Abstract
Our previous studies demonstrated that inflammatory reaction and neuronal apoptosis are the most important pathological mechanisms in ischemia-induced brain damage. Propofol has been shown to attenuate ischemic brain damage via inhibiting neuronal apoptosis. The present study was performed to evaluate the effect of propofol on brain damage and inflammatory reaction in rats of focal cerebral ischemia. Sprague–Dawley rats underwent permanent middle cerebral artery occlusion, then received treatment with propofol (10 or 50 mg/kg) or vehicle after 2 h of ischemia. Neurological deficit scores, cerebral infarct size and morphological characteristic were measured 24 h after cerebral ischemia. The enzymatic activity of myeloperoxidase (MPO) was assessed 24 h after cerebral ischemia. Nuclear factor-kappa B (NF-κB) p65 expression in ischemic rat brain was detected by western blot. Cyclooxygenase-2 (COX-2) expression in ischemic rat brain was determined by immunohistochemistry. ELISA was performed to detect the serum concentration of tumor necrosis factor-α (TNF-α). Neurological deficit scores, cerebral infarct size and MPO activity were significantly reduced by propofol administration. Furthermore, expression of NF-κB, COX-2 and TNF-α were attenuated by propofol administration. Our results demonstrated that propofol (10 and 50 mg/kg) reduces inflammatory reaction and brain damage in focal cerebral ischemia in rats. Propofol exerts neuroprotection against ischemic brain damage, which might be associated with the attenuation of inflammatory reaction and the inhibition of inflammatory genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemia-induced brain damage develops from a series of pathological mechanisms including excitotoxicity, oxidative stress, blood–brain barrier disruption, inflammatory reaction and neuronal apoptosis [1]. Our previous studies demonstrated that inflammatory reaction is one of the most important pathological mechanisms in ischemic brain damage [2, 3]. Inhibition of inflammatory reaction would reduce ischemic brain damage in a rat model of permanent focal cerebral ischemia [4, 5].
Propofol, one of the widely used anesthetic agents for maintenance of anesthesia for surgical procedures, has also been shown to attenuate brain damage in a rat model of cerebral ischemia–reperfusion injury [6]. As we know, animal models of focal cerebral ischemia comprise with focal cerebral ischemia/reperfusion model and the permanent focal cerebral ischemia model [7]. There are some different pathological mechanisms between the focal cerebral ischemia/reperfusion model and the permanent focal cerebral ischemia model [8]. To our knowledge, it is unclear that whether also propofol provides a neuroprotection in a rat model of permanent focal cerebral ischemia. The present study was performed to evaluate the neuroprotective effect of propofol on ischemic brain damage in rats of permanent focal cerebral ischemia.
Several reports demonstrated that propofol has been demonstrated to possess anti-inflammatory property in some disease models. Chen et al. [9] showed that propofol reduces the biosyntheses of inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and nitric oxide in lipopolysaccharide-activated macrophages. Sun et al. [10] demonstrated that propofol inhibits the inflammatory mediators nuclear factor-kappa B (NF-κB), TNF-α and IL-6 and provides the protective effect on the intestine following traumatic brain injury in rats. During aortic surgery, propofol was demonstrated to protect renal function through modulation of the systemic inflammatory reaction [11]. In cerebral ischemia, inflammatory reaction and inflammatory genes mediates the pathophysiological processes of ischemic brain damage. In the present study, we first try to investigate whether propofol provides a neuroprotection against brain injury in permanent focal cerebral ischemia in rats, then further explored the effect of propofol on inflammatory reaction and the potential molecular mechanisms.
Materials and Methods
Animal Model
All animal experiments were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals. All surgical procedures were performed by sterile/aseptic techniques in accordance with institutional guidelines. Clean adult male Sprague–Dawley rats weighing 250–300 g were obtained from Shanghai Laboratory Animal Center, Chinese Academy of Sciences. Animals were housed in a colony room under controlled temperature (22 °C), and a 12:12 light–dark cycle, with food and water available. Rats were anesthetized with an intraperitoneal injection of chloral hydrate (300 mg/kg) and subjected to middle cerebral artery occlusion (MCAO) described previously [12]. In brief, the right common carotid artery, external carotid artery (ECA) and internal carotid artery (ICA) were carefully exposed through a ventral midline neck incision. A 3-0 nylon monofilament suture, whose tip was rounded by heating near a flame and coated with silicone (0.32 mm diameter) was inserted from the ECA into the ICA to occlude the origin of the right middle cerebral artery (MCA). The suture was inserted 18–20 mm from the carotid bifurcation, confirmed with mild resistance to occlude the MCA. Sham operation was manipulated in the same way, but the MCA was not occluded. During the surgery, rectal temperature was controlled at 37 °C with a heating pad and warm light. Rats were allowed to freely access to food and water after recovery from anesthesia.
Experimental Groups
A total of 48 clean adult male Sprague–Dawley rats were used in the present study. Rats were divided into four groups (four rats in each group): (1) sham control group, which underwent sham operation and received vehicle; (2) MCAO group, which was subjected to MCAO and received vehicle; (3) propofol 10 mg/kg group (P10), which was subjected to MCAO and treated with propofol 10 mg/kg; (4) propofol 50 mg/kg group (P50), which was subjected to MCAO and treated with propofol 50 mg/kg. Rats were treated with normal saline as the vehicle control at the same volume as propofol.
Assessment of Neurological Deficit Scores
Neurological deficit scores were assessed at 24 h after MCAO described previously [13]: 0, no observable deficit; 1, contralateral forelimb flexion; 2, decreased resistance to lateral push without circling; 3, circling to the contralateral side.
Assessment of Cerebral Infarct Size
Fresh rat brain was removed and coronally sliced into 2.0 mm-thick sections. Brain slices were incubated in 2 % 2,3,5-triphenyltetrazolium chloride (Amresco) for 20 min. Cerebral infarct size was calculated according to the previous reports [14]. The degree of cerebral infarct size was expressed as a percentage of the contralateral hemisphere size.
Biochemical Analysis
Neutrophil infiltration was evaluated by measuring the enzymatic activity of myeloperoxidase (MPO). MPO activity in ischemic rat brain was measured according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, China). The results were expressed as U/g tissue.
Histological Examination
The rats were euthanized with deep anesthesia and perfused with 4 % paraformaldehyde in phosphate-buffered saline. Then the brains were removed, fixed and embedded in paraffin. The paraffin-embedded sections (4 μm thick) were deparaffinized with xylene and rehydrated with graded alcohol for hematoxylin and eosin (HE) staining and immunohistochemical analysis. For HE staining, coronal sections were counterstained with HE. The sections were visualized with a microscope (Olympus, Japan).
Western Blot
Brain samples were used and total protein was extracted using protein extraction kit (Beyotime Biotech. CO., China) according to the manufacturer’s instructions. Protein samples (50 μg) were separated on 10 % SDS polyacrylamide gels, transferred to nitrocellulose membranes. The membranes were incubated at 4 °C for 2 h with a mouse monoclonal antibody against NF-κB p65 (1:500, Santa Cruz). The nitrocellulose membranes were incubated with horseradish-peroxidase conjugated secondary antibodies (1:2,000, KPL Inc) for 2 h at 25 °C and developed with an enhanced chemiluminescence detection system (KPL Inc). GAPDH was used as a loading control. The optical densities of protein bands were analyzed by the Quantity one software (Bio-Rad).
Immunohistochemical Analysis
For immunohistochemical analysis, the paraffin-embedded sections were deparaffinized with xylene and rehydrated with graded alcohol. Brain sections were incubated in 3 % H2O2 for 15 min to block endogenous peroxidase activity. After washing in PBS, sections were incubated overnight at 4 °C with a rabbit polyclonal antibody against cyclooxygenase-2 (COX-2) (1:100, Zhongshan Biotechnology Co. Ltd, Beijing). Biotinylated mouse anti-rabbit IgG (Zhongshan Biotechnology Co. Ltd, Beijing) was used as a secondary antibody. Diaminobenzidine was used as a color substrate. The sections were visualized with a microscope (Olympus, Japan). Three random high area (200×) in peri-infarct zone of rat brains were counted.
Measurement of Serum TNF-α
Twenty-four hours after MCAO, blood samples (1 ml) were drawn from femoral vein of rats. After centrifugation at 3,000 rpm for 15 min, the supernatant was collected and stored at –80 °C in refrigerator. Serum contents TNF-α were measured using a rat TNF-α ELISA kits according to the manufacturer’s instructions (Shanghai Xitang Biological Technology, China).
Statistical Analysis
Four rats in each group was used in this study, experimental data were presented as mean ± SD. Statistical analysis was performed using ANOVA followed by Bonferroni test for individual comparisons between group means (SPSS13.0 for windows, USA). A value of p < 0.05 was considered statistically significant.
Results
Effect of Propofol on Neurological Deficit and Cerebral Infarct Size
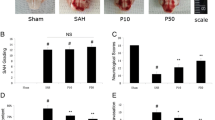
Sham-operated rats did not have any neurological deficit scores and cerebral infarct size. Propofol 50 mg/kg significantly reduced neurological deficit scores 24 h after MCAO (p < 0.01, Fig. 1a), but propofol 10 mg/kg has not difference (p > 0.05, Fig. 1a). Both of propofol at 10 and 50 mg/kg reduced cerebral infarct size 24 h after MCAO (p < 0.05 and p < 0.01, Fig. 1b).
Propofol reduced neurological deficit and cerebral infarct size. Neurological deficit scores (a) were significantly reduced by propofol 50 mg/kg (p < 0.01), but not propofol 10 mg/kg (p > 0.05). Cerebral infarct size (b) were significantly reduced by propofol 10 and 50 mg/kg (p < 0.05 and p < 0.01). n = 4, pro 10 propofol 10 mg/kg, pro 50 propofol 50 mg/kg
Effect of Propofol on Neuronal Damage
HE staining showed the morphological characteristic of sham-operated neuron vehicle-treated neuron and propofol-treated neuron in rat. No neuronal damage was observed in sham-operated rats (Fig. 2a). Many injured neurons appeared shrunken with triangulated pyknotic nuclei and cavitation were observed after 24 h of MCAO, (Fig. 2b). Experimental results showed that propofol (10 and 50 mg/kg) attenuated the neuronal damage and necrotic neurons 24 h after MCAO (Fig. 2c, d).
Morphological changes of cortical neurons following cerebral ischemia. Representative microphotographs of neuronal damage from sham-operated (a), vehicle-treated (b) and propofol-treated rats (c, d). Necrotic and ischemic injured neurons (arrow) were obviously reduced by propofol administration. Scale bar 50 μm
Effect of Propofol on Neutrophil Infiltration
Experimental results demonstrated that the enzymatic activity of MPO was increased 24 h after MCAO, which was significantly attenuated by administration of propofol 50 mg/kg (p < 0.01), but not 10 mg/kg (p > 0.05, Fig. 3).
Effect of Propofol on NF-κB Expression
Experimental results showed that MCAO causes an increased expression of NF-κB p65 following cerebral ischemia in rats. The upregulated expression of p65 subunit is an index of nuclear translocation. Propofol 50 mg/kg inhibited the expression of NF-κB p65 by the method of western blot analysis (p < 0.05), but propofol 10 mg/kg has not difference (p > 0.05, Fig. 4).
Propofol reduced the expression of NF-κB p65. Representative protein bands of NF-κB p65 expression in sham-operated, vehicle-treated and propofol-treated groups were detected by western blot (a), and the data were summarized in (b). Cerebral ischemia caused an increased expression of NF-κB p65 (p < 0.01), which was significantly inhibited by propofol 50 mg/kg (p < 0.05), but not 10 mg/kg (p > 0.05). n = 4, pro 10 propofol 10 mg/kg, pro 50 propofol 50 mg/kg
Effect of Propofol on COX-2 Expression
Experimental results showed that MCAO caused an increase of COX-2 immunopositive cells in rat brain 24 h after cerebral ischemia. Propofol 50 mg/kg significantly down-regulated the expression of COX-2 (p < 0.05), but propofol 10 mg/kg has not difference compared with vehicle group (p > 0.05, Fig. 5).
Propofol reduced the expression of COX-2. Representative microphotographs of COX-2 immunopositive cells (arrow) in ipsilateral hemisphere from sham-operated (a), vehicle-treated (b), propofol 10 mg/kg treated rats (c) and propofol 50 mg/kg treated rats (d), and the data were summarized in (e). Cerebral ischemia caused an increased expression of COX-2 (p < 0.01), which was significantly inhibited by propofol 50 mg/kg (p < 0.05), but not 10 mg/kg (p > 0.05). n = 4, pro 10 propofol 10 mg/kg, pro 50 propofol 50 mg/kg. Scale bar 50 μm
Effect of Propofol on Serum Content of TNF-α
Experimental results showed that serum TNF-α was increased 24 h after MCAO, which was significantly attenuated by propofol 50 mg/kg (p < 0.05), but not propofol 10 mg/kg (p > 0.05, Fig. 6).
Discussion
The present study demonstrated that propofol, an anesthetic agents for maintenance of anesthesia for surgical procedures, provides neuroprotective effect against ischemia-induced cerebral damage. Further study showed that propofol attenuates inflammatory reaction in rats of focal cerebral ischemia. Moreover, propofol was demonstrated to inhibit the expression of inflammatory signaling NF-κB/COX-2/TNF-α.
Several reports demonstrated that propofol reduces ischemic brain damage in rats [15–18]. Li et al. [15] showed that propofol protects against cerebral ischemia damage via inhibiting neuronal apoptosis, and propofol’s anti-apoptotic property might be mediated by the inhibition of caspase-3 expression and the increase of Bcl-2 expression. Xi et al. [16] demonstrated that propofol improves neurobehavioral outcome in rats of cerebral ischemia–reperfusion by regulating Bcl-2 and Bax expression. Cai et al. [17] showed that propofol provides neuroprotective effect against brain ischemia in rats, which might be mediated by the glutamatergic signaling pathway. Taken together, previous studies indicates that the neuroprotective effect of propofol in ischemic brain damage is associated with its anti-apoptotic property.
To our knowledge, inflammatory reaction and neuronal apoptosis are the two most important pathological mechanisms in the processes of ischemic brain damage [19]. Propofol has been demonstrated to protect cerebral ischemia damage via inhibiting neuronal apoptosis. Feng et al. [20] showed that propofol could attenuate inflammation in cerebral ischemia–reperfusion injury. As reported earlier, there are some different pathological mechanisms between the focal cerebral ischemia/reperfusion model and the permanent focal cerebral ischemia model [8]. Therefore, it is unknown that whether propofol reduces ischemic brain damage via inhibiting inflammatory reaction in rats of permanent cerebral ischemia. This is the first paper, to our best knowledge, indicating that propofol could inhibit inflammatory reaction and down-regulate the expression of inflammatory signals in rats of permanent focal cerebral ischemia.
In this study, we first demonstrated that the administration of propofol significantly reduces neurological deficit scores, cerebral infarct size and neuronal injury 24 h after permanent focal cerebral ischemia in rats. Then, we found that propofol inhibits MPO activity in a rat model of permanent focal cerebral ischemia. MPO was usually examined for quantitative indication of neutrophil infiltration and inflammatory reaction in previous study. Therefore, propofol reduces inflammatory reaction in rats of cerebral ischemia. Then, it is necessary to further investigate the potential molecular mechanisms in the subsequent experiments.
NF-κB, an inflammatory transcription factor activated in response to ischemic stroke, is known to mediated the inflammatory processes in ischemic brain damage [21]. Inhibition of NF-κB attenuates brain damage in both transient and permanent cerebral ischemia models, suggesting that NF-κB plays a detrimental role in ischemic stroke [22]. In cerebral ischemia, NF-κB has been shown to regulate the expression of downstream inflammatory genes that contributes to the evolution of ischemic brain damage. COX-2, an inflammatory enzyme that mediates the process of ischemic brain damage, was over-expressed after focal cerebral ischemia. TNF-α, an inflammatory cytokine that also aggravates ischemic brain damage, was up-regulated after focal cerebral ischemia.
In order to provide the molecular mechanisms underlying the propofol’s neuroprotection against ischemia-induced inflammatory reaction, we determined the effect of propofol on the expression of inflammatory signaling NF-κB/COX-2/TNF-α. The experimental results demonstrated that propofol significantly inhibits the activity of NF-κB, down-regulates the expression of COX-2 and reduces the secretion of TNF-α following focal cerebral ischemia.
In summary, to our knowledge, this is the first demonstration of propofol’s effect on inflammatory reaction and inflammatory signaling in rats of cerebral ischemia. The neuroprotective effect of propofol in cerebral ischemia may be associated with the inhibition of inflammatory reaction and inflammatory signaling NF-κB/COX-2/TNF-α.
References
Mehta SL, Manhas N, Raghubir R (2007) Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 54(1):34–66
Tu XK, Yang WZ, Shi SS, Wang CH, Zhang GL, Ni TN, Chen CM, Wang R, Jia JW, Song QM (2010) Spatio-temporal distribution of inflammatory reaction and expression of TLR2/4 signaling pathway in rat brain following permanent focal cerebral ischemia. Neurochem Res 35(8):1147–1155
Tu XK, Yang WZ, Shi SS, Chen Y, Wang CH, Chen CM, Chen Z (2011) Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral ischemia. Inflammation 34(5):463–470
Guo Y, Xu X, Li Q, Li Z, Du F (2010) Anti-inflammation effects of picroside 2 in cerebral ischemic injury rats. Behav Brain Funct 6:43
Park JS, Shin JA, Jung JS, Hyun JW, Van Le TK, Kim DH, Park EM, Kim HS (2012) Anti-inflammatory mechanism of compound k in activated microglia and its neuroprotective effect on experimental stroke in mice. J Pharmacol Exp Ther 341(1):59–67
Chen L, Xue Z, Jiang H (2008) Effect of propofol on pathologic time-course and apoptosis after cerebral ischemia–reperfusion injury. Acta Anaesthesiol Scand 52(3):413–419
Sobrado M, Delgado M, Fernández-Valle E, García-García L, Torres M, Sánchez-Prieto J, Vivancos J, Manzanares R, Moro MA, Pozo MA, Lizasoain I (2011) Longitudinal studies of ischemic penumbra by using 18F-FDG PET and MRI techniques in permanent and transient focal cerebral ischemia in rats. Neuroimage 57(1):45–54
Xue X, Qu XJ, Yang Y, Sheng XH, Cheng F, Jiang EN, Wang JH, Bu W, Liu ZP (2010) Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor κB p65 activation. Biochem Biophys Res Commun 403(3–4):398–404
Chen RM, Chen TG, Chen TL, Lin LL, Chang CC, Chang HC, Wu CH (2005) Anti-inflammatory and antioxidative effects of propofol on lipopolysaccharide-activated macrophages. Ann NY Acad Sci 1042:262–271
Sun J, Wang L, Shen J, Wang Z, Qian Y (2007) Effect of propofol on mucous permeability and inflammatory mediators expression in the intestine following traumatic brain injury in rats. Cytokine 40(2):151–156
Rodríguez-López JM, Sánchez-Conde P, Lozano FS, Nicolás JL, García-Criado FJ, Cascajo C, Muriel C (2006) Laboratory investigation: effects of propofol on the systemic inflammatory response during aortic surgery. Can J Anaesth 53(7):701–710
Tu XK, Yang WZ, Wang CH, Shi SS, Zhang YL, Chen CM, Yang YK, Jin CD, Wen S (2010) Zileuton reduces inflammatory reaction and brain damage following permanent cerebral ischemia in rats. Inflammation 33(5):344–352
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17:472–476
Lin TN, He YY, Wu G, Khan M, Hsu CY (1993) Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 24:117–121
Li J, Han B, Ma X, Qi S (2010) The effects of propofol on hippocampal caspase-3 and Bcl-2 expression following forebrain ischemia–reperfusion in rats. Brain Res 1356:11–23
Xi HJ, Zhang TH, Tao T, Song CY, Lu SJ, Cui XG, Yue ZY (2011) Propofol improved neurobehavioral outcome of cerebral ischemia–reperfusion rats by regulating Bcl-2 and Bax expression. Brain Res 1410:24–32
Cai J, Hu Y, Li W, Li L, Li S, Zhang M, Li Q (2011) The neuroprotective effect of propofol against brain ischemia mediated by the glutamatergic signaling pathway in rats. Neurochem Res 36(10):1724–1731
Wang H, Luo M, Li C, Wang G (2011) Propofol post-conditioning induced long-term neuroprotection and reduced internalization of AMPAR GluR2 subunit in a rat model of focal cerebral ischemia/reperfusion. J Neurochem 119(1):210–219
Zhang ZG, Sun X, Zhang QZ, Yang H (2013) Neuroprotective effects of ultra-low-molecular-weight heparin on cerebral ischemia/reperfusion injury in rats: involvement of apoptosis, inflammatory reaction and energy metabolism. Int J Mol Sci 14(1):1932–1939
Feng CS, Ma HC, Yue Y, Zhang YQ, Qu XD (2004) Effect of propofol on the activation of nuclear factor-kappa B and expression of inflammatory cytokines in cerebral cortex during transient focal cerebral ischemia–reperfusion: experiment with rats. Zhonghua Yi Xue Za Zhi 84(24):2110–2114
Xu M, Yang L, Hong LZ, Zhao XY, Zhang HL (2012) Direct protection of neurons and astrocytes by matrine via inhibition of the NF-κB signaling pathway contributes to neuroprotection against focal cerebral ischemia. Brain Res 1454:48–64
Maddahi A, Kruse LS, Chen QW, Edvinsson L (2011) The role of tumor necrosis factor-α and TNF-α receptors in cerebral arteries following cerebral ischemia in rat. J Neuroinflamm 8:107
Acknowledgments
The National Natural Science Foundation of China (81100987), the Doctoral Program Foundation of Institutions of Higher Education of China (20113518120005), the Natural Science Foundation of Fujian Province of China (2011J05066), the Clinical Key Subject (Neurosurgery) Funding of Fujian Medical University, and the Key Laboratory (Neurosurgical Department) Funding from the Affiliated Union Hospital of Fujian Medical University, supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, Ss., Yang, Wz., Chen, Y. et al. Propofol Reduces Inflammatory Reaction and Ischemic Brain Damage in Cerebral Ischemia in Rats. Neurochem Res 39, 793–799 (2014). https://doi.org/10.1007/s11064-014-1272-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1272-8