Abstract
Many studies have demonstrated that apoptosis play an important role in cerebral ischemic pathogenesis and may represent a target for treatment. Neuroprotective effect of quercetin has been shown in a variety of brain injury models including ischemia/reperfusion. It is not clear whether BDNF–TrkB–PI3K/Akt signaling pathway mediates the neuroprotection of quercetin, though there has been some reports on the quercetin increased brain-derived neurotrophic factor (BDNF) level in brain injury models. We therefore first examined the neurological function, infarct volume and cell apoptosis in quercetin treated middle cerebral artery occlusion (MCAO) rats. Then the protein expression of BDNF, cleaved caspase-3 and p-Akt were evaluated in either the absence or presence of PI3K inhibitor (LY294002) or tropomyosin receptor kinase B (TrkB) receptor antagonist (K252a) by immunohistochemistry staining and western blotting. Quercetin significantly improved neurological function, while it decreased the infarct volume and the number of TdT mediated dUTP nick end labeling positive cells in MCAO rats. The protein expression of BDNF, TrkB and p-Akt also increased in the quercetin treated rats. However, treatment with LY294002 or K252a reversed the quercetin-induced increase of BDNF and p-Akt proteins and decrease of cleaved caspase-3 protein in focal cerebral ischemia rats. These results demonstrate that quercetin can decrease cell apoptosis in the focal cerebral ischemia rat brain and the mechanism may be related to the activation of BDNF–TrkB–PI3K/Akt signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is one of the main causes of death and disability. The World Health Organization has estimated that about 15 million people worldwide suffer from stroke annually [1]. Due to the increased risk of hemorrhage, plasminogen activator is restricted to administration within 3 h of stroke onset and is used only in 3 % of patients [2]. Therefore, existing stroke therapies need to be improved and new strategies need to be explored.
Apoptosis after cerebral ischemia is one of the major pathways that lead to the process of cell death [3]. In response to the oxidative load in mitochondria, the outer membrane of mitochondria becomes permeabilized [4], resulting in the translocation of Bax from the cytosol to the mitochondria and the release of cytochrome c. This proapoptotic protein translocation is controlled by the family of Bcl-2 proteins [5]. Cytochrome c was released into the cytosol leads to the formation of the apoptosome. The apoptosome permits the autoactivation of procaspase-9, which is followed by the activation of procaspase-3 [6]. Active caspase-3 leads to DNA fragmentation [7]. Some studies have demonstrated that apoptosis contributes to the development of ischemic infarction with DNA fragmentation. Thus, the ideal preventive or therapeutic approach would indeed target apoptosis after cerebral ischemia.
BDNF is an important neuroprotective factor for ischemic brain injury in vivo by anti-excitatory amino acids, inhibiting inflammatory factor and decreasing apoptosis [8, 9]. The survival function of BDNF is mediated through the activation of two cell surface receptors, the TrkB and the p75 neurotrophin (p75NTR) receptor [10]. Activation of TrkB has been shown to be essential for the survival-promoting actions of BDNF [11]. Through TrkB receptor, BDNF activates many intracellular signaling pathways, including the activation of PI3K/Akt, thereby affecting both development and function of the nervous system [12] Akt phosphorylates the pro-apoptotic Bcl-2 family member activity thereby inhibiting the pro-apoptotic function [13]. Akt also directly phosphorylates and inhibits the caspase proteases including caspase-9 [14].
Quercetin (3,5,7,3′,4′-pentahydroxyflavone) is a naturally-occurring flavonoid found in vegetable, fruits and tea. It has a flavone nucleus composed of two benzene rings linked through a heterocyclic pyrone ring. Because of its strong antioxidant and anti-inflammatory activities, it can prevent many diseases, including neurodegenerative disorders, diabetes, cancer and obesity [15–17]. Recently the efficacy of quercetin on cerebral ischemia/reperfusion injury has been reported. And it also was found that the neuroprotective mechanisms of quercetin on cerebral ischemia are related to the reduction of matrix metalloproteinases-9 and prevention of free radicals production [18, 19]. Tchatchou et al. [20] and Hou et al. [21] reported that quercetin enhanced the levels of BDNF in the Alzheimer’s disease mice brain. In our previous study, we found that quercetin could protect oligodendrocyte precursor cells from oxygen/glucose deprivation injury in vitro via the activation of PI3K/Akt signaling pathway [22]. However, it is not clear whether the neuroprotective mechanisms of quercetin against cerebral ischemia is related to the activation BDNF–TrkB–PI3K/Akt signaling pathway.
On the basis of above considerations, we investigated the relations between neuroprotection of quercetin and the BDNF–TrkB–PI3K/Akt signaling pathway in the rat brain after stroke. We showed that TrkB receptor antagonist (K252α) or PI3K inhibitor (LY294002)strongly attenuated the increase of BDNF and p-Akt proteins induced by quercetin in focal cerebral ischemia rats. However, the cleaved caspase-3 protein was elevated in the rats treated with quercetin/K252α or quercetin/LY294002 compared with rats treated with quercetin alone.
Materials and Methods
Animals
Male Sprague-Dawley rats (n = 132) weighing from 250 to 270 g were purchased from the Shanghai Slac Laboratory Animal Co. Ltd. The rats were housed with the dam in the home cage under a 12:12-h light:dark cycle, with food and water freely available throughout the study.
MCAO Animal Model and Drug Treatment
For the test of neurological function, the rats were randomly divided into four groups: (1) sham-operated group (n = 12), (2) vehicle-treated (dH2O/0.1 % tween-80) ischemic model group (n = 12), (3) 10 mg/(kg day) quercetin-treated ischemic group (n = 12), (4) 20 mg/(kg day) quercetin-treated ischemic group (n = 12).
For infarct size and apoptosis and protein expression assay, the rats were randomly divided into four groups: (1) sham-operated group (n = 12), (2) vehicle-treated ischemic model group (n = 12), (3) 10 mg/(kg day) quercetin-treated ischemic group (n = 12), (4) 20 mg/(kg day) quercetin-treated ischemic group (n = 12).
For observing whether TrkB receptor activation is required for quercetin protection against ischemia-induced apoptosis, the MCAO model rats were randomly divided into three groups: (1) K252a group (n = 6), (2) 20 mg/kg quercetin-treated group (n = 6), (3) 20 mg/kg quercetin/K252a -treated group (n = 6).
To ascertain whether Akt activation is a major contributor to the antiapoptotic effect of quercetin, the MCAO model rats were randomly divided into three groups: (1) LY294002 (LY) group (n = 6), (2) 20 mg/kg quercetin-treated group (n = 6), (3) 20 mg/kg quercetin/LY-treated group (n = 6).
The MCAO was induced by the intraluminal filament technique. Rats were anesthetized with 10 % chloral hydrate (0.4 ml/kg, IP). Then, a piece of nylon monofilament was inserted into the left internal carotid artery. After 90 min of ischemia, the filament was withdrawn. In sham-operated animals, the middle cerebral artery was not occluded. Quercetin in 0.1 % tween-80 containing distilled water was administered intragastrically once a day at the doses of 10 and 20 mg/(kg day), respectively, starting 3 h after MCAO till the rats were sacrificed. The vehicle control was given distilled water (dH2O/0.1 % tween-80) without quercetin.
To confirm how the neuroprotective effects of quercetin might be affected if the TrkB–PI3K pathway was initially blocked, a PI3K inhibitor, LY294002 (Cell Signaling) or K252a (Cell Signaling) were intraventricularly administered to rats with or without quercetin after cerebral ischemia. For intracerebroventricular injection, the rats were placed on ear bars of a stereotaxic instrument under anesthesia. The scalp was incised on the midline and the skull was exposed. LY294002 (10 μL, 100 nmol in 25 % dimethyl sulfoxide in PBS) or K252a (10 μL, 0. 5 μg/μL in 1 % DMSO) were injected using a Hamilton microsyringe at a rate of 1 μL/min. (0.8 mm posterior and 1.5 mm lateral to the bregma and 3.3 mm from the duramater) at 1 h after MCAO.
The present research reported in this paper was conducted in accordance with the Chinese legislation on the use and care of laboratory animals and was approved by their respective university committees for animal experiments.
Neurological Functional Test
Modified neurological severity score (mNSS) measurement is a composite of motor, sensory, reflex and balance tests, as previously described [23]. In the present study, mNSS was used to determine the sensorimotor deficit 7, 14, 21 and 28 days after MCAO. Only animals that showed no or incomplete forelimb placing with rotational asymmetry 24 h after MCAO were included in the subsequent analysis.
Tissue Preparation
For histological analyses, rats were anesthetized with 10 % chloral hydrate and then perfused through the left ventricle with normal saline and 4 % phosphate-buffered paraformaldehyde. Brains were then removed and immersion fixed in 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) overnight at 4 °C, cryoprotected in 30 % (wt/vol) sucrose in 0.1Mphosphate buffer (pH 7.4). Serial (40-μm) coronal sections were cut on a freezing sliding microtome and stored at −20 °C. For Western blotting, after brains were removed, tissues from the cortex were rapidly dissected and stored at −80 °C.
Measurements of Infarct Volume
Seven sections at bregma levels +4.70, +2.70, +0.70, −1.30, −3.30, −5.30, and −7.30 mm were stained with hematoxylin and eosin (HE). Measurement of infarct volume was measured on the 7 HE stained coronal sections with the use of a Global Laboratory Image analysis program (Data Translation). Briefly, the area of both hemispheres and the infarct area (mm2) were calculated by tracing the area on the computer screen. Infarct volume (mm3) was determined by multiplying the appropriate area by the section interval thickness. The infarct volume is presented as the percentage of infarct volume of the contralateral hemisphere [24].
TdT Mediated dUTP Nick End Labeling (TUNEL) Assay
To assess DNA damage in the cortex after MCAO, three sections per brain (n = 4 for each group) were selected. The sections were treated as instructed with an in situ cell death detection kit. Diaminobenzidine was used as a chromogen. TUNEL-positive cells displayed brown staining within the nucleus of apoptotic cells. The number of TUNEL positive cells for 5 fields (200 × 200 μm2 for each field) in primary somatosensory cortex of each section was counted under high-power magnification (400×). An average for the three slices per brain was taken. The data of TUNEL positive cells was expressed as mean ± SE.
Immunohistochemistry
Immunohistochemistry staining for brain tissue was performed on ice-cold sections (40 μm). Briefly, sections were blocked with 0.01 mol/L phosphate-buffered saline (PBS) containing 5 % goat serum for 1 h at RT. Then the sections were incubated with rabbit polyclonal Bcl-2 (1:200, Santa Cruz), rabbit polyclonal Bax (1:1,000, Sigma), rabbit polyclonal-BDNF (1:100, Santa Cruz), rabbit polyclonal-TrkB (1:200, Santa Cruz) antibodies 48 h at 4 °C. After three washes with PBS for 5 min each, the secondary antibody biotin conjugated goat anti-rabbit IgG (1:500, Sigma) were added to the sections and incubated for 1 h at RT. Then after washing with PBS for 5 min each, the avidin conjugated horseradish peroxidase (1:200, Sigma) were added to the sections and incubated for 30 min at RT. Coloration with DAB/0.03 % H2O2 for 5–10 min at RT. Cell images were captured with a microscope (Nikon Eclipse E800; Germany) equipped with a Spot RT digital camera.
For semi-quantitative measurement of BDNF andTrkB positive cell pixel counts, Image Pro-Plus 5.0 software (Media Cybernetics, Inc., Bethesda, MD, USA) was used. Three sections per brain (n = 4 for each group) were selected between bregma levels +1.60 and −0.2 mm every 9th section. The digitalized images were then contrast-enhanced to clearly differentiate positivity from background, and a thresholding procedure was established to determine the proportion of immunoreactive area within 5 fields (200 × 200 μm2for each field) of view for each section. Data are presented as a percentage of area, in which the BDNF or TrkB-immunopositive areas in each field were divided by the total areas in the field.
Western Blotting
It is about 3 mm (coronal) around the infarcet area was selected for western blotting. Tissue samples were lysed in 1 % sodium dodecylsulfate buffer (pH 7.6, 20 mM HEPES containing protease inhibitor), and lysates were collected and sonicated. Protein concentrations were determined using the Bio-Rad Dc Protein assay (Bio-Rad, Hercules, CA). Protein extracts and a biotinylated molecular weight marker (Cell Signaling Technology, Beverly, MA, USA) were denaturated in Laemmli sample loading buffer at 95 °C, separated by 4–20 % polyacrylamide gel electrophoresis, and electrotransferred in transfer buffer to a polyvinyl diflouride membrane (Bio-Rad). The membrane was treated with blocking solution (5 % nonfat dry milk in TBST) and incubated overnight at 4 °C with rabbit polyclonal Bcl-2 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal Bax (1:1,000, Sigma), rabbit polyclonal cleaved caspase-3 (1:1,000, Cell Signaling), mouse monoclonal phospho-Akt (1:1,000, chemicon), polyclonal -BDNF(1:300, Santa Cruz), rabbit polyclonal –TrkB (1:500, Santa Cruz) or mouse monoclonal Actin antibody (1:2,000; Sigma). Secondary incubations were performed with horseradish peroxidase-linked anti-mouse (1:2,000; Sigma) or anti-rabbit (1:2,000; Sigma) antibodies. Bands were visualized using enhanced chemiluminescence, and serial exposures were made to radiographic film.
Statistical Analysis
Data are expressed as mean ± standard error of the mean (s.e.m.), and n refers to the number of rats. Data were analyzed by SPSS 13.0 using Windows software to conduct one way ANOVA (equal variances assumed by S–N–K). P value < 0.05 was considered significant.
Results
Quercetin Improved the Neurological Function in MCAO Rats
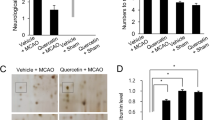
A modified neurological severity score test was performed after MCAO. The higher score means the poorer function. The results showed that mNSS scores significantly increased in MCAO rats compared to sham group rats. The treatment starting at 3 h after MCAO with 10 and 20 mg/(kg day) quercetin significantly decreased mNSS scores at 7, 14, 21 and 28 days compared with the vehicle-treated group, respectively (Fig. 1). This data indicates that quercetin was effective to improve neurological function at both early stage and late stage after focal cerebral ischemia.
Effect of quercetin on neurological function in MCAO rats. Quercetin was intragastrically administered to the rats starting from 3 h after the onset of MCAO. The vehicle-treated ischemic model group received an equal volume of normal saline. n = 12 for each group. Values are expressed as mean ± SE. Quercetin versus vehicle-treated group *P < 0.05; **P < 0.01
Quercetin Reduced Infarct Volume After MCAO
To study the effect of quercetin on the infarct volume, the damaged area was analyzed in animals decapitated 7 days after MCAO by HE staining. There was no infarct found in the sham rats brain (Fig. 2a). The infarcted area was obvious in the vehicle group rats brain (Fig. 2b, c) compared to the quercetin treated rats (Fig. 2d). The percentage of infarct volume of the contralateral hemisphere in the vehicle group was 36.2 ± 4.8 %; in the 10 mg/(kg day) quercetin group was 24.8 ± 2.7 %; in the 20 mg/(kg day) quercetin group was 21.7 ± 3.2 %. The treatment with quercetin resulted in significant decrease of infarct volume compared with vehicle control group (P < 0.05; Fig. 2e).
Quercetin treatment reduced infarct volume after MCAO. H-E staining shows the cells in the cortex of sham rats (a). The infarcted area was obvious in the vehicle rats. The line shows the boundary between infarcted area and penumbra (b). c Shows the infarcted core area in the vehicle rats. Treatment with quercetin resulted in significant decrease of infarct volume compared to the vehicle group (d, e). n = 4 for each group. Quercetin versus vehicle group *P < 0.05
Quercetin Decreased the Number of TUNEL-Positive Cells
In the present study, we observed a significant increase in TUNEL-positive cells at 7 day in the cortex of the vehicle-treated group compared with sham group (P < 0.01; Fig. 3). The quercetin treatment significantly reduced the number of TUNEL-positive cells in the cortex as compared to the vehicle-treated group (P < 0.05, 10 mg/(kg day) quercetin vs. vehicle group; P < 0.01, 20 mg/(kg day) quercetin vs. vehicle group; Fig. 3).
Quercetin decreased the number of TUNEL-positive cells in cortex of MCAO rat. Representative images demonstrating TUNEL-labeled cells in the cortex in the sham (a), vehicle (b) and 20 mg/(kg day) quercetin (c) groups. Bar = 100 μm in (c). d TUNEL-positive cells were significantly enhanced in the vehicle group compared to the sham group, treatment with querectin showed reduced number of TUNEL-positive cells. n = 4 for each group. Sham versus vehicle group # P < 0.01. Quercetin versus vehicle group *P < 0.05; **P < 0.01
Quercetin Regulated the Expression of Apoptosis Related Proteins
Figure 4 shows the results of western blotting with anti- Bcl-2 and anti- Bax antibodies in cortex of rats 7 days after MCAO. Compared with the sham-operated rats, the protein expression of Bcl-2 significantly decreased (P < 0.01) and Bax increased (P < 0.01) in the vehicle-treated rats. Treatment with quercetin significantly increased the protein expression of Bcl-2 (P < 0.05, 10 mg/(kg day) quercetin vs. vehicle group; P < 0.01, 20 mg/(kg day) quercetin vs. vehicle group) and decreased the protein expression of Bax (P < 0.01, both 10 and 20 mg/(kg day) quercetin vs. vehicle group).
Quercetin regulated apoptosis related protein expression in cortex of rats after MCAO. The quantitative analysis of Bcl-2 and Bax are expressed as a fraction of the respective level of beta-actin. n = 4 for each group. Sham versus vehicle group **P < 0.01. Quercetin versus vehicle group # P < 0.05; ## P < 0.01
The expression of cleaved caspase-3 also was analyzed by western blotting. The data shows that treatment with quercetin significantly decreased the protein expression of cleaved caspase-3 (P < 0.01, both 10 and 20 mg/(kg day) quercetin vs. vehicle group; Fig. 5).
Quercetin decreased the protein expression of cleaved caspase-3 in cortex of rats after MCAO. The quantitative analysis of cleaved caspase-3 is expressed as a fraction of the respective level of total caspase-3. n = 4 for each group. Sham versus vehicle group **P < 0.01. Quercetin versus vehicle group # P < 0.01
Quercetin Promoted the Survival of BDNF and TrkB Positive Cells
The results of this present study showed that in the vehicle-treated ischemic rats, the number of BDNF and its receptor TrkB immunoreactive positive cells decreased in the ischemic ipsilateral cortex 7 days after MCAO compared to the sham-operated group (P < 0.01). Treatment with quercetin significantly increased the number of BDNF positive cells (P < 0.05, both 10 and 20 mg/(kg day) quercetin vs. vehicle group) and TrkB positive cells (P < 0.01, both 10 and 20 mg/(kg day) quercetin vs. vehicle group) compared to the vehicle-treated group (Fig. 6).
Quercetin promoted the survival of BDNF and TrkB positive cells in cortex of MCAO rats. a–f is the images of BDNF and TrkB immunohistochemistry staining cells in the cortex. a, d sham group; b, e vehicle group; c, f quercetin 20 mg (kg day) group; Bar = 100 μm in (f). g is the quantitative analysis of the positive pixel counts of BDNF and its receptor TrkB. n = 4 for each group. Sham versus vehicle group **P < 0.01. Quercetin versus vehicle group # P < 0.05; ## P < 0.01
Quercetin Increased the Protein Levels of BDNF and TrkB and p-Akt
We further examined the expression of BDNF and TrkB and p-Akt in cortex of rats 7 days after MCAO by western blotting analysis. MCAO significantly decreased the protein expression of BDNF and TrkB compared to the sham-operated group (P < 0.01; Fig. 7). The protein expression of BDNF (P < 0.01, both 10 and 20 mg/(kg day) quercetin vs. vehicle group; Fig. 7) and TrkB (P < 0.01, both 10 and 20 mg/(kg day) quercetin vs. vehicle group; Fig. 7) significantly increased treatment with quercetin compared to the vehicle-treated ischemic rats. The examination of p-Akt showed that MCAO significantly decreased the expression of p-Akt protein (P < 0.01; Fig. 8). Oral administration of quercetin to MCAO induced ischemic rats reversed the decrease of p-Akt protein. The protein expression of p-Akt significantly increased compared with the vehicle-treated ischemic rats (P < 0.01, both 10 and 20 mg/(kg day) quercetin vs. vehicle group; Fig. 8). All of the BDNF and TrkB and p-Akt protein levels in quercetin group almost restored to the sham-operated group.
Quercetin Protects Neuronal Apoptosis Through BDNF–TrkB–p-Akt Signaling Pathway
To examine whether TrkB activation was required for the neuroprotective effect of quercetin, we examined the effect of K252a, an inhibitor of Trk family members, on focal cerebral ischemic injury. In the presence of K252a, quercetin failed to show neuroprotection against ischemia—induced apoptosis. The protein expression of BDNF and p-Akt significantly decreased both in the K252a group and quercetin/K252a group compaired to quercetin group. (both BDNF and p-Akt: P < 0.01, K252a group vs. quercetin group and quercetin/K252a group vs quercetin group; Fig. 9). However, the protein expression of cleaved caspase-3 increased in the presence of K252a (P < 0.01, K252a group vs. quercetin group and quercetin/K252a group versus quercetin group; Fig. 9). There were no significant differences between the K252a group and quercetin/K252a group in the protein levels of BDNF, p-Akt and cleaved caspase-3.
To establish the role of PI3K/Akt pathway in neuroprotective effect of quercetin, MCAO rats were treated with PI3K inhibitor, LY294002. We observed the similar results in LY294002 treated rats and in the K252a treated rats. The protein expression of BDNF and p-Akt significantly decreased both in the LY group and quercetin/LY group compared to quercetin group (both BDNFand p-Akt: P < 0.01, LY group vs. quercetin group and quercetin/LY group vs. quercetin group; Fig. 10). However, the protein expression of cleaved caspase-3 increased in the presence of LY (P < 0.01, LY group vs. quercetin group and quercetin/LY group vs. quercetin group; Fig. 10). There were no significant differences between the LY group and quercetin/LY group in the protein levels of BDNF, p-Akt and cleaved caspase-3.
Quercetin protects against ischemia-induced apoptosis is mediated by activation of PI3K/Akt pathway. Treatment with LY294002 reversed the quercetin-induced increase of BDNF and p-Akt proteins and decrease of cleaved caspase-3 protein in focal cerebral ischemia rats. n = 6 for each group. Quercetin versus LY group **P < 0.01; quercetin/LY versus quercetin group ## P < 0.01
Discussion
Previous studies have demonstrated that MCAO causes neurological deficits and infarct, and neuronal cell apoptosis plays an important role in the evolution of ischemic injury in the brain [25, 26]. In the present study, we found that treatment with quercetin could significantly improve neurological function at 7, 14, 21 and 28 days after cerebral ischemia by mNSS. Our findings in accordance with the earlier studies carried out by others where neurological function has been improved by treatment with antioxidants or quercetin [18, 27, 28].
A classical measure of histological outcome used to evaluate neuroprotection following MCAO is the infarct volume. Traditional histochemical staining, 2,3,5 Triphenyltetrazolium chloride (TTC) methods was recently suspected to overestimate infarct size [29]. We therefore quantified the infarct volume at 7 days after reperfusion using H-E staining [30] and found quercetin treatment reduced the infarct size. The result was consistent with others studies which indicated that quercetin administered after cerebral ischemia is effective in reducing infarct volume and lead to improvements in neurological outcome [18, 31, 32].
Since most of delayed neuronal degeneration is due to apoptosis, we further investigated the number of apoptotic cells by TUNEL staining. Results showed less TUNEL positive cells in quercetin treatment rats than in the vehicle treatment rats. The activation of the PI3K/Akt pathway is critical for neuroprotection from ischemia-induced apoptosis. To determine whether Akt activation is a major contributor to quercetin against apoptosis induced by ischemia, we therefore investigated the phosphorylation of Akt after MCAO by western blot. Treatment with quercetin significantly increased the expression of p-Akt compared to the vehicle-treated group. Then the expression of p-Akt downstream signaling proteins were further examined. Results showed that decreased protein level of Bcl-2 and increased protein levels of Bax and cleaved-caspase-3 in MCAO rats. Interestingly, quercetin treatment was associated with pronounced upregulation of Bcl-2 and downregulation of Bax and cleaved-caspase-3 proteins. Previous studies have suggested that the neuroprotective mechanisms of quercetin could be due to its inhibition of sodium channels, MMP-9 activity, NF-kappaB activation and increasing GSH levels in cerebral ischemia rats [32–34]. This present study found quercetin could regulate the expression of Bcl-2 family member and inhibit the caspase-3 activation after MCAO. These data provided further evidence that quercetin can reduce cell apoptosis during ischemia through inhibiting mitochondria-dependent caspase apoptotic pathway.
In the current study, we demonstrated that quercetin also significantly increased BDNF and TrkB protein levels. It is reported that BDNF can inhibit the caspase-3 activation and cell apoptosis caused by hypoxia-ischemic injury in vivo [35]. Neuroprotection of BNDF was shown to be dependent on the activation of the TrkB receptor, which was verified by its phosphorylation. BDNF-TrkB leads to the activation of the mitogen activated protein kinase (MAPK), phospholipase C (PLC)-1, and PI3K/Akt [12]. However, PI3K/Akt pathway is particularly important for mediating neuronal survival under a wide variety of circumstances [36] and has been found to be sufficient and, in some cases, necessary for the trophic-factor-induced cell survival of several neuronal cell types. To determine whether quercetin exerts antiapoptotc effects via BDNF–TrkB–PI3K/Akt signaling pathway, we further examined the protein expression of BDNF, p-Akt and cleaved caspase-3 in the presence of a TrkB receptor antagonist K252a or PI3K inhibitor LY294002. We confirmed inhibition of TrkB receptor signaling with K252a prevented the increase of BDNF and phosphorylation of Akt induced by quercetin. Meanwhile, the quercetin induced decrease of cleaved caspase-3 also diminished. Blocking PI3K/Akt signaling with the pharmacological inhibitor LY294002 significantly disturbed the antiapoptotic effect of quercetin. This suggests that quercetin protects against apoptosis induced by ischemia mediated by PI3K/Akt pathway.
Conclusion
Collectively, our present findings demonstrate that the neuroprotection of quercetin is related to the activation of PI3K/Akt signaling through phosphorylation of the TrkB receptor from ischemia-induced apoptosis. Further investigation into the role and mechanisms of anti-apoptosis of quercetin may have a therapeutic value for the treatment of stroke.
References
Paul SL et al (2007) The large and growing burden of stroke. Curr Drug Targets 8(7):786–793
Heuschmann PU et al (2004) Predictors of in-hospital mortality in patients with acute ischemic stroke treated with thrombolytic therapy. JAMA 292(15):1831–1838
Mattson MP et al (2001) Neurodegenerative disorders and ischemic brain diseases. Apoptosis 6(1–2):69–81
Kowaltowski AJ et al (2001) Mitochondrial permeability transition and oxidative stress. FEBS Lett 495(1–2):12–15
Kuwana T et al (2002) Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111(3):331–342
Green DR et al (1998) Mitochondria and apoptosis. Science 281(5381):1309–1312
Li P et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91(4):479–489
Islam O et al (2009) Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res 6(1):42–53
Ploughman M et al (2009) Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke 40(4):1490–1495
Huang EJ et al (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 361(1473):1545–1564
Patapoutian A et al (2001) Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11(3):272–280
Datta SR et al (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91(2):231–241
Cardone MH et al (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282(5392):1318–1321
Cho JY et al (2006) Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global cerebral ischemia. Neurosci Lett 404(3):330–335
Comalada M et al (2005) In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol 35(2):584–592
Dok-Go H et al (2003) Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res 965(1–2):130–136
Ahmad A et al (2011) Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem Res 36(8):1360–1371
Lee JK et al (2011) Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochir (Wien) 153(6):1321–1329
Tchantchou F et al (2009) Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. J Alzheimers Dis 18(4):787–798
Hou Y et al (2010) Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology 58(6):911–920
Wang XQ et al (2011) Oligodendrocyte precursor cells from oxygen/glucose deprivation injury in vitro via the activation of PI3K/Akt signaling pathway. Brain Res Bull 86(3–4):277–284
Jin K et al (2003) Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci 24(1):171–189
Pu F et al (2007) Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci 104(4):329–334
Rizk NN et al (1053) (2005) Cerebral ischemia induced apoptosis and necrosis in normal and diabetic rats. Brain Res 1–2:1–9
Solaroglu I et al (2006) Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience 143(4):965–974
Khan MM et al (2009) Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res 1292:123–135
Yousuf S et al (2009) Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res 1250:242–253
Benedek A et al (2006) Use of TTC staining for the evaluation of tissue injury in the early phases of reperfusion after focal cerebral ischemia in rats. Brain Res 1116(1):159–165
Wang YH et al (2006) Taxifolin ameliorates cerebral ischemia-reperfusion injury in rats through its anti-oxidative effect and modulation of NF-kappa B activation. J Biomed Sci 13(1):127–141
Kovalenko TM et al (2006) Neuroprotective effect of quercetin during experimental brain ischemia. Fiziol Zh 52(5):21–27
Rivera F et al (2008) Reduction of ischemic brain damage and increase of glutathione by a liposomal preparation of quercetin in permanent focal ischemia in rats. Neurotox Res 13(2):105–111
Hwang IK et al (2009) Neuroprotective effects of onion extract and quercetin against ischemic neuronal damage in the gerbil hippocampus. J Med Food 12(5):990–995
Yao Y et al (2010) Quercetin improves cognitive deficits in rats with chronic cerebral ischemia and inhibits voltage-dependent sodium channels in dhippocampal CA1 pyramidal neurons. Phytother Res 24(1):136–140
Han BH et al (2000) BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci 20(15):5775–5781
Brunet A et al (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K–Akt signaling pathway. Curr Opin Neurobiol 11(3):297–305
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 30801526 to Ruiqin Yao), the Natural Science Foundation for Universities in Jiangsu Province (No.09KJD310010 to Ruiqin Yao) and the Foundation of Xuzhou Medical College (No.09KJZ14 to Ruiqin Yao).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, RQ., Qi, DS., Yu, HL. et al. Quercetin Attenuates Cell Apoptosis in Focal Cerebral Ischemia Rat Brain Via Activation of BDNF–TrkB–PI3K/Akt Signaling Pathway. Neurochem Res 37, 2777–2786 (2012). https://doi.org/10.1007/s11064-012-0871-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0871-5