Abstract
Childhood absence epilepsy (CAE) is an epilepsy syndrome with seizures occurring in the early childhood, highlighting that seizures susceptibility in CAE is dependent on brain development. The Notch 1 signalling pathway is important in brain development, yet the role of the Notch1 signalling pathway in CAE remains elusive. We here explored Notch1 and its modulator notchless homologue 1 (NLE1) expression in WAG/Rij and control rats using immunohistochemistry. Functional Notch 1 effects were assessed in WAG/Rij rats in vivo. WAG/Rij rats lack the developmental increase in cortical Notch1 and NLE 1 mRNA expression seen in controls, and Notch 1 and NLE1 mRNA and protein expression were lower in somatosensory cortices of WAG/Rij rats when compared to controls. This coincided with an overall decreased cortical GFAP expression in the early development in WAG/Rij rats. These effects were region-specific as they were not observed in thalamic tissues. Neuron-to-glia ratio as a marker of the impact of Notch signalling on differentiation was higher in layer 4 of somatosensory cortex of WAG/Rij rats. Acute application of Notch 1 agonist Jagged 1 suppressed, whereas DAPT, a Notch antagonist, facilitated spike and wave discharges (SWDs) in WAG/Rij rats. These findings point to Notch1 as an important signalling pathway in CAE which likely shapes architectural organization of the somatosensory cortex, a region critically involved in developmental epileptogenesis in CAE. More immediate effects of Notch 1 signalling are seen on in vivo SWDs in CAE, pointing to the Notch 1 pathway as a possible treatment target in CAE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic generalized epilepsy (IGE) syndromes, such as childhood absence epilepsy (CAE), are electroclinical syndromes characterized by a sudden loss of consciousness associated with generalized spike and wave discharges recorded on scalp EEG (Loiseau and Panayiotopoulos 2005). It is intriguing that CAE occurs between 4 and 8 years of life, highlighting the importance of brain development in the pathogenesis of the disease. Although CAE is not inherited in a mendelian fashion, familial predisposition is common in IGE, and environmental factors likely play a minor role highlighting the role of genetics in this type of epilepsy (Durner et al. 2001; Greenberg et al. 1992). The electrographic hallmark of CAE is spike and wave complexes which occur both interictally and during the absence seizures forming an interictal-to-ictal continuum. The previous studies have shown that these spike and wave discharges are a feature of abnormal oscillations between the thalamus and neocortex (Gorji et al. 2011), with both human (Ferri et al. 1995; Niedermeyer 1996) and animal studies (Meeren et al. 2002; Polack et al. 2007, 2009), demonstrating a leading role of the somatosensory neocortex in generating these discharges.
Wistar Albino Glaxo from Rijswijk (WAG/Rij) rats are a faithful disease model for absence epilepsy and are widely used to study the disease (Coenen and Van Luijtelaar 2003; Sarkisova and van Luijtelaar 2011). The similarities of the WAG/Rij disease model to human epilepsy are the developmental occurrence of spike and wave complexes which appear at several months of age and increase by 6 months of age (Coenen and Van Luijtelaar 2003). Thus, the animal model reproduces several features of the human disease; most importantly, the developmental aspect of this type of epilepsy is represented in this animal model.
Brain development and brain maturation are complex processes which involve multiple signalling cascades. Notch signalling has been identified as such a signalling cascade with particular importance in brain development. Notch signalling is a conserved signalling pathway which is linked to multicellular organisms and the subsequent need for cell-to-cell communication for a coordinate development (Ables et al. 2011; Artavanis-Tsakonas et al. 1999). Notch family proteins are large transmembrane receptors which interact with membrane bound proteins of the Delta and Serrate/Jagged family (Schroeter et al. 1998). Notch 1 has been shown as a pivotal signalling pathway in the subventricular zone where it promotes radial glia formation during embryogenesis (Gaiano et al. 2000; Patten et al. 2003). Postnatally, Notch1 signalling promoted periventricular astrocyte formation (Gaiano et al. 2000). Not only astrocytes are affected by Notch signalling, but dendritic formation in neurons is critically modulated by Notch signalling which promotes dendritic branching while inhibiting dendritic growth (Redmond et al. 2000). Direct regulators of the Notch signalling pathway have been identified. Such a regulator is Notchless (NLE1) which has been identified through a genetic screen for suppressors of the notchoid mutant in drosophila (Royet et al. 1998). Interestingly, more recent data suggest that besides cell fate decisions, Notch signalling has more immediate effects on excitation and synaptic plasticity (Salama-Cohen et al. 2006; Sha et al. 2014; Wang et al. 2004). Notch signalling was shown to impact on seizures in a temporal lobe model of epilepsy with inhibition of Notch 1 suppressing seizure activity. Surprisingly, despite its role in development, very little is known about the impact of Notch 1 in CAE, where seizure occurrence in humans is coupled to developmental stages. We thus aimed to explore Notch1 expression and its modulator NLE1 expression in the neocortex and thalamus at different developmental stages in WAG/Rij rats. Furthermore, experiments were performed to determine how these changes impact on glial and neuronal expressions in different regions of the somatosensory cortex and thalamus, and areas which are critically involved in the generation of spike and wave discharges (SWDs) in WAG/Rij rats. Finally, we investigated whether modulation of the Notch1 pathway is functionally relevant in spike and wave discharges.
Materials and methods
Animals
Wistar and WAG/Rij rats were housed under controlled conditions (ambient temperature = 22 ± 2 °C, humidity = 40%; 12-h light/dark cycle) with food and water ad libitum. All experiments were carried out according to the protocol approved by the ethics committee of the Shefa Neuroscience Research Center, Tehran, Iran.
Electrocorticogram (ECoG) recording
2- and 6-month-old Wistar and WAG/Rij rats were anesthetized by chloral hydrate (350 mg/kg) and the heads were put in the stereotaxic frame. Soft tissues of the skull at the incision and at contact points were anesthetized using local application of 0.5% lidocaine hydrochloride (Bastany et al. 2016). Recording electrodes were implanted on the dura mater of the left and right parietal cortex. ECoG was recorded by connecting the silver recording electrodes to the amplifier (EXT-02 F, NPI, Germany). Sedation was maintained with repeated injections of fentanyldihydrogencitrat every 20–30 min (0.03 lg/kg body weight per hour, i.p.; Janssen-Cilag, Issy-Les-Moulineaux, France) and dehydrobenzperidol (3.79 mg/kg/h; i.p.). WAG/Rij rats display spike and wave discharges after puberty with increasing spike and wave discharges recorded at 6 months of age (Coenen and Van Luijtelaar 2003). Thus, multiple control groups were assigned to maintain best possible control for age related effects and rat strain-related effects. 2- and 6-month-old Wistar as well as 2-month-old WAG/Rij rats with no epileptic form activity in their ECoG were chosen as non-epileptic controls, whereas 6-month-old WAG/Rij rats displaying SWDs in their ECoG recording were considered as epileptic rats. ECoG was recorded for at least 6 h under sedation to evaluate SWDs occurrence.
RNA extraction and reverse transcription
New-born, 2- and 6- month-old Wistar and WAG/Rij rats (n = 6) were decapitated and the thalamus and parietal neocortex were dissected manually. Total RNA was isolated from tissue samples using Gene ALL Ribospin Kit. A DNase treatment was performed to remove genomic DNA by (RNase-Free DNase Set “DNase I”, Qiagen). Total RNA (500 ng) was used for cDNA synthesis using Maxima first-strand cDNA synthesis kit (Thermo Scientific; Germany) according to recommended instructions.
Real-time PCR
β-Actin primer sequences were obtained from the previous studies (Peinnequin et al. 2004). The Notch1, NLE1, NeuN, and GFAP primers were designed using AlleleID® 7.50 (Premier Biosoft International; USA). The primer pairs used in this study are either spanning exon–exon junctions or located on different exons. The optimal primer concentration was determined for each primer pair. The efficiency of qRT-PCR was approximately 2 for each pair of primers, which was calculated using serial 1:2 dilutions of template cDNA, on a CFX 96 Real-Time System. Real-time PCR reactions were performed in duplicate on a CFX 96 Real-Time System (Bio-Rad, Germany). The primer sequences for the gene and internal control are mentioned in the Table 1. Reactions were prepared in a total volume of 20 μl containing: 5 μl template, 0.5 μl of each 10 μM primer (Macrogen, The Netherlands), 4 μl of 5× HOT FIREPol® EvaGreen® qPCR Mix Plus (Solis BioDyne, Estonia), and 10 μl RNase/DNase-free sterile water (Sigma, Germany). The cycle conditions included an initial denaturation at 95 °C for 15 min, followed by 45 cycles of denaturation at 95 °C for 30 s. PCR was followed by a melting curve analysis to determine product specificity.
Histology analysis
2- and 6-month-old Wistar and WAG/Rij rats (n = 6) were deeply anesthetized by chloral hydrate and perfused transcardially with 200 ml of saline followed by 600 ml of 1% paraformaldehyde (PFA) solution. The brains were extracted and kept in 1% PFA for at least 4 days at 4 °C (Khodaie et al. 2015). Serial (8 µm) coronal sections were prepared. Ten sections from each animal were selected randomly and stained with toluidine blue. The number of neurons and glial cells was counted in the fourth and sixth layers of somatosensory cortex under a light microscope (BX51, Olympus, Japan) linked to a digital camera (100× objective). Neurons and glial cells were distinguished by their characteristic morphology. In addition, for differentiation of astrocytes from other glial cells, we evaluated distribution of GFAP by immunohistochemistry. The first section of each pair was considered as the reference and the second one was evaluated (Jafarian et al. 2015). On each section, at least five microscopic fields were evaluated randomly using un-biased frame and physical dissector counting rule. Neurons were differentiated to glial cells by staining pattern of nucleus and nucleolus. Nucleus of neurons was stained in light blue and nucleolus in dark blue. In contrast, nucleus of glial cells was coloured intensively in dark blue and the nucleolus was not visible. In each field, we measured the number of neurons and the number of glial cells.
Immunohistochemistry analysis
Paraffin embedded sections in 1.8–3.3 mm posterior to the bregma (Paxinos and Watson 1997) from 2- and 6-month-old Wistar and WAG/Rij rats were cleared and rehydrated through series of xylol and alcohol and washed with phosphate buffered saline (PBS) three times and boiled in citrate buffer at 95 °C for 10 min. After cooling at room temperature, the slides were incubated in 10% normal goat serum and 0.2% Twin 20 in PBS for 1 h at room temperature (Sadeghian et al. 2012). Slides were incubated over night at 4 °C with commercial rabbit polyclonal anti-rat antibodies: GFAP (1:100; Santa Cruz, Germany), NueN (1:300; Abcam, UK), NLE1 (1:350; Santa Cruz, Germany), and Notch1 (1:350; Santa Cruz, Germany).
The antibodies were diluted in a solution containing 1–5% normal goat serum (NGS) in 0.3% Triton X-100 and 0.1 M PBS at pH of 7.4. The sections were then rinsed three times in PBS (10 min each), and incubated with FITC goat anti-rabbit secondary antibody (Santa Cruz, Germany) diluted at 1:500 in PBS with 0.3% Triton X-100 and 5% NGS at 22 °C for 1 h. Nuclei were counterstained by immersing sections in a solution of propidium iodide (75 µg/ml) for 5 min at 37 °C. The slides were mounted on a cover slip with 90% glycerol mounting buffer and visualized with a fluorescent microscope (Olympus, Japan). Control for the specificity of immunostaining was performed by the omission of the primary antibody. Images for analysis were acquired with a digital camera attached to the microscope (40× objective). The number of cells labelled with the primary antibodies was counted per mm2 in the fourth and sixth layers of somatosensory cortex as well as in different thalamic nuclei.
Drug application
6-month-old WAG/Rij (epileptic) rats were divided to vehicle, agonist, and antagonist groups. Recording electrodes were stereotaxically implanted on the dura mater of the left and right parietal cortex. A hole was made on the skull bone (AP = −4.3 mm, ML = ±5.2 mm) for local drug application on the somatosensory cortex according to the coordinates provided by the atlas Paxinos and Watson (1997). After appearance of SWDs and before application of any drugs, ECoG was recorded for 30 min to establish a baseline activity. Then, the recombinant protein of Jagged1; agonist of Notch1 receptor (R&D systems, USA), DAPT; antagonist of Notch1 receptor (Sigma, Germany); or vehicle were injected (0.5 μl/2 min) in the somatosensory cortex. The recombinant protein of Jagged1 was dissolved in PBS (10 µg/1 µl). DAPT was dissolved in DMSO and was injected in a concentration of 50 µM (Sha et al. 2014). PBS containing 0.5% DMSO was injected at the same volume and rate in the vehicle group. ECoG was recorded 6 h after application of drugs and vehicle. The amplitude and duration of SWDs were calculated using the AxoScope 10 software (Axon Instruments, USA).
Statistical analysis
All data are given as mean ± S.E.M. The immunohistochemical and Real-Time PCR data were analysed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc tests. The amplitude and duration of SWDs were statistically analysed by the Friedman and the Wilcoxon test. Significance was established by p ≤ 0.05.
Results
6-month-old WAG/Rij rats with typical spike-wave complexes during ECoG recording were selected as epileptic animals. SWDs (5–43, n = 45) appeared at a frequency of 5–10 Hz and peak amplitudes between 250 and 1500 µV. The SWDs intervals were between 0.4 and 12 min with a duration from 400 ms to 3 min (average, 6 ± 0.5 s; n = 45). 2-month-old WAG/Rij rats as well as 2- and 6-month-old Wistar rats with no SWDs during 6 h recording of ECoG served as non-epileptic control animals (Fig. 1).
Real-time PCR assessment
Cortical expression of Notch1, NLE 1, GFAP, and NeuN
The expression of Notch 1, NLE 1, NeuN, and GFAP genes was evaluated in the parietal neocortex of newborn, 2- and 6- month-old Wistar and WAG/Rij rats (Fig. 2). Notch 1 as well as NLE1 showed significantly higher expression in 2- and 6-month-old Wistar rats when compared to Wistar newborn rats (p < 0.001). In contrast, there was no significant difference in Notch 1 and NLE1 gene expression between different ages of the WAG/Rij rats. In addition, the expression of Notch 1 and NLE1 in 2- and 6-month-old WAG/Rij was significantly lower than age-matched Wistar rats (p < 0.001).
mRNA levels of Notch 1, NLE1, GFAP, and NeuN in the parietal cortex. Bar charts summarizing cortical mRNA levels of Notch 1, NLE1, GFAP, and NeuN in 2- and 6-month-old WAG/Rij and age-matched Wistar rats (a). Note that the mRNA levels of Notch 1, NLE1, GFAP, and NeuN increase during development in Wistar rats, whereas WAG/Rij rats lack this increase in Notch 1 and NLE1 mRNA expression, and exhibit a proportionally small increase in GFAP mRNA expression when compared to Wistar rats. *p < 0.05 **p < 0.01 and ***p < 0.001. Scatter plots visualizing correlations between mRNA expression of Notch 1 and NLE1 (b), GFAP (c), and NeuN (d), respectively. Note that all three correlations are strong (Correlation coefficient r = 0.80 (b), r = 0.73 (c), and r = 0.57 (d) all p < 0.001)
GFAP, a marker of astrocytes, was highly expressed during cortical development in both strains. There was a significant increase of GFAP expression in 2- and 6-month-old Wistar and WAG/Rij rats compared to newborn rats (p < 0.001). The expression was significantly decreased in 2-month-old WAG/Rij rats compared to age-matched Wistar rats (p < 0.001).
The expression of NeuN as a neural marker increased significantly in 2-month-old Wistar when compared to newborn Wistar pups (p < 0.001). This developmental increase in NeuN expression was not seen in WAG/Rij rats. Furthermore, comparison of WAG/Rij rats with age-matched Wistar rats did not show significant difference.
Correlation between the cortical expression of Notch 1 and NLE 1, and GAFP and NeuN showed that an increase in expression of Notch1 was accompanied by an increase in expression of NLE 1 (r = 0.80, Fig. 2b) as well as GAFP (r = 0.73, Fig. 2c) and NeuN (r = 0.57, Fig. 2d) during development of brain in Wistar and WAG/Rij rats (p < 0.001).
Thalamic expression of Notch1, NLE 1, GFAP, and NeuN
The expression of Notch 1, NLE 1, NeuN, and GFAP genes were evaluated in the thalamus of newborn, 2- and 6-month-old Wistar and WAG/Rij rats (Fig. 3a). Thalamic expression of Notch 1 increased significantly in 2- and 6-month-old Wistar and WAG/Rij rats when compared to newborn rats (p < 0.01). There was no significant difference in Notch1 gene expression between Wistar and WAG/Rij rats. The variation of NLE1 expression was not significant during the three developmental stages in both Wistar and WAG/Rij rats. The expression of GFAP increased significantly in 2- and 6-month-old Wistar and WAG/Rij rat compared to newborn rats (p < 0.01 and p < 0.001, respectively). In addition, the expression of GFAP was significantly lower in 2-month-old WAG/Rij rats compared to age-matched Wistar rats (p < 0.05). The expression of NeuN significantly increased in 2- and 6-month old Wistar rats compared to newborn rats (p < 0.001). NeuN expression was significantly higher in 6-month-old WAG/Rij rats when compared to newborn rats (p < 0.001). There was no correlation between the expression of Notch 1, NLE 1, GAFP, and NeuN in the thalamus.
mRNA levels of Notch 1, NLE1, GFAP, and NeuN in the thalamus. Bar charts summarizing mRNA levels of Notch 1, GFAP, and NeuN in the thalamus of 2- and 6-month-old WAG/Rij and age-matched Wistar rats. Note that thalamic mRNA level of Notch 1, GFAP, and NeuN increases significantly during development. There are no significant differences between the strains except for a greater increase of GFAP in 2-month-old Wistar rats when compared to age-matched WAG/Rij rats. *p < 0.05** p < 0.01 and ***p < 0.001
Histological assessment
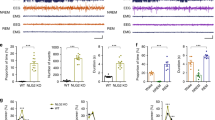
We evaluated the mean number of neurons with respect to glial cells (N/G index) in the fourth and sixth layers of somatosensory cortex. In the fourth cortical layer, the mean N/G index was higher in 2- and 6- month-old WAG/Rij rats when compared to age-matched-Wistar controls (p < 0.01; Fig. 4). There was no significant difference between age-matched Wistar and WAG/Rij strains in the sixth cortical layer.
Neuron-to-glia ratio (N/G index) in the somatosensory cortex. Immunhistochemistry (40× magnification) of rat somatosensory cortex of 2- and 6-month-old Wistar and WAG/Rij rats stained with toluidine blue (a). Bar charts summarizing mean ± SEM of the N/G index in the fourth and sixth layers of somatosensory cortex. p < 0.01 comparing rats of the same age
Immunohistochemistry assessment
Distributions of Notch1, NLE 1, GFAP, and NeuN expression were evaluated in the fourth and sixth layers of somatosensory cortex as well as dorsal, lateral, and medial thalamic nuclei in 2- and 6-month-old Wistar and WAG/Rij rats.
Notch1
Notch1 receptor expression decreased significantly in the fourth and sixth layers of somatosensory cortex (40%, p < 0.05) as well as dorsal thalamic nuclei (50%, p < 0.05) of 6-month-old when compared to 2-month-old in WAG/Rij rats (Fig. 5). Furthermore, in medial thalamic nuclei, the expression of Notch 1 decreased significantly in 2-month-old when compared to 6-month-old in Wistar rats (50%, p < 0.05).
Notch 1 expression in the somatosensory cortex and different thalamic nuclei. Representative immunohistochemistry photomicrographs of Notch 1 expression (green). Nuclei are stained in red. Different cortical and thalamic brain areas in 2- and 6-month-old Wistar and WAG/Rij rats are shown (a). Bar charts summarizing Notch 1 expression densities in different brain areas of 2- and 6-month-old Wistar rats and WAG/Rij rats (b). *p < 0.05
NLE1
NLE1 expression decreased significantly in the fourth and sixth layers in 6-month-old WAG/Rij rats when compared to 2-month-old WAG/Rij (65% and 73%, respectively, p < 0.001) and 6-month-old Wistar rats (67% and 75%, respectively, p < 0.001). In dorsal and lateral thalamic nuclei, NLE1 expression decreased (45.5% and 43%, respectively, p < 0.01) significantly in 6-month-old WAG/Rij rats when compared to age-matched Wistar rats (Fig. 6).
NLE1 expression in the somatosensory cortex and different thalamic nuclei. Representative immunohistochemistry photomicrographs of NLE1 expression (green). Nuclei are stained in red. Different cortical and thalamic brain areas in 2- and 6-month-old Wistar and WAG/Rij rats are shown (a). Bar charts summarizing NLE1 expression densities in different brain areas of 2- and 6-month-old Wistar rats and WAG/Rij rats (b) ***p < 0.001. **p < 0.01
GFAP
With regard to GFAP expression in different brain areas, significant differences were seen in fourth cortical layer and lateral thalamic nuclei. In the other cortical and thalamic brain areas, no significant differences were seen. In the fourth cortical layer, GFAP-positive cells increased significantly in 6-month-old Wistar rats compared to 2-month-old Wistar rats (p < 0.01). In contrast, there was no significant difference between 2- and 6-month-old WAG/Rij rats (Fig. 7). In the lateral thalamic nuclei of WAG/Rij rats, GFAP-positive cells decreased significantly in 6-month olds compared to 2-month olds (p < 0.001). In addition, a significant increase was observed in 2-month-old WAG/Rij when compared to age-matched Wistar rats (p < 0.01).
GFAP expression in the somatosensory cortex and different thalamic nuclei. Representative immunohistochemistry photomicrographs of GFAP expression (green). Nuclei are stained in red. Different cortical and thalamic brain areas in 2- and 6-month old Wistar and WAG/Rij rats are shown (a). Bar charts summarizing GFAP expression densities in different brain areas of 2- and 6-month-old Wistar rats and WAG/Rij rats (b). **p < 0.01, ***p < 0.001
NeuN
Assessment of NeuN expression showed a significant increase (p < 0.001) in the fourth layer of somatosensory cortex in 6 months old when compared to 2-month-old Wistar rats (Fig. 8).
NeuN expression in the somatosensory cortex and different thalamic nuclei. Representative immunohistochemistry photomicrographs of NeuN expression (green). Nuclei are stained in red. Different cortical and thalamic brain areas in 2- and 6-month-old Wistar and WAG/Rij rats are shown (a). Bar charts summarizing NeuN expression densities in different brain areas of 2- and 6-month-old Wistar rats and WAG/Rij rats (b). ***p < 0.001 **p < 0.01
In dorsal thalamic nuclei, NeuN expression decreased significantly in 6-month-old WAG/Rij rats compared to age-matched Wistar rats (p < 0.01).
Drug administration
Given these striking differences in Notch and its counter player NLE1 expression between epileptic rats and non-epileptic controls, we next asked whether Notch modulation translates into a functional effect, such as altered excitability as measures by the frequency of spike and wave discharges. Micro injection of Notch1 agonist (Jagged 1) on the somatosensory cortex reduced the amplitude of SWDs (Fig. 9a, b, p < 0.01). The mean of SWDs amplitude before and after agonist application was 0.79 ± 0.02 and 0.62 ± 0.01 mV, respectively. In addition, the duration of SWDs decreased (1.6 ± 0.3 s) after application of Notch 1 agonist (Fig. 9a, c, p < 0.05), whereas SWDs duration increased (2.6 ± 0.2 s) after application of Notch 1 antagonist (Fig. 9a, c, p < 0.01).
Discussion
Here, we demonstrate that WAG/Rij rats lack the developmental increase in cortical Notch1 and NLE 1 mRNA expression as it is seen in wild-type rats. In addition, cortical Notch 1 and NLE1 mRNA and protein expression were altogether diminished in WAG/Rij when compared to control rats. This resulted in an overall decreased GFAP expression in the early development in WAG/Rij rats when compared to control. Interestingly, thalamic Notch1, NLE1, GFAP, and NeuN mRNA expression was not different between epileptic rats and controls, indicating a brain region-specific effect. Neuron-to-glia ratio as a marker of the impact of Notch signalling on differentiation of these cell types was higher in layer 4 somatosensory cortex of WAG/Rij rats when compared to Wistar controls indicating a relative lack of astrocytes. Notch1 signalling had an impact on excitability in WAG/Rij rats as spike and wave discharges were suppressed by agonists at the Notch1 receptor and facilitated by Notch1 antagonism.
Notch signalling has been identified as an important signalling route in brain development. Neuronal induction of radial glia formation is mediated by Notch1 signalling (Patten et al. 2003). Notch signalling is pivotal in the subventricular zone where it regulates radial glia differentiation and neural stem call renewal (Nyfeler et al. 2005; Patten et al. 2006). Radial glia cells have the potential to differentiate into astrocytes, neurons, and oligodendrocytes. Multiple studies have confirmed the impact of Notch1 on neurogenesis which is stimulated by Notch expression (Hatakeyama and Kageyama 2006). This was also shown in the adult hippocampus where Notch regulates cell fate and dendrite morphology of neurons (Breunig et al. 2007). Data from knockout experiments show that null heterozygous mutations in Notch1 result in spatial learning and memory deficits without affecting other forms of learning, motor control, or exploratory activity (Costa et al. 2003). Notch1 homozygos mice are lethal (Conlon et al. 1995), highlighting again the importance of Notch signalling for normal brain development. Strong postnatal Notch1 expression has been shown in the subventricular zone and in the dentate gyrus, regions which are involved in adult neurogenesis (Stump et al. 2002). Notch1 expression was also found to be particularly high in hippocampus and in the cerebellum (Berezovska et al. 1998). In the forebrain, Notch is expressed in different cell populations, such as neurons, astrocytes, precursors, ependymal cells, and endothelium (Breunig et al. 2007; Carlén et al. 2009; Sestan et al. 1999; Stump et al. 2002). Notch signalling has been shown to be involved in gliosis (Shimada et al. 2011; Zhang et al. 2015). Several neurological diseases are associated with brain gliosis. Perhaps, the most prominent example is epilepsy where gliotic tissue may be seen in hippocampal sclerosis. Data about notch signalling in epilepsy are scarce and focus on temporal lobe epilepsy. Liu and colleagues were able to show that Notch1 expression was increased in temporal neocortex and hippocampus in an experimental status epilepticus animal model and in an animal model of recurrent spontaneous seizures. Moreover, they found increased Notch expression in human tissue from patients undergoing temporal lobe resections for pharmacoresistant seizures (Liu et al. 2014). Interestingly, Sibbe and colleagues found diminished Notch signalling in neural stem cells (NSCs) in the dentate gyrus after status epilepticus in mouse model of TLE using unilateral intrahippocampal kainic acid, showing that excessive seizure activity, such as is seen in status epilepticus, affects the stem cell niche (Sibbe et al. 2012). We also found diminished Notch1 expression in somatosensory cortex in WAG/Rij when compared to controls. The occurrence of spike and wave discharges and thus increased excitability is one explanation why Notch1 expression was diminished and would parallel the findings of Sibbe and colleagues. In fact, our data show that Notch1 expression in layer four and six of somatosensory cortex and in some regions of the thalamus decreases in 6-month-old WAG/Rij rats when compared to 2-month-old rats. 6-month-old WAG/Rij rats display robust spike and wave activity, and have developed the phenotype of CAE coinciding with decreased Notch signalling. We found higher neuron-to-glia ratios in somatosensory cortex of WAG/Rij rats when compared to controls. Notch expression levels were also lower in somatosensory cortex of WAG/Rij rats when compared to controls. The somatosensory cortex has been shown to be the initiation site of spike and wave discharges in the WAG/Rij model of epilepsy and human studies in absence seizures have underscored this finding (Ferri et al. 1995; Meeren et al. 2002; Niedermeyer 1996; Polack et al. 2007, 2009). It is interesting that detailed current source density analysis of spike and wave discharges in the WAG/Rij epilepsy model revealed that a strong sink was found in layer 4 of the somatosensory cortex. These findings might be explained by a difference in tissue composition such as is reflected in the differences in neuron-to-glia ratio observed in our study.
Very little attention has been payed to notchless NLE1, a regulator of the Notch1 pathway (Royet et al. 1998). NLE1 interacts directly with the intracellular domain of the Notch receptor and regulates the activity of the Notch signalling pathway (Chantha et al. 2007).
Regulation of Notch1 by NLE1 is complex and reducing NLE1 activity has been found to increase Notch activity, whereas overexpression surprisingly also increased Notch1 activity suggesting a dominant-negative effect of NLE1 on Notch1 (Royet et al. 1998). In our study, NLE1 changes paralleled Notch1 changes, a finding which could well be explained by a dominant-negative effect. Overall, similar to Notch1 levels, NLE1 cortical mRNA expression and protein expression as measured by NLE1 positive cells were diminished in epileptic rats when compared to non-epileptic Wistar rats and strain-specific controls. This finding suggest that Notch changes and NLE1 changes are interconnected.
A more recent study has focussed on Notch signalling and its effect on angiogenesis after status epilepticus (Zhai et al. 2015). They found a stimulation of angiogenesis via Notch1 signalling in reactive astrocytes. Notch signalling is traditionally viewed as a developmental pathway, but, recently, more immediate effects of Notch signalling have been described. Notch signalling has been shown to impact on neuronal excitation and inhibition (Salama-Cohen et al. 2006; Sha et al. 2014; Wang et al. 2004). Decreased dendrite outgrowth and increased inhibitory (GABAergic) synaptogenesis have been described after activation of the Notch signalling pathway in hippocampal neurons (Salama-Cohen et al. 2006). Reduced long-term potentiation (LTP) at hippocampal CA1 has been shown in mice with reduced Notch levels and Jagged1 enhanced LTP in control mice, showing that Notch1 is important in memory function (Wang et al. 2004). Effects of Notch activation on acute and chronic seizures activity have been reported in an animal model of temporal lobe epilepsy (Sha et al. 2014). Sha and colleagues found endogenous upregulation of Notch signalling after kainic acid induced epilepsy. This upregulation was seen as soon as 3 h after seizure induction by kainic acid. Using the same animal model, it has been revealed that a downregulation of Notch signalling (Sibbe et al. 2012). These controversial findings might be explained by different cell population studies. In their study, Sibbe and colleagues focussed on NSCs which are distinct from mature neurons studied by Sha and colleagues. In our study, seizure activities did not affect Notch signalling as there was no difference in Notch 1 expression between non-epileptic (new-born and 2-month-old) and epileptic (6-month-old) WAG/Rij rats. This suggests that downregulation of Notch1expression in WAG/Rij rats (compared to controls) may play a role in the occurrence of SWDs in older WAG/Rij rats and is not a result of seizure activity.
Notch signalling does not only impact on development but has been shown to have more acute effects on excitability. A recent study showed that DAPT, an inhibitor of Notch, inhibited ictal activity in the kainate model of epilepsy. Jagged1, an agoinst at the Notch1 receptor, had proconvulsant effects (Sha et al. 2014). In contrast to this, we found that Jagged1 had an anticonvulsant effect, as it decreased the duration and amplitude of spike and wave discharges, and DAPT had a proconvulsant effect and increased the duration of spike and wave discharges. These findings point to a specific effect of Notch1 activation depending on the type of epilepsy with proconvulsant properties in temporal lobe and anticonvulsant properties in absence epilepsy. Such opposing responses to substances are well known, and in fact, anticonvulsant drugs which are efficient in one type of epilepsy might not be suitable for other epilepsy syndromes. Such an example is carbamazepine, which is well suited in focal epilepsies, but is known to exacerbate seizures in IGE (Genton 2000).
Our findings support a critical role of Notch1 signalling as a developmental pathway in an absence model of epilepsy. In addition, modulation of the Notch1 pathway in this established absence epilepsy model affected SWDs and thus may represent a novel potential treatment target for CAE.
Change history
02 August 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s00429-024-02843-y
References
Ables JL, Breunig JJ, Eisch AJ, Rakic P (2011) Not(ch) just development: notch signalling in the adult brain. Nat Rev Neurosci 12:269–283. doi:10.1038/nrn3024
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776. doi:10.1126/science.284.5415.770
Bastany ZJ, Askari S, Dumont GA, Speckmann EJ, Gorji A (2016) Non-invasive monitoring of spreading depression. Neuroscience 333:1–12. doi:10.1016/j.neuroscience.2016.06.056
Berezovska O, Xia MQ, Hyman BT (1998) Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J Neuropathol Exp Neurol 57:738–745. doi:10.1097/00005072-199808000-00003
Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P (2007) Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci USA 104:20558–20563. doi:10.1073/pnas.0710156104
Carlén M, Meletis K, Göritz C, Darsalia V, Evergren E, Tanigaki K et al (2009) Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci 12:259–267. doi:10.1038/nn.2268
Chantha S-C, Tebbji F, Matton DP (2007) From the notch signaling pathway to ribosome biogenesis. Plant Signal Behav 2:168–170. doi:10.1007/s00425-006-0420-z
Coenen AML, Van Luijtelaar ELJM (2003) Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet 33:635–655. doi:10.1023/A:1026179013847
Conlon RA, Reaume AG, Rossant J (1995) Notch1 is required for the coordinate segmentation of somites. Development 121:1533–1545
Costa RM, Honjo T, Silva AJ (2003) Learning and memory deficits in Notch mutant mice. Curr Biol 13:1348–1354. 10.1016/S0960-9822(03)00492-5
Durner M, Keddache MA, Tomasini L, Shinnar S, Resor SR, Cohen J et al (2001) Genome scan of idiopathic generalized epilepsy: evidence for major susceptibility gene and modifying genes influencing the seizure type. Ann Neurol 49:328–335. doi:10.1002/ana.69
Ferri R, Iliceto G, Carlucci V (1995) Topographic EEG mapping of 3/s spike-and-wave complexes during absence seizures. Ital J Neurol Sci 16:541–547. doi:10.1007/BF02282912
Gaiano N, Nye JS, Fishell G (2000) Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 26:395–404. 10.1016/S0896-6273(00)81172-1
Genton P (2000) When antiepileptic drugs aggravate epilepsy. Brain Dev 22:75–80. 10.1016/S0387-7604(99)00113-8
Gorji A, Mittag C, Shahabi P, Seidenbecher T, Pape H-C (2011) Seizure-related activity of intralaminar thalamic neurons in a genetic model of absence epilepsy. Neurobiol Dis 43:266–274. doi:10.1016/j.nbd.2011.03.019
Greenberg DA, Durner M, Delgado-Escueta AV (1992) Evidence for multiple gene loci in the expression of the common generalized epilepsies. Neurology 42:56–62. 10.1086/302371
Hatakeyama J, Kageyama R (2006) Notch1 expression is spatiotemporally correlated with neurogenesis and negatively regulated by Notch1-independent Hes genes in the developing nervous system. Cereb Cortex 16(Suppl 1):i132–i137. doi:10.1093/cercor/bhj166
Jafarian M, Karimzadeh F, Alipour F, Attari F, Lotfinia AA, Speckmann E-J et al (2015) Cognitive impairments and neuronal injury in different brain regions of a genetic rat model of absence epilepsy. Neuroscience 298:161–170. doi:10.1016/j.neuroscience.2015.04.033
Khodaie B, Lotfinia AA, Ahmadi M, Lotfinia M, Jafarian M, Karimzadeh F et al (2015) Structural and functional effects of social isolation on the hippocampus of rats with traumatic brain injury. Behav Brain Res 278:55–65. doi:10.1016/j.bbr.2014.09.034
Liu X, Yang Z, Yin Y, Deng X (2014) Increased expression of Notch1 in temporal lobe epilepsy: animal models and clinical evidence. Neural Regen Res 9: 526–533. doi:10.4103/1673-5374.130083
Loiseau P, Panayiotopoulos CP (2005) Childhood absence epilepsy. Available at http://www.ilae-epilepsy.org/Visitors/Centre/ctf. Ref Type: Internet Communication. Accessed 10 Sept 2016
Meeren HKM, Pijn JPM, Van Luijtelaar ELJM, Coenen AML, Lopes da Silva FH (2002) Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci 22:1480–1495
Niedermeyer E (1996) Primary (idiopathic) generalized epilepsy and underlying mechanisms. Clin Electroencephalogr 27:1–21. doi:10.1177/155005949602700103
Nyfeler Y, Kirch RD, Mantei N, Leone DP, Radtke F, Suter U et al (2005) Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J 24:3504–3515. doi:10.1038/sj.emboj.7600816
Patten BA, Peyrin JM, Weinmaster G, Corfas G (2003) Sequential signaling through Notch1 and erbB receptors mediates radial glia differentiation. J Neurosci 23:6132–6140
Patten BA, Sardi SP, Koirala S, Nakafuku M, Corfas G (2006) Notch1 signaling regulates radial glia differentiation through multiple transcriptional mechanisms. J Neurosci 26:3102–3108. doi:10.1523/JNEUROSCI.4829-05.2006
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates, Compact 3rd edn. Academic Press, San Diego
Peinnequin A, Mouret C, Birot O, Alonso A, Mathieu J, Clarençon D et al (2004) Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol 5:3. doi:10.1186/1471-2172-5-3
Polack P-O, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S (2007) Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence seizures. J Neurosci 27:6590–6599. doi:10.1523/JNEUROSCI.0753-07.2007
Polack P-O, Mahon S, Chavez M, Charpier S (2009) Inactivation of the somatosensory cortex prevents paroxysmal oscillations in cortical and related thalamic neurons in a genetic model of absence epilepsy. Cereb Cortex 19:2078–2091. doi:10.1093/cercor/bhn237
Redmond L, Oh SR, Hicks C, Weinmaster G, Ghosh A (2000) Nuclear Notch1 signaling and the regulation of dendritic development. Nat Neurosci 3:30–40. doi:10.1038/71104
Royet J, Bouwmeester T, Cohen SM (1998) Notchless encodes a novel WD40-repeat-containing protein that modulates Notch signaling activity. EMBO J 17:7351–7360. doi:10.1093/emboj/17.24.7351
Sadeghian H, Jafarian M, Karimzadeh F, Kafami L, Kazemi H, Coulon P et al (2012) Neuronal death by repetitive cortical spreading depression in juvenile rat brain. Exp Neurol 233:438–446. doi:10.1016/j.expneurol.2011.11.017
Salama-Cohen P, Arévalo M-A, Grantyn R, Rodríguez-Tébar A (2006) Notch and NGF/p75NTR control dendrite morphology and the balance of excitatory/inhibitory synaptic input to hippocampal neurones through Neurogenin 3. J Neurochem 97:1269–1278. doi:10.1111/j.1471-4159.2006.03783.x
Sarkisova K, van Luijtelaar G (2011) The WAG/Rij strain: a genetic animal model of absence epilepsy with comorbidity of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35: 854–876. doi:10.1016/j.pnpbp.2010.11.010
Schroeter EH, Kisslinger JA, Kopan R (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393:382–386. doi:10.1038/30756
Sestan N, Artavanis-Tsakonas S, Rakic P (1999) Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science 286:741–746. doi:10.1126/science.286.5440.741
Sha L, Wu X, Yao Y, Wen B, Feng J, Sha Z et al (2014) Notch signaling activation promotes seizure activity in temporal lobe epilepsy. Mol Neurobiol 49:633–644. doi:10.1007/s12035-013-8545-0
Shimada IS, Borders A, Aronshtam A, Spees JL (2011) Proliferating reactive astrocytes are regulated by Notch-1 in the peri-infarct area after stroke. Stroke 42:3231–3237. doi:10.1161/STROKEAHA
Sibbe M, Häussler U, Dieni S, Althof D, Haas CA, Frotscher M (2012) Experimental epilepsy affects Notch1 signalling and the stem cell pool in the dentate gyrus. Eur J Neurosci 36:3643–3652. doi:10.1111/j.1460-9568.2012.08279.x
Stump G, Durrer A, Klein A-L, Lütolf S, Suter U, Taylor V (2002) Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech Dev 114:153–159. 10.1016/S0925-4773(02)00043-6
Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK et al (2004) Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci USA 101:9458–9462. doi:10.1073/pnas.0308126101
Zhai X, Liang P, Li Y, Li L, Zhou Y, Wu X et al (2015) Astrocytes regulate angiogenesis through the jagged1-mediated Notch1 pathway after status epilepticus. Mol Neurobiol 53(9):5893–5901. doi:10.1007/s12035-015-9492-8
Zhang Y, He K, Wang F, Li X, Liu D (2015) Notch-1 signaling regulates astrocytic proliferation and activation after hypoxia exposure. Neurosci Lett 603:12–18. doi:10.1016/j.neulet.2015.07.009
Acknowledgements
This work was supported by the Shefa Neuroscience Research Center, Tehran, Iran, grant related to Dr-Thesis 33817, and Iran National Science Foundation, Tehran, Iran (INSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s00429-024-02843-y
About this article
Cite this article
Karimzadeh, F., Modarres Mousavi, S.M., Alipour, F. et al. RETRACTED ARTICLE: Developmental changes in Notch1 and NLE1 expression in a genetic model of absence epilepsy. Brain Struct Funct 222, 2773–2785 (2017). https://doi.org/10.1007/s00429-017-1371-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1371-9