Abstract
Protein kinase C (PKC) signaling pathway is recognized as an important molecular mechanism of Alzheimer’s disease (AD) in the regulation of neuronal plasticity and survival. Genistein, the most active molecule of soy isoflavones, exerts neuroprotective roles in AD. However, the detailed mechanism has not been fully understood yet. The present study aimed to investigate whether the neuroprotective effects of genistein against amyloid β (Aβ)-induced toxicity in cultured rat pheochromocytoma (PC12) cells is involved in PKC signaling pathway. PC12 cells were pretreated with genistein for 2 h following incubation with Aβ25–35 for additional 24 h. Cell viability was assessed by MTT. Hoechst33342/PI staining was applied to determine the apoptotic cells. PKC activity, intracellular calcium level and caspase-3 activity were analyzed by assay kits. The results showed that pretreatment with genistein significantly increased cell viability and PKC activity, decreased the levels of intracellular calcium, attenuated Hoechst/PI staining and blocked caspase-3 activity in Aβ25–35-treated PC12 cells. Pretreatment of Myr, a general PKC inhibitor, significantly attenuated the neuroprotective effect of genistein against Aβ25–35-treated PC12 cells. The present study indicates that PKC signaling pathway is involved in the neuroprotective action of genistein against Aβ25–35-induced toxicity in PC12 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly. Substantial evidence indicates that Aβ contributes significantly to the pathological cascades in AD by various mechanisms, such as by generating reactive oxygen species (ROS), elevating intracellular free Ca2+, and other cytotoxic stimuli [1]. Apoptosis is increasingly recognized as an important mode of neuronal death in many different pathological settings including AD [2]. Accordingly, DNA fragmentation [3], expression of apoptosis-related genes [4] and activation of caspases [5] are associated with the neurodegenerative disorder [6]. A consistent neuropathological aspect is the formation of amyloid deposits [7]. The amyloid peptide in the plaque cores, called amyloid (Aβ), is a 39–43 amino acid sequence that is initially found within a longer protein, the amyloid-β protein precursor (AβPP) [8]. Deposition of amyloid β-protein (Aβ) in the brain is one of major pathological features in AD [9]. It has also been found that the fragment 25–35 of Aβ (Aβ25–35) has the central functional domain of the whole molecule Aβ that is required for neurotoxic effect [10].
Protein kinase Cs (PKCs) are a family of Ser/Thr protein kinases that play important roles in many cellular processes including proliferation, tumor promotion, differentiation and apoptosis [11]. Many factors have been shown to influence the processing and metabolism of AβPP. It is reported that PKC signaling pathway regulates amyloid precursor protein release in Swiss 3T3 fibroblasts [12]. Several lines of evidence have demonstrated that PKC lead to neuronal apoptosis and elevated intracellular calcium levels in neurodegenerative disorders, such as AD [13]. Especially, human studies have shown that PKC is deficient in an area selective manner in AD [14]. In addition, PKC protects neural cells against apoptosis induced by Aβ through decreasing intracellular calcium levels and oxidative stress [2]. Therefore, it is well established that Aβ induces apoptosis through inhibiting the activation of PKC signaling pathway.
Genistein (4′,5,7-trihydroxyisoflavone, Fig. 1), the most active component of soy isoflavones, showing an affinity to estrogen receptors (ERs) [15], possesses antioxidative [16] and antiapoptotic activities [17] in the prevention of central nervous system. Furthermore, there is growing interest in the beneficial effects of genistein on Aβ-induced neurotoxicity [18]. Previous study showed that Genistein suppresses Aβ25–35-induced ROS overproduction in isolated rat brain synaptosomes [19]. However, whether genistein exerts its beneficial neuroprotective effects involved in PKC signaling pathway remains largely unknown.
In this study, we demonstrated the protective effect of genistein on cultured PC12 cells against Aβ25–35-induced apoptosis and genistein inhibited the elevation of intracellular free Ca2+ levels. Using specific PKC antagonist, we also demonstrated that genistein attenuates neurotoxicity induced by Aβ25–35 in PC12 cells via PKC signaling pathway.
Materials and Methods
Reagents
PC12 cells were kindly donated by Dr. Rikang Wang from School of Pharmaceutical University, Sun Yat-sen University, Guangzhou, China. DMEM medium, penicillin (100 U/mL), and streptomycin (100 mg/mL) were obtained from Gibco (Gaithersburg, Maryland, USA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, Utah, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl (MTT), Genistein, Aβ25–35 were purchased from Sigma (StLouis, Missouri, USA). Hoechst33342/propidium (PI) double staining and Caspase-3 assay fluorometric kit were obtained from Nanjing Kaiji Biotech Ltd. (Nanjing, China). BCA Protein Assay Kit was obtained from Pierce Biotechnology (Rockford, USA). Fluo-3/AM and paraformaldehyde were purchased from Beijing Dingguo Biotech Ltd. (Beijing, China). The PepTag® Assay for Non-Radioactive Detection of Protein kinase C was from Promega (Madison, USA).
Cell Culture
Pheochromocytoma (PC12) (ATCC, USA) were cultured in Dulbecco’s moditi ed Eagle’s medium (Gibco, USA) containing 10 % (v/v) fetal bovine serum, 2 mM glutamine, 0.2 % (w/v) sodium bicarbonate, penicillin (100 U/mL), and streptomycin (100 μg/mL). Cells were maintained at 37 °C in a humiditied 5 % CO2 incubator and were subcultured at 50–80 % confluence using 0.05 % trypsin–0.02 % EDTA (Gibco).
For the experimental studies, PC12 cells were grown to confluence in the presence of 10 % FBS and then made quiescent by serum starvation in DMEM for 24 h. Cells were pre-treated by genistein at different dosages (0, 12.5, 25, 50, 100 μmol/L) for 2 h, then treated by 20 μmol/L Aβ25–35 for 24 h.
MTT Assay
To determine cell viability, PC12 cells were seeded in 96-well plates at 5 × 104 cells/mL and incubated in a 37 °C, 5 % CO2 incubator. After treatment with genistein (Sigma-Aldrich) in serum-free DMEM for 24 h, the cells were incubated for an additional 3 h in the presence of 0.5 mg/mL MTT reagent. After careful removal of the MTT reagent and disruption of cells with 150 μL dimethyl sulphoxide (DMSO)/well, incubation at 37 °C for 10 min with horizontal shaking followed. The absorbance was measured at 570 nm using an automated microplate reader (BioTek, Winooski, VT, USA). Data are presented as the percentage of viability relative to vehicle-treated control.
PKC Activity
PKC activity was determined using a non-radioactive method as previously described [20]. Briefly, after treatment, neurons were lysed in a modified PKC extraction buffer (25 mM Tris, 0.05 % Triton X-100, 10 mM β-mercaptoethanol, protease and phosphatase inhibitors). After protein extraction and quantification, equal amounts of protein were used in each PKC reaction following the PepTag® Assay for Non-Radioactive Detection of Protein Kinase C protocol (Promega, Madison, USA). Samples were then incubated with a positively-charged, fluorescent, PKC-specific peptide for 30 min and separated on agarose gels. The phosphorylated, negatively-charged peptide was separated from the non-phosphorylated, positively-charged peptide and visualized under UV light. Resulting bands were quantified by densitometry and normalized to controls.
Apoptosis Analysis by Hoechst33342/PI Staining
PC12 cells were trypsinized, collected by centrifugation at 1,000 rpm for 5 min, and washed twice with PBS. The cells were fixed with 3.7 % paraformaldehyde at room temperature for 2 h, centrifuged, and washed with PBS, stained with 40 mL of Hoechst33342/PI at 1:1 ratio for 30 min at 37 °C. At the end of incubation, the cells were washed and resuspended in PBS for the observation of nuclear morphology under a fluorescence microscope (Nikon E800, Nikon, Tokyo, Japan). The percentage of apoptosis was calculated from the ratio of apoptotic cells to total cells.
Measurement of Caspase-3 Activity
The activity of caspase-3 activity was determined using the caspase-3 activity kit. To evaluate the activity of caspase-3, cell lysates were prepared after their respective treatment with various designated treatments. Assays were performed on 96-well microtiter plates by incubating 10 mL protein of cell lysate per sample in 80 mL reaction buffer (0.1 % NP-40, 20 mM Tris–HCl, pH 7.5, 137 mM Nad, and 10 % glycerol) containing 10 mL caspase-3 substrate (Ac-DEVD-pNA) (2 mM). Lysates were incubated at 37 °C for 4 h. Samples were measured with an ELISA reader at an absorbance of 405 nm. The detailed analysis procedure was described in the manufacturer’s protocol.
Measurement of Intracellular Calcium Concentration
The intracellular calcium levels were determined by the method of Fluo-3/AM double wavelength fluorescence scanning as described previously with minor modification [21]. Briefly, at the end of the treatment, the supernatant were disposed and the cells (5 × 105 cells/mL) were collected and incubated with the complete medium containing 5 μM Fluo-3/AM at 37 °C for 40 min. Subsequently, the cells were washed twice with Hank’s solution and resuspended in Hank’s solution containing 0.2 % bovine serum albumin. The intracellular calcium concentration was determined by alternating excitation wavelengths of between 340 and 380 nm with emission at 510 nm, using a fluorescence spectrophotometer (Shimadzu, RF-5301, Japan), and the data were analyzed with customized software provided by Shimadzu. The concentration of intracellular calcium was expressed as percentage of non-treated control.
Statistical Analysis
Data were presented as mean ± SD. Multiple group comparison test was performed by one-way analysis of variance (ANOVA) followed by the LSD test. Calculations were performed using the SPSS for Windows version 17.0 statistical package (SPSS, Chicago, IL). P < 0.05 was considered statistically significant. Each experiment was performed three times using three batches of cells with the same treatment.
Results
Effect of Genistein on Aβ25–35-Induced Cytotoxicity in PC12 Cells
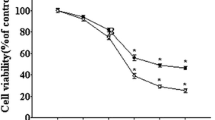
To observe the protective roles of genistein in the neurotoxicity of PC12 cells induced by Aβ25–35 and rule out the possibility of cytotoxicity of genistein, cells were pretreated with various concentrations (12.5–100 μM) of genistein for 2 h and subsequently treated with 20 μM Aβ25–35 for 24 h. As shown in Fig. 2, Aβ25–35 (20 μM) significantly decreased the cell viability compared with control group (56.41 % of control, P < 0.01). When 12.5–100 μM genistein was applied to PC12 cells in the presence of Aβ25–35, cells were observed to be increased viability compared with Aβ25–35 treatment alone. Pretreatment with genistein demonstrated a dose-dependent recovery in Aβ25–35-induced decreases of cell viability that showed maximal effects at 25 μM (Fig. 2). However, the cell viability in the genistein treatment group at the concentrations of 50–100 μM were markedly decreased compared to 25 μM group, leading us to choose a lower dose (25 μM) for the in vitro experiments.
Effect of genistein on the viability of PC12 cell treated by Aβ25–35. a Cells were treated with genistein (0, 12.5, 25, 50 and 100 μM) for 24 h. b Cells were pretreated with genistein (0, 12.5, 25, 50 and 100 μM) for 2 h followed by exposure to 20 μM Aβ25–35 for 24 h. Cell viability was evaluated by MTT assay. Values were expressed as the mean ± SD (n = 6). *P < 0.01 versus control; # P < 0.05, ## P < 0.01 versus Aβ25–35 group
Genistein Protects PC12 Cells Against Aβ25–35-Induced Toxicity
The MTT assay (Fig. 3) showed that genistein pretreatment reversed the cell viability inhibited by Aβ25–35 in PC12 cells. However, the co-pretreatment with PKC inhibitor Myr (50 μM) significantly decreased the cell viability compared with genistein pretreatment alone (P < 0.05). These data revealed that genistein protected PC12 cells against Aβ25–35-induced toxicity via PKC signaling pathway.
Effect of Myr on the protective role of genistein in the cell viability of PC12 cells treated by Aβ25–35. Cells were pretreated with genistein (25 μM) for 2 h and Myr (50 μM) for 3 h followed by exposure to 20 μM Aβ25–35 for 24 h. Cell viability was evaluated by MTT assay. Values were expressed as the mean ± SD (n = 6). *P < 0.01 versus control; # P < 0.01 versus Aβ25–35 group; △P < 0.05 versus genistein + Aβ25–35 group
Genistein Increases PKC Activity in Aβ25–35-Treated PC12 Cells
Since it has been previously shown that the activation of PKC is closely associated with cell survival [22], we next investigated the effect of genistein on the activity of PKC. As shown in Fig. 4, the activity of PKC was decreased after exposure to Aβ25–35. However, the pretreatment of genistein abolished the inhibitory effect of Aβ25–35 on the PKC activity. As expected, treatment of Myr significantly blocked the increased activity of PKC induced by genistein.
Effect of genistein on PKC activity in Aβ25–35-treated PC12 cells. Cells were pretreated with genistein (25 μM) for 2 h and Myr (50 μM) for 3 h followed by exposure to 20 μM Aβ25–35 for 24 h. Cell lysates were collected and equal amounts of proteins were incubated with a fluorescent PKC substrate as described in Sect. "Materials and Methods". At the end of the reaction, the mixture was separated on an agarose gel and the bands corresponding to the phosphorylated peptide were quantified and normalized to controls. Results were expressed as the mean ± SD of three independent experiments. *P < 0.01 versus control; # P < 0.01 versus Aβ25–35 group; △P < 0.01 versus genistein + Aβ25–35 group
Genistein Decreases the Intracellular Calcium Levels in PC12 Cells Induced by Aβ25–35
As shown in Fig. 5, compared with control group, the intracellular calcium levels were significantly increased in PC12 cells treated with 20 μM Aβ25–35 for 24 h (P < 0.01). When cells were pretreated with genistein for 2 h, followed by treated with Aβ25–35 for 24 h, the intracellular calcium levels were markedly decreased as comparison to the Aβ25–35 group (P < 0.01). However, the intracellular calcium levels increased significantly in PC12 cells pretreated with combination of genistein and Myr compared with genistein pretreatment alone (P < 0.01). These data suggested that genistein inhibited intracellular calcium overload in PC12 cells induced by Aβ25–35 through PKC signaling pathway.
Effect of genistein on intracellular calcium concentration in Aβ25–35-treated PC12 cells. Cells were pretreated with genistein (25 μM) for 2 h and Myr (50 μM) for 3 h followed by exposure to 20 μM Aβ25–35 for 24 h. Results were expressed as the mean ± SD of three independent experiments. *P < 0.01 versus control; # P < 0.01 versus Aβ25–35 group; △P < 0.01 versus genistein + Aβ25–35 group
Genistein Inhibits Apoptosis of PC12 Cells Induced by Aβ25–35
Next, we examined the effect of genistein on the apoptosis of PC12 cells induced by Aβ25–35. Cellular apoptosis was assessed by measuring the extent of Hoechst33342/PI staining and caspase-3 activity. As shown in Fig. 6, treatment of PC12 cells Aβ25–35 for 24 h caused a significant augmentation in the amount of apoptotic cells compared with the control group (P < 0.01). When PC12 cells were pretreated with genistein for 2 h, followed by exposure to Aβ25–35 for 24 h, the extent of apoptotic cells was significantly decreased (P < 0.01) compared with Aβ25–35 treatment alone. However, the extent of apoptotic cells increased significantly in PC12 cells pretreated with combination of genistein and Myr compared with genistein pretreatment alone (P < 0.01).
Effect of genistein on Aβ25–35-induced PC12 cell apoptosis using Hoechst33342/propidium (PI) staining. a Untreated cells (Control group). b PC12 cells were treated with Aβ25–35 for 24 h (Aβ25–35). c PC12 cells were pretreated with genistein for 2 h and treated with Aβ25–35 for additional 24 h (Aβ25–35 + genistein). d PC12 cells were pretreated with genistein (25 μM) for 2 h and Myr (50 μM) for 3 h followed by exposure to Aβ25–35 for additional 24 h (Aβ25–35 + genistein + Myr). Original magnification ×100. e Percentage of apoptosis was calculated from the ratio of apoptotic cells to total cells. Results were expressed as the mean ± SD of three independent experiments. *P < 0.01 versus control; # P < 0.01 versus Aβ25–35 group; △P < 0.05 versus genistein + Aβ25–35 group
Caspase-3 activity was examined because it is an important biomarker of apoptosis process. The effect of genistein on the caspase-3 activity induced by Aβ25–35 was shown in Fig. 7. Caspase-3 activity in the cells treated with Aβ25–35 (20 μM) for 24 h increased up to 221.7 % of the control level. Genistein caused a 42.8 % reduction of the increased caspase-3 activity induced by Aβ25–35, whereas Myr significantly abolished the inhibitory effect of genistein on caspase-3 activity.
Effect of genistein on the activity of caspase-3 in Aβ25–35-treated PC12 cells. Cells were pretreated with genistein (25 μM) for 2 h and Myr (50 μM) for 3 h followed by exposure to 20 μM Aβ25–35 for 24 h. Results were expressed as the mean ± SD of three independent experiments. *P < 0.01 versus control; # P < 0.01 versus Aβ25–35 group; △P < 0.01 versus genistein + Aβ25–35 group
Discussion
In the current study, the neuroprotective mechanism of action of genistein was characterized in PC12 cell apoptosis induced by Aβ25–35. Our study demonstrated that genistein exerted a protective effect against Aβ25–35-induced neurotoxicity in PC12 cells. The protective effect of genistein was through activation of PKC signaling pathway and inhibition of intracellular calcium influx and neuronal apoptosis.
Treatment of PC12 cells with Aβ induces overload of intracellular calcium influx, oxidative stress and cell apoptosis [23, 24]. Accordingly, the model of Aβ25–35-induced PC12 cell apoptosis was used to investigate the neuroprotective effect of genistein. In our study, Aβ25–35 treatment on PC12 cells induced neurotoxicity as indicated by enhanced cell apoptosis and death, increased intracellular calcium level and caspase-3 activity. These observations are consistent with the previous findings [24, 25]. Treatment with genistein significantly reversed these changes in PC12 cells, suggesting the protective effects of genistein against Aβ25–35–induced neurotoxicity.
Several mechanisms may underlie the neuroprotection of genistein against Aβ25–35-induced neurotoxicity [1, 16]. For example, genistein has been shown to posses signficant free-radical scavenging properties in PC12 cells [16], suggesting that genistein was an antioxidant against ROS-induced toxicity. PKC, a phospholipid- and calcium-dependent kinase, is widely distributed throughout the brain. The PKC pathway has been described to function as a main neuronal survival pathway [26]. Accumulating evidences indicate a close link between PKC and AD [9, 27]. For example, chronic accumulation of Aβ into the rat brain reduced activation of PKC [28]. Treatment of neuronal cultures with Aβ caused PKC isoforms downregulation [29]. In addition, bryostatin, a PKC activator, significantly reduced Aβ in the brains of APP transgenic mice and a more recently developed AD double-transgenic mouse [30]. In this study, genistein was shown to increased PKC activity and concentration-dependently protected against Aβ25–35-induced toxicity in cultured PC12 cells. To determine whether PKC could be involved in the neuroprotective effects of genistein, a specific inhibitor of PKC, Myr, was used in this study. The results showed that Myr markedly prevented the protective effects of genistein on the cell viability, PKC activity, intracellular calcium levels and caspase-3 activity, suggesting that the PKC pathway is an important pathway mediating the genistein neuroprotective effects.
The intracellular calcium concentration plays a critical role in the neuronal development. Recent researches have revealed that the neurotoxicity induced by Aβ25–35 is mediated by the overload of intracellular calcium in primary neurons such as hippocampal neurons and cortical neurons [31]. On the other hand, the increase in intracellular calcium levels has been considered as an important mediator of by Aβ-induced toxicity in PC12 cells [23]. Aβ, which is a major contributor to the pathogenesis of AD, induces many kinds of neurotoxicity, such as the disruption of intracellular homeostasis of calcium, induction of oxidative stress, increased tau phosphorylation and caspase-3 activity [32]. Notably, elevation of the calcium level may act as a sensor in the apoptotic process. Therefore, the blockage of Aβ-induced overload of intracellular calcium might provide neuroprotection against Aβ-induced cell death in PC12 cells. In this study, treatment of PC12 cells with Aβ25–35 significantly increased intracellular calcium levels, whereas pretreatment with genistein attenuated the intracellular calcium influx, suggesting the neuroprotective effect of genistein results from the inhibition of the overload of intracellular calcium.
Caspases play an important role in the apoptotic process in two ways: the extrinsic pathway and the intrinsic pathway, and caspase-3 acts as an apoptotic executor [33]. The protein level of caspase-3 is increased in AD brain [34]. It has been reported that caspase-3 is activated by Aβ25–35 [35]. Additionally, caspase-3 is activated by both PKC [2] and the disruption of intracellular homeostasis of calcium [36]. Our data showed that genistein inhibited Aβ-triggered activation of caspase-3 through its regulatory function in the stabilization of intracellular calcium homeostasis and activation of PKC. Due to the importance that the reduction of Aβ-induced neurotoxicity has for the treatment of AD, our present results can rule out a new mechanism of action of genistein via PKC. As described by Maines MD [37], biliverdin reductase (BVR) is one of the main modulator of PKC. Further study is needed to be investigated that genistein could represent a good modulator of PKC by preventing for example Aβ-induced BVR oxidation as observed in AD.
Taken together, the current study suggests that the PKC signaling pathway plays a pivotal role in the neuroprotective properties of genistein against Aβ25–35-induced toxicity in PC12 cells. The relevance of our findings to in vivo clinical situations remains to be demonstrated. Further studies are needed to investigate the neuroprotective effects in vivo of genistein on Aβ25–35-induced neurotoxicity in AD.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid β-peptide
- PKC:
-
Protein kinase C
- PC12:
-
Pheochromocytoma
References
Zeng H, Chen Q, Zhao B (2004) Genistein ameliorates beta-amyloid peptide (25–35)-induced hippocampal neuronal apoptosis. Free Radic Biol Med 36:180–188
Xie J, Guo Q, Zhu H, Wooten MW, Mattson MP (2000) Protein kinase C iota protects neural cells against apoptosis induced by amyloid beta-peptide. Brain Res Mol Brain Res 82:107–113
Su JH, Anderson AJ, Cummings BJ, Cotman CW (1994) Immunohistochemical evidence for apoptosis in Alzheimer’s disease. Neuroreport 5:2529–2533
Tortosa A, Lopez E, Ferrer I (1998) Bcl-2 and Bax protein expression in Alzheimer’s disease. Acta Neuropathol 95:407–412
Masliah E, Mallory M, Alford M, Tanaka S, Hansen LA (1998) Caspase dependent DNA fragmentation might be associated with excitotoxicity in Alzheimer disease. J Neuropathol Exp Neurol 57:1041–1052
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1:120–129
Tomas M, Garcia N, Santafe MM, Lanuza M, Tomas J (2009) Protein kinase C involvement in the acetylcholine release reduction induced by amyloid-beta(25-35) aggregates on neuromuscular synapses. J Alzheimers Dis 18:877–884
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82:4245–4249
Zhang JF, Qi JS, Qiao JT (2009) Protein kinase C mediates amyloid beta-protein fragment 31-35-induced suppression of hippocampal late-phase long-term potentiation in vivo. Neurobiol Learn Mem 91:226–234
Ashenafi S, Fuente A, Criado JM, Riolobos AS, Heredia M, Yajeya J (2005) Beta-Amyloid peptide25-35 depresses excitatory synaptic transmission in the rat basolateral amygdala “in vitro”. Neurobiol Aging 26:419–428
Zhang L, Xing D, Zhu D, Chen Q (2008) Low-power laser irradiation inhibiting Abeta25-35-induced PC12 cell apoptosis via PKC activation. Cell Physiol Biochem 22:215–222
Slack BE, Nitsch RM, Livneh E, Kunz GM Jr, Eldar H, Wurtman RJ (1993) Regulation of amyloid precursor protein release by protein kinase C in Swiss 3T3 fibroblasts. Ann N Y Acad Sci 695:128–131
Cecchi C, Fiorillo C, Baglioni S, Pensalfini A, Bagnoli S, Nacmias B, Sorbi S et al (2007) Increased susceptibility to amyloid toxicity in familial Alzheimer’s fibroblasts. Neurobiol Aging 28:863–876
Battaini F, Pascale A (2005) Protein kinase C signal transduction regulation in physiological and pathological aging. Ann N Y Acad Sci 1057:177–192
Kurzer MS, Xu X (1997) Dietary phytoestrogens. Annu Rev Nutr 17:353–381
Ma W, Yuan L, Yu H, Ding B, Xi Y, Feng J, Xiao R (2010) Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by beta-amyloid peptides 25-35 in PC12 cells. Int J Dev Neurosci 28:289–295
Yu HL, Li L, Zhang XH, Xiang L, Zhang J, Feng JF, Xiao R (2009) Neuroprotective effects of genistein and folic acid on apoptosis of rat cultured cortical neurons induced by beta-amyloid 31-35. Br J Nutr 102:655–662
Valles SL, Borras C, Gambini J, Furriol J, Ortega A, Sastre J, Pallardo FV et al (2008) Oestradiol or genistein rescues neurons from amyloid beta-induced cell death by inhibiting activation of p38. Aging Cell 7:112–118
Andersen JM, Myhre O, Fonnum F (2003) Discussion of the role of the extracellular signal-regulated kinase-phospholipase A2 pathway in production of reactive oxygen species in Alzheimer’s disease. Neurochem Res 28:319–326
VanDemark KL, Guizzetti M, Giordano G, Costa LG (2009) Ethanol inhibits muscarinic receptor-induced axonal growth in rat hippocampal neurons. Alcohol Clin Exp Res 33:1945–1955
Berts A, Minneman KP (2000) Tyrosine kinase inhibitors and Ca2+ signaling: direct interactions with fura-2. Eur J Pharmacol 389:35–40
Levites Y, Amit T, Youdim MB, Mandel S (2002) Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem 277:30574–30580
Sul D, Kim HS, Lee D, Joo SS, Hwang KW, Park SY (2009) Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci 84:257–262
Qu M, Li L, Chen C, Li M, Pei L, Chu F, Yang J et al (2011) Protective effects of lycopene against amyloid beta-induced neurotoxicity in cultured rat cortical neurons. Neurosci Lett 505:286–290
Xian YF, Lin ZX, Mao QQ, Ip SP, Su ZR, Lai XP (2012) Protective effect of isorhynchophylline against beta-amyloid-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol 32:353–360
Maher P (2001) How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci 21:2929–2938
Fowler CJ (1997) The role of the phosphoinositide signalling system in the pathogenesis of sporadic Alzheimer’s disease: a hypothesis. Brain Res Brain Res Rev 25:373–380
Olariu A, Yamada K, Mamiya T, Hefco V, Nabeshima T (2002) Memory impairment induced by chronic intracerebroventricular infusion of beta-amyloid (1-40) involves downregulation of protein kinase C. Brain Res 957:278–286
Kuperstein F, Reiss N, Koudinova N, Yavin E (2001) Biphasic modulation of protein kinase C and enhanced cell toxicity by amyloid beta peptide and anoxia in neuronal cultures. J Neurochem 76:758–767
Etcheberrigaray R, Tan M, Dewachter I, Kuiperi C, Van der Auwera I, Wera S, Qiao L et al (2004) Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc Natl Acad Sci USA 101:11141–11146
Zhu JT, Choi RC, Xie HQ, Zheng KY, Guo AJ, Bi CW, Lau DT et al (2009) Hibifolin, a flavonol glycoside, prevents beta-amyloid-induced neurotoxicity in cultured cortical neurons. Neurosci Lett 461:172–176
Ekinci FJ, Linsley MD, Shea TB (2000) Beta-amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Brain Res Mol Brain Res 76:389–395
Grutter MG (2000) Caspases: key players in programmed cell death. Curr Opin Struct Biol 10:649–655
Shimohama S, Tanino H, Fujimoto S (1999) Changes in caspase expression in Alzheimer’s disease: comparison with development and aging. Biochem Biophys Res Commun 256:381–384
Harada J, Sugimoto M (1999) Activation of caspase-3 in beta-amyloid-induced apoptosis of cultured rat cortical neurons. Brain Res 842:311–323
McGinnis KM, Wang KK, Gnegy ME (1999) Alterations of extracellular calcium elicit selective modes of cell death and protease activation in SH-SY5Y human neuroblastoma cells. J Neurochem 72:1853–1863
Maines MD, Miralem T, Lerner-Marmarosh N, Shen J, Gibbs PE (2007) Human biliverdin reductase, a previously unknown activator of protein kinase C betaII. J Biol Chem 282:8110–8122
Acknowledgments
This study is supported by the Science and Technology Plan of Agricultural Research Projects of Guangdong Province (2010B020312016) and the Social Development Plan of Industrial Projects of Guangdong Province (2005B10401005).
Conflict of interest
Nothing to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sijing Luo and Tian Lan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Luo, S., Lan, T., Liao, W. et al. Genistein Inhibits Aβ25–35 –Induced Neurotoxicity in PC12 Cells via PKC Signaling Pathway. Neurochem Res 37, 2787–2794 (2012). https://doi.org/10.1007/s11064-012-0872-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0872-4