtubule (MAP2; 1:50; Boster, China) for the purpose of identifying hippocampal neurons.
Hippocampal Neuronal SREDs Model
To induce the hippocampal neuronal SREDs pattern, the neuronal cultures had been treated with a solution containing no added MgCl2 (Mg2+-free) for 3 h on the 14th day according to previous study [10, 11]. Briefly, after the removal of maintenance media, neurons were gently washed with physiological basal recording solution (pBRS) containing (in mM): pH 7.3, 0.001 glycine, 10 glucose, 2 CaCl2, 10 HEPES, 2.5 KCl as well as 145 NaCl, and osmolarity adjusted to 325 ± 5 mOsm with sucrose and and then allowed to incubate in this solution at 37 °C under 5% CO2/95% air atmosphere for 3 h. Mg2+-free treatment was carried out with pBRS without MgCl2, which was used as Mg2+-free group. However, neurons treated with pBRS containing 1 mm MgCl2 were used as control group. At the end of the 3-h period, neurons returned to the maintenance medium and incubated at 37 °C under 5% CO2/95% air atmosphere for 12 h. Then, neurons were harvested, and whole cell current clamp recordings, quantitative reverse-transcription polymerase chain reaction (qRT-PCR) and western blot analysis were performed.
Transfection and Drug Administration
The negative control miRNA (NC; 5′-CAGUACUUUUGUGUAGUACAA-3′), miRNA-34a inhibitor (5′-ACAACCAGCUAAGACACUGCCA-3′) as well as miRNA-34a mimics (5′-UGGCAGUGUCUUAGCUGGUUGU-3′) had been composed by Guangzhou RiboBio, Co., Ltd. in accordance with the researches before [12]. The neurons had been transfected with 40 nM inhibitors or 20 nM miRNA-34a-5p mimics after 3-h Mg2+-free treatment by adopting Lipofectamine RNAiMax (Invitrogen Corp., Carlsbad, CA, America) according to the manufacturer’s instructions for 12 h. For drug administration, γ-secretase inhibitor N-[N-(3,5-di uorophenacetyl)-1-alanyl]-S-phenylglycine t-butylester (DAPT, Sigma-Aldrich, St. Louis, MO, USA) was used to inhibit Notch signaling. 1 µM DAPT was added into pBRS after 3-h Mg2+-free treatment for 12 h [13]. Then, neurons were harvested, and whole cell current clamp recordings, qRT-PCR and western blot analysis were performed.
Whole Cell Current Clamp Recordings
Whole cell current clamp recordings were performed using previously established procedures [10, 11]. Briefly, cell culture dish was placed on the stage of an inverted microscope (IX71, Olympus) continuously perfused with pBRS. A Brown-Flaming P-97 electrode puller (Sutter Instruments, Novato, CA, USA) was used to pull the patch electrodes with a resistance of 2–4 mΩ. Patch electrodes were filled with a solution containing (in mM): 140 K+ gluconate, 1.1 EGTA, 1 MgCl2, and 10 Na-HEPES, pH 7.2, osmolarity adjusted to 290 ± 10 mOsm with sucrose. Whole-cell recordings were carried out using an Axopatch 200B amplifier (Molecular Devices, Foster City, CA, USA). Data were acquired using a Multiclamp 700B amplifier (Axon, Sunnyvale, CA, USA) and Digidata 1440A (Axon, Sunnyvale, CA, USA). The recorded data were analyzed by the Mini Analysis program (Synaptosoft, Leonia, NJ) and Clampfit 10.3 software (Molecular Devices, Sunnyvale, CA, USA). The frequency of action potentials (AP) was recorded and analyzed.
QRT-PCR
In accordance with the protocol of the manufacturer, the total RNA had been extracted from harvested neurons by adopting an RNAiso Plus kit. In accordance with the protocol of the manufacturer, almost 2 µl of RNA had been adopted for obtaining complementary DNA (cDNA) by reversed transcription through adopting particular reversed transcription primers for U6 (RiboBio) and miRNA-34a as well as a Prime Script RT reagent kit with gDNA Eraser (TaKaRa). According to the protocol of the manufacturer, the qRT-PCR had been conducted by adopting a CFX96 Real-time PCR Detection System (BioRad Laboratories, Inc.) as well as a SYBR Premix Ex TaqII to discuss the expression of U6 and miRNA-34a adopting a particular Bulge-Loop™ miRNA qRT-PCR Primer Set. The miRNA-34a’s expression had been normalized to the levels of U6 and expressed as fold using comparative Ct method [14].
Western Blot Analysis
The total proteins were extracted from harvested neurons by adopting the extraction kits of proteins (Beyotime, Shanghai, China). The total proteins were separated by using 12% sodium dodecyl sulphate–polyacrylamide gels and transferred to PVDF membranes. The membranes have been incubated by using some corresponding primary antibodies at 4 °C overnight. The primary antibodies below had been adopted: rabbit anti-Bcl-2-associated X protein (Bax, 1:1000, Abcam), rabbit anti-B-cell lymphoma-2 (Bcl-2, 1:1000, Abcam), rabbit anti-active caspase-3 (1:1000, Abcam), rabbit anti-Hes5 (1:1000, Proteintech), Enhancer-of-split homologues 1 and rabbit anti-mammalian hairy (Hes1, 1:1000, Abcam, New York, UK), rabbit anti-Notch2 (1:1000, Abcam), rabbit anti-Notch1 (1:1000, Proteintech, Chicago, America), or rabbit anti-GAPDH (1:2000, Proteintech). The membranes had been washed for three times by using TBST and then incubated with horseradish peroxidase (HRP) labeled goat anti-rabbit secondary antibody (Santa Cruz, CA, America) at normal temperature for 1 h. At last, the bands had been illuminated by adopting Immobilon ECL (Millipore). The target strip’s gray value had been discussed by adopting Image J software;relative protein expression = gray value of target protein/gray value of GAPDH.
TUNEL Staining
Neuronal apoptosis had been detected via TUNEL staining, which had been conducted by adopting Apoptosis Detection Kit (Beyotime) following the instructions of the manufacturer. Rabbit anti-MAP2 (1:50; Boster) and corresponding secondary antibody goat anti-mouse Alex Flour 555 (1:50, Beyotime) had been adopted for labeling neurons.
Statistical Analysis
Statistics have been shown to be the mean ± SEM and discussed through adopting the t-test for the comparisons between two groups as well as one-way ANOVA for the comparison among various groups followed by the test of post hoc Tukey. Different points had been regarded to be statistically important for p < 0.05.
Results
SREDs Induces Increase of miR-34a Expression and Decrease of Notch Pathway Expression
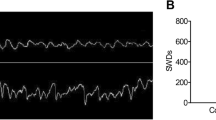
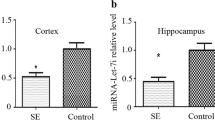
Immunofluorescence had been conducted with MAP2 and DAPI on the 7th day of culture. The results showed that over 90% of the cells positively stained for MAP2, which would be used for further studies (Fig. 1a). The recordings of the whole cell current clamp gained from neurons within cultures half a day after the treatment with low-Mg2+ (Mg2+-free group) for 3 h demonstrated significantly higher frequency of AP when compared with control group (Fig. 1b, n = 5 each group, p < 0.05). The expression of miRNA-34a in the cultured neurons of the Mg2+-free and control groups of cultured neurons was detected by qRT-PCR, and significantly higher level of miRNA-34a was detected in the Mg2+-free group when compared with control group (Fig. 1c, n = 5 each group, p < 0.05). Moreover, western blot was used to detect the expression of Notch signaling (including Notch1, Notch2, Hes1 and Hes5), and we found that those proteins were significantly decreased in Mg2+-free group when compared with control group (Fig. 1d, n = 5 each group, p < 0.05).
SREDs induces increase of miR-34a expression and decrease of Notch signaling expression a Immunofluorescence staining for DAPI and MAP2. Scale bars: 50 µm. b Representative trace of AP in hippocampal neurons within the Mg2+-free group as well as the control group. c The miRNA-34a’s expression within the Mg2+-free group as well as the control group had been detected by qRT-PCR. d The expression of Hes5, Hes1, Notch2 as well as Notch1 within the Mg2+-free group as well as the control group had been detected by western blot, *p < 0.05 vs. control group
SREDs Induces Neuronal Apoptosis
Neuronal apoptosis was detected by TUNEL staining and western blot. TUNEL staining revealed that the apoptosis rate in the Mg2+-free group was significantly higher than those in the control group (Fig. 2a, n = 5 each group, p < 0.05). Quantification by western blot showed that the expression of active caspase-3 was increased in the Mg2+-free group when compared with control group (Fig. 2b, n = 5 each group, p < 0.05). The expression of pro-apoptotic protein Bax was significantly higher, but anti-apoptotic protein Bcl-2 was significantly decreased in Mg2+-free group than that of control group (Fig. 2b, n = 5 each group, p < 0.05).
SREDs induce neuronal apoptosis a TUNEL staining of hippocampal neurons within the Mg2+-free group and the control group. Scale bars: 50 µm. b The expression of proteins which are related to apoptosis (active caspase-3, Bax as well as Bcl-2) within the Mg2+-free group as well as the control group had been detected by western blot. *p < 0.05 vs. control group
Inhibition of miRNA-34a Suppresses Epileptiform Discharges and Upregulates Notch Signaling
After treatment with NC, miRNA-34a mimics and miRNA-34a inhibitor, neurons were harvested and the expression of miRNA-34a was detected by qRT-PCR. As shown in Fig. 3a, the miRNA-34a expression was significantly increased in the mimics group when compared with the NC group; however, the miRNA-34a expression was significantly decreased in the inhibitor group when compared with the NC group (n = 5 each group, p < 0.05). To clarify the effect of miRNA-34a on epileptiform discharges, we performed electrophysiological evaluations of hippocampal neurons treated with miRNA-34a mimics, miRNA-34a inhibitor and NC. Our experiment showed that miRNA-34a inhibitor treatment significantly decreased the AP frequency when compared with the NC group (Fig. 3b, n = 5 each group, p < 0.05). In contrast, miRNA-34a mimics treatment significantly increased the AP frequency when compared with the NC group (Fig. 3b, n = 5 each group, p < 0.05). Those data suggest that miRNA-34a could significantly inhibit epileptiform discharges in our SREDs model of cultured hippocampal neurons. In addition, the expression of Notch signaling (including Notch1, Notch2, Hes1 and Hes5) had been detected by western blot. It was discovered that the proteins had been greatly reduced in the mimics group and increased in the inhibitor group when compared with NC group, indicating that inhibition of miRNA-34a could up-regulate Notch signaling (Fig. 3d, n = 5 each group, p < 0.05).
Inhibition of miRNA-34a suppresses epileptiform discharges and upregulates Notch signaling a The miRNA-34a’s expression within hippocampal neurons treated with miRNA-34a inhibitor, miRNA-34a mimics or NC was detected by qRT-PCR. b Representative trace of AP in hippocampal neurons treated with miRNA-34a inhibitor, miRNA-34a mimics or NC. c The expression of Notch1, Notch2, Hes1 and Hes5 in hippocampal neurons treated with NC, miRNA-34a mimics or miRNA-34a inhibitor had been detected by western blot. *p < 0.05 vs. NC group
Inhibition of miRNA-34a Inhibits Neuronal Apoptosis
After treatment with miRNA-34a mimics, miRNA-34a inhibitor and NC, neuronal apoptosis was also detected by TUNEL staining and western blot. TUNEL staining revealed that the apoptosis rate in the inhibitor group was significantly lower than those in the NC group (Fig. 4a, n = 5 each group, p < 0.05). However, apoptosis rate in the mimics group was significantly higher than those in the NC group (Fig. 4a, n = 5 each group, p < 0.05). Quantification by western blot showed that the expression of active caspase-3 and Bax was significantly decreased in the inhibitor group and significantly increased in the mimics group when compared with the NC group (Fig. 4b, n = 5 each group, p < 0.05). While the expression of Bcl-2 showed opposite way (Fig. 4b, n = 5 each group, p < 0.05). Those data suggest that inhibition of miRNA-34a could inhibit neuronal apoptosis.
Inhibition of miRNA-34a inhibits neuronal apoptosis a TUNEL staining of hippocampal neurons treated with miRNA-34a inhibitor, miRNA-34a mimics as well as NC. Scale bars: 50 µm. b The expression of proteins which are related with apoptosis (active caspase-3, Bax as well as Bcl-2) in hippocampal neurons treated with miRNA-34a inhibitor, miRNA-34a mimics or NC had been detected by western blot, *p < 0.05 vs. NC group
DAPT Weakens Anti-apoptosis Effect of miRNA-34a Inhibitor
Finally, to detect whether miRNA-34a regulates apoptosis through Notch signaling, we used DAPT to inhibit Notch signaling and investigated the alteration of neuronal apoptosis. We found that the expression of Hes1 and Hes5 in the inhibitor + DAPT group was higher than those in the DAPT group (Fig. 5a, n = 5 each group, p < 0.05), but lower that those in the inhibitor group (Fig. 5a, n = 5 each group, p < 0.05), indicating that DAPT suppresses miRNA-34a inhibitor-induced activation of Notch signaling. TUNEL staining revealed that the apoptosis rate in the inhibitor + DAPT group was significantly higher than that in the inhibitor group (Fig. 5b, n = 5 each group, p < 0.05). However, the apoptosis rate in the inhibitor + DAPT group was significantly lower than that in the lower group (Fig. 5b, n = 5 each group, p < 0.05). Additionally, quantification by western blot showed that the expression of active caspase-3 and Bax in the inhibitor + DAPT group was significantly higher than those in the inhibitor group (Fig. 5c, n = 5 each group, p < 0.05), but significantly lower than those in the DAPT group (Fig. 5c, n = 5 each group, p < 0.05). While the expression of Bcl-2 showed opposite way (Fig. 5c, n = 5 each group, p < 0.05). Those data suggest that DAPT weakens anti-apoptosis effect of miRNA-34a inhibitor.
DAPT weakens anti-apoptosis effect of miRNA-34a inhibitor a The expression of Hes1 and Hes5 in hippocampal neurons treated with RNA-34a inhibitor, DAPT or RNA-34a inhibitor + DAPT had been detected by western blot. b TUNEL staining of hippocampal neurons treated with RNA-34a inhibitor, DAPT or RNA-34a inhibitor + DAPT. Scale bars: 50 µm. c The expression of proteins which are related with apoptosis (active caspase-3, Bax as well as Bcl-2) in hippocampal neurons treated with RNA-34a inhibitor, DAPT or RNA-34a inhibitor + DAPT had been detected by western blot, *p < 0.05 vs. inhibitor group; #p < 0.05 vs. DAPT group
Discussion
Removal of Mg2+ from bath media can permanently transform hippocampal neurons into a network of cells spontaneously manifesting recurrent seizure discharges, which provides a novel in vitro model of sustained epileptiform discharge to elucidate the underlying biophysical, biochemical, and genetic mechanisms involved in the termination, maintenance and induction of the “epileptic condition” [11]. Similarly, in our study, the recordings of whole cell current clamp from control cultured hippocampal neurons demonstrated spontaneous excitatory (EPSPs), inhibitory (IPSPs) and occasional AP [11]. However, the neurons manifested recurrent epileptiform activity with paroxysmal depolarizing shifts or multiple AP at 12 h after treatment with Mg2+-free media, which suggested that the hippocampal neuronal SREDs pattern was successfully established. Moreover, we found obvious neuronal apoptosis in the Mg2+-free-treated neurons, which indicated close relationship between neuronal apoptosis and SREDs.
Apoptosis, a morphologically distinct form of cell death, is characterized by cytoplasmic condensation, preservation and packaging of intracellular organelles, DNA fragmentation, dispersal and phagocytosis of the cell as apoptotic bodies [15]. Accumulating evidence suggests that certain seizures could cause neuronal apoptosis, which makes contributions to the epilepsy phenotype, impairments in cognitive function or epileptogenesis [9]. In the earliest work, Pollard et al. found apoptotic DNA frag and DNA laddering within tissue samples from the brain of the rat after seizures [16]. After that, an increasing number of researchers focus on the mechanisms of seizures-induced neuronal apoptosis and tried to seek a novel or effective therapeutic strategy to suppress the progress of epilepsy through mediating neuronal apoptosis. Guo et al., for example, have found obvious neuronal apoptosis and tangeretin exerted effective neuroprotective impacts against pilocarpine-induced seizures via modulating apoptotic protein expressions [17]. In addition, many first-line antiepileptic drugs, such as sodium valproate and lamotrigine, have been proved to ameliorate seizures-induced neuronal apoptosis through various molecular mechanisms [18, 19]. However, the mechanisms of seizures-induced neuronal apoptosis are extremely complicated and require further studies.
Surprisingly, many studies indicate that a Notch signaling also plays an important role in regulating apoptosis [20, 21]. In mammalian, there are four receptors, containing Notch1, Notch2, Notch3, and Notch4, and five ligands, i.e. Jagged1, Jagged2, Delta-like-1, -3, and -4 [20]. After Notch ligand-receptor binding, Notch intracellular domain (NIC) is cleaved by γ-secretase, leading to the release of NIC and its entrance into the nucleus [20, 22]. Then, NIC activates Hes1 and Hes5 expression, which is was consistent with our results [23, 24]. It is the most widely accepted that activation of Notch signaling could inhibit apoptosis, but it remains controversial even in the same disease. For example, activation of Notch signaling suppressed the apoptosis, hippocampus neuronal injury as well as cerebral infarction [13], but acute blockage of Notch signaling improved functional outcome and inhibited neuronal apoptosis in the neonatal rat brain after stroke [25]. Similarly, the expression of Notch signaling within the brains of the patients who have epilepsy and experimental patterns also maintains to be controversial. For instance, Sha et al. have discovered Notch signaling had been up-regulated in the brain of epileptic patients and kainic acid-induced seizures mice, and that inhibition of Notch signaling could suppress seizure activity [26]. Using the same animal model, Sibbe et al. have found that a down-regulation of Notch signalling [27]. In addition, acute application of Notch 1 agonist Jagged 1 suppressed, whereas a Notch antagonist named DAPT promoted spike as well as wave discharges within WAG/Rij rats [28]. Those discrepancies could be explained by the choice of animals, cells and drug administrations. In present study, we found an increase of neuronal apoptosis and a decrease of Notch signaling in the hippocampal neuronal SREDs model, indicating negative correlation between Notch signaling and neuronal apoptosis in the hippocampal neuronal SREDs pattern.
The mechanisms of Notch signaling-mediated apoptosis have been sophisticated because of a variety of targets and signal pathways, particularly miRNA. Most of Notch members have been targeted by at least one miRNAs and miRNAs biogenesis influenced by the signal transduction [29]. Among them, miRNA-34a is widely believed to function as pro-apoptotic factor by targeting Notch signaling. A previous study has suggested that transient transfection of miRNA-34a into glioma and medulloblastoma cell lines might significantly promote apoptosis and reduce the levels of protein of Notch1 and Notch2. However, overexpression of Notch1 and Notch2 rescue partially cell cycle arrest as well as cell death induced by miR-34a in stem cells or glioma cells [30]. In addition, miRNA-34a can promote cardiac microvascular endothelial cells (CMEC) apoptosis, thus worsening CMEC damage and inhibiting angiogenesis by negatively targeting the Notch signaling [31]. This pro-apoptotic effect of miRNA-34a was also found in the neurons of epileptic rats [8]. However, whether or not this pro-apoptotic effect of miRNA-34a on epilepsy is associated with Notch signaling remains unclear. In present study, we found high level of miRNA-34a, low level of Notch signaling and obvious neuronal apoptosis in the hippocampal neuronal SREDs model. When those neurons were managed with miRNA-34a inhibitor, the level of Notch signaling was increased, which was accompanied by suppression of epileptiform discharges as well as neuronal apoptosis. However, miRNA-34a mimics showed adverse effects on epileptiform discharge, Notch pathway and neuronal apoptosis. Additionally, DAPT can accelerate apoptosis in miRNA-34a-inhibitor-treated neurons. Those data indicated that miRNA-34a regulates neuronal apoptosis through targeting Notch pathway.
In conclusion, our results demonstrate that miRNA-34a-mediated Notch signaling may participate in neuronal apoptosis after seizures, and inhibition of miRNA-34a suppresses epileptiform discharges through regulating Notch signaling-mediated neuronal apoptosis, which may provide a new potential target and therapeutic strategy for restraining the progress of epilepsy.
Abbreviations
- SREDs:
-
Spontaneous recurrent epileptiform discharges
- qRT-PCR:
-
Quantitative reverse-transcription polymerase chain reaction
- MAP2:
-
Protein-2 associated with anti-microtubule
- pBRS:
-
Physiological basal recording solution
- AP:
-
Action potentials
- Bax:
-
Bcl-2-associated X protein
- Bcl-2:
-
B-cell lymphoma-2
- HRP:
-
Horseradish peroxidase
- NIC:
-
Notch intracellular domain
References
Egbenya DL, Hussain S, Lai YC, Xia J, Anderson AE, Davanger S (2018) Changes in synaptic AMPA receptor concentration and composition in chronic temporal lobe epilepsy. Mol Cell Neurosci 92:93–103
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen HW, Mathern G, Moshé SL, Perucca E, Wiebe S, French J (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on therapeutic strategies. Epilepsia 51:1069–1077
Ma Y (2018) The challenge of microRNA as a biomarker of epilepsy. Curr Neuropharmacol 16:37–42
Reschke CR, Henshall DC (2015) microRNA and epilepsy. Adv Exp Med Biol 888:41–70
Henshall DC (2014) MicroRNA and epilepsy: profiling, functions and potential clinical applications. Curr Opin Neurol 27:199–205
Chen F, Hu SJ (2012) Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J Biochem Mol Toxicol 26:79–86
Lacombe J, Zenhausern F (2017) Emergence of miR-34a in radiation therapy. Crit Rev Oncol Hematol 109:69–78
Hu K, Xie YY, Zhang C, Ouyang DS, Long HY, Sun DN, Long LL, Feng L, Li Y, Xiao B (2012) MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. BMC Neurosci 13:115
Henshall DC, Simon RP (2005) Epilepsy and apoptosis pathways. J Cereb Blood Flow Metab 25:1557–1572
Deshpande LS, Nagarkatti N, Ziobro JM, Sombati S, DeLorenzo RJ (2008) Carisbamate prevents the development and expression of spontaneous recurrent epileptiform discharges and is neuroprotective in cultured hippocampal neurons. Epilepsia 49:1795–1802
Sombati S, Delorenzo RJ (1995) Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J Neurophysiol 73:1706–1711
Li XY, Wen JY, Jia CC, Wang TT, Li X, Dong M, Lin QU, Chen ZH, Ma XK, Wei LI, Lin ZX, Ruan DY, Chen J, Wu DH, Liu W, Tai Y, Xiong ZY, Wu XY, Zhang QI (2015) MicroRNA-34a-5p enhances sensitivity to chemotherapy by targeting AXL in hepatocellular carcinoma MHCC-97L cells. Oncol Lett 10:2691–2698
Guan J, Wei X, Qu S, Lv T, Fu Q, Yuan Y (2017) Osthole prevents cerebral ischemia-reperfusion injury via the Notch signaling pathway. Biochem Cell Biol 95:459–467
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Henshall DC (2007) Apoptosis signalling pathways in seizure-induced neuronal death and epilepsy. Biochem Soc Trans 35:421–423
Pollard H, Charriaut-Marlangue C, Cantagrel S, Represa A, Robain O, Moreau J, Ben-Ari Y (1994) Kainate-induced apoptotic cell death in hippocampal neurons. Neuroscience 63:7–18
Guo XQ, Cao YL, Hao F, Yan ZR, Wang ML, Liu XW (2017) Tangeretin alters neuronal apoptosis and ameliorates the severity of seizures in experimental epilepsy-induced rats by modulating apoptotic protein expressions, regulating matrix metalloproteinases, and activating the PI3K/Akt cell survival pathway. Adv Med Sci 62:246–253
Li Q, Li QQ, Jia JN, Cao S, Wang ZB, Wang X, Luo C, Zhou HH, Liu ZQ, Mao XY (2018) Sodium valproate ameliorates neuronal apoptosis in a kainic acid model of epilepsy via enhancing PKC-dependent GABAAR γ2 serine 327 phosphorylation. Neurochem Res 43:2343–2352
Zhang B, Zhang JW, Wang WP, Dong RF, Tian S, Zhang C (2017) Effect of lamotrigine on epilepsy-induced cognitive impairment and hippocampal neuronal apoptosis in pentylenetetrazole-kindled animal model. Synapse 71(2):e21945
Zeng C, Xing R, Liu J, Xing F (2016) Role of CSL-dependent and independent Notch signaling pathways in cell apoptosis. Apoptosis 21:1–12
Miele L, Osborne B (1999) Arbiter of differentiation and death: notch signaling meets apoptosis. J Cell Physiol 181:393–409
Chen G, Zhang Z, Cheng Y, Xiao W, Qiu Y, Yu M, Sun L, Wang W, Du G, Gu Y, Peng K, Xu C, Yang H (2014) The canonical Notch signaling was involved in the regulation of intestinal epithelial cells apoptosis after intestinal ischemia/reperfusion injury. Int J Mol Sci 15:7883–7896
Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R (1999) Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J 18:2196–2207
Kageyama R, Ohtsuka T (1999) The Notch-Hes pathway in mammalian neural development. Cell Res 9:179–188
Li Z, Wang J, Zhao C, Ren K, Xia Z, Yu H, Jiang K (2016) Acute blockage of notch signaling by DAPT induces neuroprotection and neurogenesis in the neonatal rat brain after stroke. Transl Stroke Res 7:132–140
Sha L, Wu X, Yao Y, Wen B, Feng J, Sha Z, Wang X, Xing X, Dou W, Jin L, Li W, Wang N, Shen Y, Wang J, Wu L, Xu Q (2014) Notch signaling activation promotes seizure activity in temporal lobe epilepsy. Mol Neurobiol 49:633–644
Sibbe M, Häussler U, Dieni S, Althof D, Haas CA, Frotscher M (2012) Experimental epilepsy affects Notch1 signalling and the stem cell pool in the dentate gyrus. Eur J Neurosci 36:3643–3652
Karimzadeh F, Modarres MSM, Alipour F, Hosseini RH, Kovac S, Gorji A (2017) Developmental changes in Notch1 and NLE1 expression in a genetic model of absence epilepsy. Brain Struct Funct 222:2773–2785
Yan D, Hao C, Xiao-Feng L, Yu-Chen L, Yu-Bin F, Lei Z (2018) Molecular mechanism of Notch signaling with special emphasis on microRNAs: implications for glioma. J Cell Physiol 234:158–170
Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, Johnson E, Marcinkiewicz L, Jiang J, Yang Y, Schmittgen TD, Lopes B, Schiff D, Purow B, Abounader R (2009) MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res 69:7569–7576
Li J, Gong J, Li X, Shen L, Xie Y, Zhang R (2019) MicroRNA-34a promotes CMECs apoptosis and upregulate inflammatory cytokines, thus worsening CMECs damage and inhibiting angiogenesis by negatively targeting the Notch signaling pathway. J Cell Biochem 120(2):1598–1609
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
Every animal use process was supported by the Animal Care and Use Committee of Shandong University and was in severe accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Zheng, Y., Cheng, X. et al. Inhibition of microRNA-34a Suppresses Epileptiform Discharges Through Regulating Notch Signaling and Apoptosis in Cultured Hippocampal Neurons. Neurochem Res 44, 1252–1261 (2019). https://doi.org/10.1007/s11064-019-02772-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02772-x