Abstract
Experimental studies have demonstrated that oxidative stress and apoptosis play an important role in cerebral ischemic pathogenesis and may represent a target for treatment. The purpose of this study was to determine whether the quercetin dihydrate (Q) protects against cerebral ischemia neuronal damage. Male Wistar rats were subjected to transient middle cerebral artery occlusion (MCAO) for 2 h and reperfused for 72 h. Quercetin (30 mg/kg, i.p) was administrated 30 min before the onset of ischemia and after the ischemia at interval of 0, 24, 48, and 72 h. The administration of Q showed marked reduction in infarct size, reduced the neurological deficits in terms of behaviors, suppressed neuronal loss and diminished the p53 expression in MCAO rats. Q was found to be successful in upregulating the antioxidant status and lowering the TBARS level. Conversely, the elevated activity of poly (ADP-ribose) polymerase (PARP), and activity of caspase-3 in MCAO group was attenuated significantly in Q treated group when compared with MCAO group. Our study reveals that Q, as a powerful antioxidant, could prevent free radicals associated oxidative damage and morphological changes in the MCAO rats. Thus, it may have a therapeutic value for the treatment of stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a leading cause of lasting disability. Ischemic stroke results from a transient or permanent reduction in cerebral blood flow that is restricted to the territory of a major brain artery. Oxidation of protein, lipid and nucleic acid increase at an alarming pace in brain with induction of ischemic stroke, which trigger signals to an interrelated metabolic processes such as decrease in ATP production, intracellular calcium accumulation, massive release of excitatory amino acids, free radical formation etc. preceding neuronal cell death. Oxidative stress has been implicated in the machinery of ischemia–reperfusion injury after cerebral ischemia in a number of studies [1–3], consequently producing surplus amounts of biphasic players, reactive oxygen species (ROS).
Oxidative stress is often defined as an imbalance between reactive oxygen species (ROS) and their quenching by the antioxidant system. This imbalance may originate from an overproduction of ROS or from a reduction in antioxidant defenses [4]. Brain tissue has potential sources of ROS, high level of polyunsaturated fatty acids (PUFA) and large oxidative capacity, but its ability to combat oxidative stress is limited [5]. Increasing evidence suggests that oxidative stress is a prominent and early feature in the pathogenesis of ischemic/reperfusion injury.
Animal models have been used to evaluate the deleterious mechanism involved in ischemic damage and to improve our understanding to study the potential efficiency of therapeutic strategies. Thus, it can be speculated that treatment with antioxidants may boost the defense system against neurodegeneration. Earlier our research group has been evaluated efficacy of several antioxidants against experimental model of neurodegeneration [6–8].
Quercetin, a natural flavonoid, has been reported to possess potential antioxidant and free radical scavenger and is found in high quantities in fruits and vegetables. Quercetin has been shown to have anti-inflammatory, anti-blood coagulation, anti-ischemic [9–11]. Neuroprotective effects have been demonstrated in a variety of brain injury models including ischemia/reperfusion showing improvement of morphological and functional outcomes [12–15]. Recently Lee et al. [16] have investigated and reported the efficacy of Quercetin on cerebral ischemia/reperfusion injury. But there are some reports showing quercetin at low concentration exhibits prooxiadant activity [17]. In this study we used the rat model of middle cerebral artery occlusion (MCAO) and reperfusion to confirm the neuroprotective effects of quercetin. Thus, the aim of this study was to investigate the neuroprotective effects of quercetin for cerebral ischemia.
Materials and Methods
Chemical and Reagents
Quercetin dihydrate, oxidized glutathione (GSSG), reduced glutathione (GSH), (−) epinephrine, glutathione reductase (GR), glycine, nicotinamide adenine dinucleotide phosphate reduced form (NADPH), 1-chloro-2,4- dinitrobenzene (CDNB), 5-5′-dithio-bis-2-nitrobenzoic acid (DTNB), bovine serum albumin, thiobarbituric acid (TBA), trichloroacetic acid (TCA), sulfosalicylic acid (SSA), adenosine 5-triphosphate (ATP), 2,3,5-triphenyltetrazolium chloride (TTC), ethylenediamine tetra acetic acid (EDTA), p-nitroanilide (pNA), dithiothritol (DTT), Ouabain, 2,3-diaminonaphthotriazole, 2,3-diaminonaphthalene (DAN), glacial acetic acid, sodium azide, triton X-100, haematoxylin, eosin, caspase-3 colorimetric assay kit, anti-mouse IgG and diaminobenzidine (DAB), were purchased from Sigma–Aldrich Chemicals Pvt. Ltd, India. Monoclonal antibody p53, was purchase from Biovision, USA, [3H] NAD+ was purchased from New England Nuclear (NEN), USA.
Animals
Male Wistar rats weighing 250–300 g were obtained from the Central Animal House, Jamia Hamdard, New Delhi, India. They were housed in polypropylene cages in air conditioned room and allowed free access to pellet diet and water ad libitum. The animals were used in accordance with the procedure approved by the Animal Ethics Committee of Jamia Hamdard.
Experimental Protocol
To investigate the neuroprotective effects of Quercetin in experimental model of cerebral ischemia, we used the rat MCAO model [18]. The animals were separated into four groups of eight rats each. Group I: sham-operated vehicle-treated control (S); group II: ischemia and vehicle-treated stroke (MCAO); group III: Querectin (30 mg/kg) and MCAO (Q+MCAO). Group IV: sham-operated querectin-treated control (Q+S); Querectin was dissolved in 0.1% DMSO in PBS and given in a dose of 30 mg/kg by intraperitoneal injection 1 h before the onset of ischemia and additional injections of 30 mg/kg were administered 0, 24, 48 and 72 h post-MCAO. We examined the effects of different doses of Quercetin on cerebral ischemia reperfusion injury in pilot studies to determine the optimal dose of Quercetin that provides the most neuroprotection against ischemia/reperfusion injury and also the Quercetin dose (30 mg/kg) used in this experiment was supported from previous studies showing that this amount provided the maximal protective effects in the treatment of different types of brain injury [10, 19].
Induction of Transient Cerebral Ischemia
The right middle cerebral artery occlusion was performed using an intraluminal filament model by the method of Longa et al. [18] as described by us [3]. In brief, the rats were anesthetized with chloral hydrate (400 mg/kg, i.p), silicone rubber (DOCEOL, USA) coated monofilament has a smooth, soft, and flexible tip (which reduces the risk of vessel injury and intracranial bleeding) was introduced into the external carotid artery (ECA) and advanced into the middle cerebral artery via the internal carotid artery (ICA) (17–20 mm) until a slight resistance was felt. Such resistance indicated that the filament had passed beyond the proximal segment of the anterior cerebral artery (ACA). At this point, the intraluminal suture blocks the origin of MCA and occluded all sources of blood flow from ICA, anterior cerebral artery and the posterior cerebral artery. Two hours after the induction of ischemia, the filament was slowly withdrawn and the animals were then returned to their cages. In the groups of sham-operated rats all surgical procedures except the induction of transient cerebral ischemia (MCAO) were performed. The animals were then returned to their cages and given free access to food and water.
Rota Rod (Motor Coordination Skill) Test
The neurological functions were evaluated after 72 h of reperfusion. The rotarod unit (Omni Rotor, Omnitech Electronics, Inc., Columbus, OH, USA) was used to evaluate the motor coordination skill. The rotarod unit consists of a rotating rod, 75 mm diameter, which was divided into four parts by compartmentalization to permit the testing of four rats at a time. The drug-naive animals were trained on the rod, so that they could stay on it at least for the cut-off time. After twice daily training for three successive days, the rotational speed of the test was increased to 10 rpm on the experimental day in a test session (speed 5 rpm on the first 2 days and 8 rpm on third day). The time each rat remained on the rotating rod was recorded for three trials of each rat, at a 5 min interval and a maximum trial length of 180 s per trial. The apparatus automatically records the time in 0.1 s when the rats fall of the rotating shaft. The motor deficiency was evaluated as the ability of the rat to hold the rotating rotor Data were presented as mean time on the rotating bar over the three test trials.
2, 3, 5- Triphenyltetrazolium Chloride Staining
The animals were sacrificed after 2 h occlusion and 72 h reperfusion. The brains were dissected out and kept on a rat brain matrix and 2 mm coronal sections were cut down and stained with 0.1% triphenyltetrazolium chloride (TTC) at 37°C for 15 min.The slices were observed with a stereomicroscope (Olympus SZX-12; Olympus Optical), and the infarct areas of each section were measured with the analysis of pixel counting by a computer program of Photoshop 6.0 and the volume was calculated.
Tissue Preparation
After 72 h of reperfusion periods, animals were sacrificed, and their brains were taken out quickly to dissect hippocampus and frontal cortex. The dissected brain parts were homogenized (5% w/v) in 10 mM phosphate buffer (PB, pH 7.4, having protease arrest 10 μl/ml) except in the case of Na+ K+ ATPase which was homogenized (5% w/v) in 10 mM Tris–HCl buffer (pH 7.4, having protease arrest 10 μl/ml). The homogenate was centrifuged at 800g for 5 min at 4°C to separate the nuclear debris. This supernatant was used for estimation of lipid peroxidation and Na+ K+ ATPase activity. The remaining supernatant was future centrifuged at 10,000g for 20 min at 4°C to get the post-mitochondria supernatant (PMS), which was used for other assays.
Estimation of Protein Concentration
The protein was estimated by the method of Lowry et al. [20] using bovine serum albumin (BSA) as the standard.
Thiobarbituric Acid Reactive Substances
The assay of Thiobarbituric Acid Reactive Substances (TBARS) was done according to method of Utley et al. [21] as described by us [22].The homogenate 0.25 ml was incubated at 37 ± 1°C in a metabolic shaker (120 cycles/min) for 1 h. Similarly, 0.25 ml of the same homogenate was pipette in a test tube and incubated at 0°C. After 1 h of incubation, 0.25 ml 5% chilled TCA and 0.25 ml of 0.67% TBA was added in each test tube. The mixture was centrifuged at 4,000g for 15 min and supernatant was transferred to another tube and placed in a boiling water bath for 10 min. Thereafter, the test tubes were cooled and the absorbance of the color was read at 535 nm. The rate of lipid peroxidation was expressed as nmol TBARS formed/h/g tissue using a molar extinction coefficient of 1.56 × 105 M−1 cm−1.
Glutathione Assay
Glutathione (GSH) was assayed by the method of Jollow et al. [23] with slight modification. Briefly, 0.1 ml PMS was precipitated with 0.1 ml sulfosalicylic acid (4%). The samples were kept at 4°C for 30 min. and then subjected to centrifugation at 4,000g for 10 min at 4°C. The assay mixture contained 0.1 ml supernatant, 2.7 ml phosphate buffer (0.1 M, pH 7.4) and 0.2 ml DTNB (0.4% in phosphate buffer 0.1 M, pH 7.4) in a total volume of 3.0 ml. The yellow color developed was read immediately at 412 nm using molar extinction coefficient 13.6 × 103 M−1 cm−1. The GSH content was calculated as nmol of GSH/mg protein.
Glutathione Reductase (GR) Assay
Glutathione Reductase (GR) activity was assayed by the method of Carlberg and Mannervik [24] as modified by Mohandas et al. [25]. The assay mixture consisted of phosphate buffer (0.1 M, pH 7.6), NADPH (0.1 mM), EDTA (0.5 mM) and oxidized glutathione (1 mM) and 0.05 ml of PMS in total volume of 1 ml. The enzyme activity was quantitated at room temperature by measuring the disappearance of NADPH at 340 nm and was calculated as nmol NADPH oxidized/min/mg protein using molar extinction coefficient of 6.22 × 103 M−1 cm−1.
Glutathione Peroxidase Assay
Glutathione peroxidase (GPx) activity was estimated according to the procedure described by Mohandas et al. [25]. The reaction mixture consisted of phosphate buffer (0.05 M, pH 7.0), EDTA (1 mM), sodium azide (1 mM), glutathione reductase (1 EU/ml), glutathione (1 mM), NADPH (0.2 mM), hydrogen peroxide (0.25 mM) and 0.1 ml of PMS in the final volume of 2 ml. The disappearance of NADPH at 340 nm was recorded at room temperature. The activity was calculated as nmol NADPH oxidized/min/mg/protein by using molar extinction coefficient 6.22 × 10−3 M−1 cm−1.
Glutathione-S-Transferase Assay
Glutathione-S-transferase (GST) activity was measured by the method of Habig et al. [26]. The reaction mixture consisted of phosphate buffer (0.1 M, pH 6.5), reduced glutathione (1 mM), CDNB (1 mM) and PMS in a total volume of 1.0 ml. The change in absorbance was recorded at 340 nm and enzyme activity was calculated as nmol CDNB conjugate formed/min/mg protein using a molar extinction coefficient of 9.6 × 103 M−1 cm−1.
Catalase Assay
Catalase (CAT) activity was assayed by the method of Claiborne [27]. Briefly, the assay mixture consisted of phosphate buffer (0.05 M, pH 7.0), hydrogen peroxide (0.019 M) and 0.05 ml PMS in total volume of 1.5 ml. The change in absorbance was recorded at 240 nm. Catalase activity was calculated as nmol of H2O2 consumed/min/mg/protein using molar extinction coefficient of 43.6 × 103 M−1 cm−1.
Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity was measured as described previously by Stevens et al. [28] by monitoring the autooxidation of (−)-epinephrine at pH 10.4 for 3 min at 480 nm. The reaction mixture contained glycine buffer (50 mM, pH, 10.4) and 0.2 ml of PMS. The reaction was initiated by the addition of (−)-epinephrine. The enzyme activity was calculated in terms of nmol (−)-epinephrine protected from oxidation/min/mg protein using molar extinction coefficient of 4.02 × 103 M−1 cm−1.
Na+ K+-ATPase Activity
Na+ K+-ATPase activity was measured by the method of Sovoboda and Massinger [29] with slight modification. The Na+ K+-ATPase activity was determined in two reaction mixtures, A and B. The reaction mixture A consisted of 0.2 M KCl, 1.0 M NaCl, 0.1 M MgCl2, 0.2 M Tris–HCl buffer (pH 7.4); 0.1 ml of homogenate in a total volume of 2.0 ml. The reaction mixture B consisted of 0.1 M MgCl2 10 mM Ouabain, 1.0 M NaCl, 0.2 M Tris–HCl buffer (pH 7.4); 0.1 ml of homogenate in a total volume of 2.0 ml. The reaction was started by adding 0.12 ml of 25.0 mM ATP at 37°C and terminated after 15 min by adding of 1.0 ml chilled 10% TCA. The mixture was centrifuged and supernatant (0.5 ml) was used for the estimation of inorganic phosphorous.
Poly ADP-Ribosyl Polymerase Assay
Poly (ADP-ribosyl) polymerase (PARP) activity was estimated by the method of Masmoudi et al. [30] using 0.1 mM [3H] NAD+ as substrate in reaction mixture containing 10 mM Tris/HCl (pH 8.0), 0.4 mM dithiothritol, 4.0 mM MgCl2 and 25 μl of PMS in a total volume of 125 μl. The reaction was carried out at 37°C for 10 min and terminated by the addition of 10% trichloroacetic acid containing 0.02 M sodium pyrophosphate. After 30 min at 4°C, the precipitate was collected on Whatman GF/B glass fiber paper, dried for 15 min at 100°C. Thereafter 10 ml of scintillation fluid was added to each vial and radioactivity was determined on WALLAC-1410 Liquid Scintillation Counter.
Caspase-3 Activity
The caspase-3 assay was done by colorimetric kit as given in manual (CasPASE TM colorimetric assay kit supplied with Ac-DEVD-AFC substrate).
Histopathological Studies
The brains of each group animals were perfused with a mixture of formaldehyde (40%), glacial acetic acid and methanol (1:1:8, v/v) as described by Nakayama et al. [31]. The tissues were cut into 3 mm thickness and its blocks were embedded in paraffin. Sections of 5 micrometers thick were cut in the coronal plane and stained with haematoxylin and eosin.
Immunohistochemistry
After 72 h of lesioning, the animals were anesthetized with chloral hydrate (400 mg/kg, ip) and perfused transcardially through ascending aorta, with saline followed a mixture of formaldehyde (40%), glacial acetic acid and methanol (1:1:8, v/v) as described by Nakayama et al. [31] as modified by us [3] in cold phosphate buffer saline (PBS; 0.1 M, pH 7.2). The brains were removed quickly, post fixed in the formaldehyde (40%), glacial acetic acid methanol solution for 48 h, then transferred to 30% sucrose in 0.1 M PB for at least 16 h until they sank for cryoprotection. The tissues were then kept in final sucrose solution till sectioning. The fixed tissues were embedded in OCT compound (polyvinyl glycol, polyvinyl alcohol, and water) and frozen at −20°C. Coronal sections of 10 μm thicknesses were cut on a cryostat and collected on gelatin-coated slides and immersed in wash buffer (sodium phosphate 100 mM, sodium chloride 0.5 M, Triton X-100, sodium azide) pH 7.4 for 20 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide and 10% methanol in PBS and incubated for 30 min at room temperature. Thereafter, the slides were washed with PBS and the sections were overlaid with anti p53 antibody of dilution 1:100 incubated overnight in a humid chamber at 4°C. The slides were washed again to remove the unbound antibodies and incubated with 20.0 μl solution of biotinylated anti-mouse IgG of dilution 1:5,000 for 2 h at 4°C in the humid chamber. Thereafter, the slides were exposed to streptavidin peroxidase and the labeled sites were visualized with a solution of diaminobenzidine and hydrogen peroxide. Finally the sections were dehydrated and covered with coverslip, viewed under a microscope, and photomicrographs were taken. The positively stained cells p33 in the ischemic core lesions of the cerebral cortices were counted in five randomized areas per rat brain. The results were expressed as the number of stained cells per mm2.
Statistics
Results are expressed as mean ± SEM. of eight animals. Differences between the means of experimental and control groups animals were analyzed by one-way ANOVA followed by Tukey–Kramer post-hoc test for multiple comparisons. The P-values less than 0.05 were considered statistically significant.
Results
Effect of Quercetin on Rota Rod Test
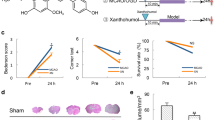
A significant decrease in muscular coordination skill was observed in MCAO group as compared to the sham group animals (Fig. 1). Animals treated with Quercetin in Q+MCAO group afforded a significant protection in muscular coordination skill, as compared to MCAO group animals; however, no significant effect was observed in Quercetin treated sham group (Q+S) as compared to sham.
Effect of middle cerebral artery occlusion on motor coordination skill of sham, MCAO and Q+MCAO rats. The time each rat remained on the rotating rod was recorded for three trials of each rat, at a 5 min interval and a maximum trial length of 180 s per trial. Values are expressed as mean ± S.E.M. of 8 animals. *P < 0.01 MCAO versus sham, #P < 0.01 Q+MCAO versus MCAO group
Infarction Volume
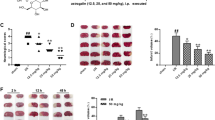
TTC acts as a proton acceptor for many pyridine nucleotide-linked dehydrogenases along with cytochrome form an integral part of the inner mitochondrial membrane and make up the electron transport chain [32, 33]. The tetrazolium salt is reduced by the enzymes into a red lipid soluble formazan. Viable tissues stain deep red while the infarct remains unstained. From the Fig. 2a it is evident that TTC staining of brain sections obtained from MCAO rats showed reproducible and readily detectable lesions in the infarct areas. The lesions were present in hippocampus, lateral striatum and the overlying cortex. Q reduced significantly the infarct volume in Q+MCAO group as compared to MCAO group as shown in the Fig. 2b.
Effect of Q on brain infarct size by TTC stain after MCAO for 2 h occlusion and reperfusion of 72 h. Representative photographs of brain sections stained with 0.1% TTC (a) and measurement of infarct volumes (b) of various groups are presented. MCAO group produced a significant infarcted neuronal damage as compared to S. Whereas MCAO+Q showed a significant (*P < 0.05) reduction in infarction volume as compared to MCAO
Q Treatment Decreased TBARS Content in Hippocampus and Frontal Cortex
TBARS are formed by lipid peroxidation due to production of free radicals in hippocampus and frontal cortex of MCAO rats. Figure 3 shows the level of TBARS in S, MCAO and Q treated rats. The level was significantly (P < 0.05) elevated in MCAO rats as compared to S. Treatment of MCAO rats with Q has depleted its level significantly (P < 0.01) while its level was not altered in Q+S group as compared to S.
Q Treatment Increased GSH Content in Hippocampus and Frontal Cortex
Protective effect of quercetin on GSH level in hippocampus and frontal cortex was observed. The level of GSH was depleted significantly (P < 0.05) in MCAO group as compared to S group. Q treatment increased its level significantly (P < 0.05) in Q+MCAO group as compared to MCAO group. Q alone treated group exhibited no significant changes in GSH level as compared to S group (Fig. 4).
Q Treatment Attenuated the Activities of Antioxidant Enzymes in Hippocampus and Frontal Cortex
Antioxidant defense system in the organisms is involved in the prevention of cellular damage by pro-oxidants. In ischemic conditions, free radicals are generated which accumulate in brain and impairs antioxidant defense system resulting in neurodegeneration. Here, the results of antioxidant enzyme assays in sham (S), ischemic (MCAO) rats & Q treated sham (Q+S) and ischemic (Q+MCAO) rats are summarized in Table 1. GPx, GR, GST, SOD and CAT of hippocampus and frontal cortical regions were selected for the present study. The activities of these antioxidant enzymes were decreased significantly in MCAO group as compared to S group and their activities were restored significantly by Q in Q+MCAO. No significant change in the activity of these enzymes was observed in Q+S group as compared to S group.
Protection of Na+ K+-ATPase Activity by Q
ATP dependent Na+ K+-ATPase regulates the transmembrane homeostasis of Na+ and K+ to maintain the optimum cell volume. In brain hypoxic condition, the ATP metabolism is greatly reduced resulting in depletion of ATP and ultimately lowering the Na+ K+-ATPase activity. The rate of Pi liberation is the measurement of ATPase activity which is lowered at the time of arterial occlusion. In the rat model, the activity of Na+ K+-ATPase was significantly decreased (P < 0.001) in MCAO group as compared to S group as shown in the Fig. 5. If these MCAO rats are treated with Q, the activity is restored significantly (P < 0.001) in both hippocampus and frontal cortex regions.
Protection of PARP Activity by Q
Recovery of damaged DNA is catalyzed by PARP at the cost of ATP. The over activation of this enzyme may lead to depletion in ATP and finally cell death. The inhibition of PARP is one of the key factors of neuroprotection in cerebral ischemia as the activity of PARP is increased in brain hypoxic condition. In hippocampus and frontal cortex the [3H] NAD+ count was double in MCAO group in comparison to S group which is shown in the Fig. 6 in the form of PARP activity. The activity of PARP was inhibited significantly (P < 0.05) in MCAO group treated with Q as compared to MCAO group, which implies that Q acts as an inhibitor of PARP.
Effect of Q on Caspase-3 Activity
In ischemic condition, the activation of PARP induced by DNA damage is also responsible for caspase-3 activation which leads to apoptosis. From Fig. 7, it is clear that in hippocampus and frontal cortical region of MCAO rats, the activity of caspase-3 was enhanced significantly (P < 0.001) as compared to sham group animals. Q treatment decreased its level significantly (P < 0.01) in Q+MCAO group as compared to MCAO group.
Morphological Changes
Figure 8 shows the histopathological changes after 2 h of occlusion and 72 h of reperfusion. Sections of the brain passing from frontal cortex of lesion, Q+MCAO and sham groups were examined. Sections of the MCAO group showed with neuronal staining and presence of numerous vacuolated spaces. Intact neurons were absent in that area. The corresponding area in the sections of Q+MCAO group showed partial loss of neuronal staining with presence of intact neurons in between the vacuolated spaces. The section of the sham group showed normal neuronal staining with no pathological change.
Heamatoxylin & eosin staining in the brain sections of sham, MCAO and Q+MCAO. a of cortical area of brain from sham group (×40) animal showing uniform distribution of cells. No clustering of glial cells is seen. b of cortical area of brain from MCAO group (×40) animal showing a large cluster of glial cells near a blood vessel (BV). c of cortical area of brain from Q+MCAO (×40) group animal showing a small cluster of glial cells
Q Diminishes the Increased Expression of p53
Upon generation of ROS in ischemic condition the apoptosis pathway is activated abruptly where the activities of PARP and caspase-3 increased leading to elevation in the p53 expression. The immunohistochemical analyses of cortical region have shown a marked elevation of p53 expression in MCAO rats (Fig. 9b) as compared to S group rats (Fig. 9a). Administration of Q to the MCAO rats attenuated the expression of p53 (Fig. 9c).
High power photomicrograph (×40) sham, MCAO and Q+MCAO. b of cortical neuronal area of brain from MCAO group animal showing significant increase in P53 expression as compared to sham group (a) and this expression was found to be decreased in Q+MCAO group (c) as compared to MCAO group (b). Quantification of p53 positive cell in cortical tissue. Reduction of the number of p53 stained cells in the quercetin-treated group (Q+MCAO) compared with the vehicle-treated (MCAO) group (*P < 0.05)
Discussion
We demonstrate that treatment with Q has a far reaching protective influence against the effects of ischemic insults. In this context, we have evaluated middle cerebral artery occlusion (MCAO) rats model for the behavioral and biochemical tests which is widely used to study neuroprotective effect of drugs because it recapitulates the biochemical and pathological features of stroke in humans [3, 8, 18], such as oxidative stress, mitochondrial dysfunction and apoptosis.
The behavioral impact is closely linked to the degree of neuronal dysfunction [34]. Functional deficits are common neurological sequel in patients with brain injuries and animal models of cerebral ischemia [3, 8]. Behavioral parameters are useful measures of functional deficits following experimental focal cerebral ischemia and the degree of sensorimotor dysfunction as an important indicator of severity of the injury [3, 7]. Furthermore the free radicals are always known to play vital role in neurobehavioral deficit in experimental models through oxidative stress so the poor neurobehavioral outcome in MCAO group rats might be attributed to oxidative stress induced free radicals. These free radicals are associated with increase in PARP activity and p53 expression [59]. The motor function was found to be disturbed in rota rod task. In this study, we proved that the motor function was impaired after ischemic insults in rat and could be significantly ameliorated by supplementation with Q. Our findings correlate well with the earlier studies carried out by us and others where motor deficits have been attenuated by treatment with antioxidants [3, 7, 8].
Infarction volume in the brain is an important determinant in assessing the consequences of ischemic stroke which leads to severe neuronal damage in the different brain parts with subsequent neurological impairment. TTC methods have been used to detect the morphological features of infarct tissue after ischemic injury [33, 35]. In the present study, MCAO group showed a prominent infarct size along with significantly altered behavioral outputs. Quercetin treatment not only reduced the infarct size but also improved behavioral deficits in MCAO group treated with Q. Animal experiments have indicated that Q administered after cerebral ischemia are effective in reducing infarct volume and lead to improvements in neurological outcome [9, 10, 36].
The biochemical mechanism immersed in the thriving of ischemia-induced neuronal injury is well studied [37–39]. ROS are critical spoilage in neuronal injury [40, 41]. The over production of ROS can be detoxified by endogenous antioxidants, causing their cellular reservoir to be depleted [42]. Oxidative stress prospers lipid peroxidation and alters the antioxidant defense system in the brain under ischemic circumstances [43] which was protected prominently by the treatment with quercetin. In the brain cells, neurons are highly vulnerable to oxidative insults due to low levels of GSH, which provides protection to the cells from oxidative damage by reducing disulphide groups of proteins and other cellular molecules or by scavenging free radicals and active oxygen species. Additionally it is vital for the regulation of the redox state. Furthermore GSH plays a crucial and critical role in the regulation of expression of several anti-inflammatory genes. Thus, GSH inhibition in cerebral ischemia would increase the susceptibility of plasma membranes towards peroxide attacks. However, the main cause of GSH loss during oxidative stress in brain ischemia is the formation of protein glutathione mixed disulphide (PrSSG) and loss of thiol proteins [44]. The loss of GSH and formation of PrSSG in the brain results the various membrane dysfunction, such as inhibition on Na+ K+ ATPase activity. The enzyme is relevant for cellular excitability and is very susceptible to free radical reaction and lipid peroxidation because it is wedged in cell membrane and requires phospholipids for the maintenance of the activity [45, 46]. There are several reports about the modulatory effect of Q on lipid peroxidation, glutathione and antioxidant enzymes following brain injury [9, 10, 12, 47]. In agreement with this finding, we also found Q significantly reduced the TBARS level along with increase in level of glutathione and antioxidant enzymes. SOD scavenges superoxide radicals by catalyzing the conversion of two of these radicals into hydrogen peroxide and molecular oxygen [48]. The hydrogen peroxide formed by SOD and by other processes is scavenged by GPx and CAT, a ubiquitous protein that catalyzes the dismutation of hydrogen peroxide into water and molecular oxygen. GPx uses hydrogen peroxide to oxidize GSH [49]. Thus, GPx is low molecular weight antioxidant and play a key role in detoxifying hydrogen peroxide [50]. GST catalyses the detoxification of oxidized metabolites of catecholamines (o-quinone) and may serve as an antioxidant system preventing degenerative cellular processes [51]. On the other hand, CAT detoxifies hydrogen peroxide and its depletion is known to be a factor that contributes to brain injury and cerebral edema. In the current work, GSH content was significantly reduced due to ischemic insult and also significant decline in activity of the endogenous antioxidant enzymes.
Besides defending against oxidant stress, another existing and encouraging finding that Q significantly attenuated histological changes i.e. it caused minimal glial cell infiltration, less neural damage with small vacuolated space along with presence of intact neuron in the neuronal tissue as compared to ischemic cortical neuronal loss. The section of sham group exhibited normal neuronal staining and did not show any significant histological changes. Our findings are consistent with other studies where quercetin improves the morphological changes after stroke [9, 10].
Apoptosis is a process of cell death which occurs in various kinds of pathological and physiological conditions. It has been known that apoptosis is one of the major neuronal cell death mechanisms in the experimental model of cerebral ischemia [3, 8, 36]. Various signaling pathways are involved in apoptotic cell death. MCAO induced oxidative damage to the cortical neurons involves activation of p53 expression in rat brain [3, 52]. Together these findings, our immunohistochemistry reports also suggest that neurons are clearly susceptible to p53-induced cell death in MCAO group while Q+MCAO group attenuate the expression of p53 transcription factor and inhibit caspase-3 activity.
PARP, activated by cleaved DNA strands, utilizes nicotinamide adenine dinucleotide (NAD+) as its substrate and synthesizes long branched and negatively charged polymers of ADP-ribose which are covalently bound to chromatin associated proteins and to PARP itself. Resynthesis of NAD+ leads to ATP depletion and necrotic cell death. There are also proposition that PARP induced NAD+ utilization may first release apoptosis inducing factor (AIF) from mitochondria and activate caspase-independent mechanisms of apoptotic cell death [53]. PARP and caspase-3 activation, in the ischemic stroke induces apoptotic processes in the cortical region via a mitochondria-mediated pathway. PARP is also known for its interaction with several transcription factors engrossed in the regulation of apoptosis, among them is p53 [54, 55, 59]. Both factors play a critical role in transcription of many proteins, regulation of DNA repair and in immune or inflammatory responses. PARP inhibited by Q protected cells against death provoked by brain ischemic/reperfusion injury. So, quercetin act as PARP inhibitor, besides their specific inhibitory action on the enzyme, may possess some anti oxidative properties, affecting their pharmacological specificity. p53 might primarily serve a protective role by activating DNA repair mechanisms after mild to moderate insults but could become detrimental in response to severe ischemic insults [56]. A fraction of induced p53 translocates to the mitochondria at the onset of p53-dependent apoptosis in cerebral ischemia [3]. Bypassing the nucleus by targeting p53 to mitochondria is yet profuse to launch apoptosis [55]. This evidence suggests the contribution of p53 to apoptosis by direct signaling at the mitochondria. They propounded that mitochondrial translocation of p53 triggers a rapid proapoptotic response that jump starts and amplifies the slower transcription-dependent response. Quercetin plays an innovative role for treating neurologic ailments that involve caspase-mediated cell dysfunction and cell death. It has been reported that blockade of p53 activation and PARP inhibition by pharmacological agents are associated with inhibition of apoptosis [57, 58]. Together our data and similar evidence in MCAO model of cerebral ischemia further support the neuroprotective potential of quercetin dihydrate.
Conclusion
Our present findings indicate that MCAO causes behavioral deficits and oxidative stress due to abrupt generation of fee radical causing weakening of antioxidant systems. Quercetin offered significant neuroprotection in MCAO rats, which may be attributed to inhibition of neurological deficit, lipid peroxidation, PARP activity, caspase-3 activity, p53 expression and increase in endogenous antioxidant defense enzymes. Thus, these findings suggest that the naturally occurring polyphenol quercetin may be an attractive and useful intervention for the treatment of stroke. Further investigation into the role and mechanisms of antioxidant action of quercetin is needed to determine whether it can be an effective remedy for stroke.
References
Chan PH (1994) Oxygen radicals in focal cerebral ischemia. Brain Pathol 4:59–65
Kinouchi H, Epstein CJ, Mizui T et al (1991) Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA 88:11158–11162
Khan MM, Ahmad A, Ishrat T et al (2009) Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res 1292:123–135
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Halliwell B, Gutteridge J, Cross CE (1992) Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med 119:598–620
Zafar KS, Siddiqui A, Sayeed I et al (2003) Dose-dependent protective effect of selenium in rat model of Parkinson’s disease: neurobehavioral and neurochemical evidences. J Neurochem 84:438–446
Saleem S, Ahmad M, Ahmad AS, Yousuf S et al (2006) Effect of Saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J Med Food 2:246–253
Yousuf S, Atif F, Ahmad M, Hoda N et al (2009) Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res 1250:242–253
Kovalenko TM, Osadchenko IO, Tsupykov OM, Pivneva TA, Shalamai AS, Moibenko OO, Skybo HH (2006) Neuroprotective effect of quercetin during experimental brain ischemia. Fiziol Zh 52:21–27
Rivera F, Costa G, Abin A, Urbanavicius J et al (2008) Reduction of ischemic brain damage and increase of glutathione by a liposomal preparation of quercetin in permanent focal ischemia in rats. Neurotox Res 13:105–114
Zhang ZJ, Cheang LC, Wang MW, Lee SM (2011) Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. Int J Mol Med 27:195–203
Kumar A, Sehgal N, Kumar P, Padi SS, Naidu PS (2008) Protective effect of quercetin against ICV colchicine-induced cognitive dysfunctions and oxidative damage in rats. Phytother Res 22:1563–1569
Das S, Mandal AK, Ghosh A, Panda S, Das N, Sarkar S (2008) Nanoparticulated quercetin in combating age related cerebral oxidative injury. Curr Aging Sci 1:169–174
Shutenko Z, Henry Y, Pinard E, Seylaz J, Potier P, Berthet F, Girard P, Sercombe R (1999) Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem Pharmacol 57:199–208
Schultke E, Kamencic H, Zhao M, Tian GF, Baker AJ, Griebel RW, Juurlink BH (2005) Neuroprotection following fluid percussion brain trauma: a pilot study using quercetin. J Neurotrauma 22:1475–1484
Lee JK, Kwak HJ, Piao MS, Jang JW, Kim SH, Kim HS (2010) Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochir (Wien)
Filipe P, Haigle J, Silva JN, Freitas J, Fernandes A, Mazière JC, Mazière C, Santus R, Morlière P (2004) Anti- and pro-oxidant effects of quercetin in copper-induced low density lipoprotein oxidation. Quercetin as an effective antioxidant against pro-oxidant effects of urate. Eur J Biochem 271:1991–1999
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Haleagrahara N, Radhakrishnan A, Lee N, Kumar P (2009) Flavonoid quercetin protects against swimming stress-induced changes in oxidative biomarkers in the hypothalamus of rats. Eur J Pharmacol 621:46–52
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Utley HC, Bernhein F, Hochslein P (1967) Effects of sulfhydryl reagent on peroxidation in microsomes. Arch Biochem Biophys 260:521–531
Islam F, Zia S, Sayeed I, Zafar KS, Ahmad AS (2002) Selenium induced alteration on lipids, lipid peroxidation, and thiol group in circadian rhythm centers of rat. Biol Trace Elem Res 90:1–12
Jollow DJ, Mitchell JR, Zampaghone N, Gillete JR (1974) Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic intermediate. Pharmacology 11:161–169
Carlberg I, Mannerviek B (1975) Glutathione reductase levels in rat brain. J Biol Chem 250:5475–5480
Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller D (1984) Differential distribution of glutathione and glutathione related enzymes in rabbit kidneys: possible implication in analgesic neuropathy. Cancer Res 44:5086–5091
Habig WH, Pabst M, Jakoby WB (1986) Glutathione S-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Claiborne A (1985) Catalase activity. In: Green Wald RA (ed) CRC hand book of methods for oxygen radical research. CRC Press, Boca Raton, pp 283–284
Stevens MJ, Obrosova I, Cao X et al (2000) Effect of DL-alpha-lipidic acid on peripheral nerve conduction, blood flow, energy metabolism and oxidative stress in experimental diabetic neuropathy. Diabetes 49:1006–1015
Sovoboda P, Mossinger B (1981) Catecholamines and brain microsomal Na+ K+ -ATPase protection against lipoperoxidative damage. Biochem Pharmacol 30:527–532
Masmoudi A, Islam F, Mandal P (1998) ADP Ribosylation of high purified rat brain mitochondria. J Neurochem 51:188–193
Nakayama H, Ginsberg MD, Dietrich WD (1998) (S)-Emopamil, 539 a novel calcium channel blocker and serotonin S2 antagonist, 540 markedly reduces infarct size following middle cerebral artery 541 occlusion in rat. Neurology 38:1667–1673
Altman FP (1976) Tetrazolium salts and formazans. Prog Histochem Cytochem 9:1–56
Bederson JB, Pitts LH, Germano SM et al (1986) Evaluation of 2, 3, 5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17:1304–1308
Schwarting RK, Steiner H, Huston JP (1991) Asymmetries in thigmotactic canning: evidence for a role of dopaminergic mechanisms. Psychopharmacology 103:19–27
Liszczak TM, Hedley-Whyte ET, Adams JF et al (1984) Limitation of tetrazolium salts in delineating infarcted brain. Acta Neuropathol (Berl) 65:150–157
Lao CJ, Lin JG, Kuo JS et al (2005) Microglia, apoptosis and interleukin-1beta expression in the effect of sophora japonica l. on cerebral infarct induced by ischemia-reperfusion in rats. Am J Chin Med 33:425–438
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–69
Evans PH (1993) Free radicals in brain metabolism and pathology. Br Med Bull 49:577–587
Petito C, Kraig RP, Pulsinelli WA (1987) Light and electron microscopic evaluation of hydrogen ion-induced brain necrosis. J Cereb Blood Flow Metab 5:625–632
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21:2–14
Flamm ES, Demopoulos HB, Seligman ML et al (1978) Free radicals in cerebral ischemia. Stroke 9:445–447
Candelario-Jalil E, Mhadu NH, Al-Dalain SM et al (2001) Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci Res 41:233–241
Thiyagrajan M, Sharma S (2004) Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia. Life Sci 74:969–985
Reed DJ (1990) Glutathione: toxicological implications Annu. Rev Pharmacol Toxicol 30:603–631
Furui T, Tanaka I, Iwata K (1990) Alterations in Na+-K+-ATPase activity and beta-endorphin content in acute ischemic brain with and without naloxone treatment. J Neurosurg 72:458–462
Ildan F, Polat S (1996) The effects of the pretreatment of intravenous high dose ethylprednisolone on Na(+)-K(+)/Mg(+2) ATPase and lipid peroxidation and early ultrastructural findings following middle cerebral artery occlusion in the rat. Acta Neurochir (Wien) 138:338–345
Coldiron AD, Sanders RA (2002) Watkins JB 3rd. Effects of combined quercetin and coenzyme Q (10) treatment on oxidative stress in normal and diabetic rats. J Biochem Mol Toxicol 4:197–202
Freeman A, Crapo JD (1982) Biology of disease: free radicals and tissue injury. Lab Investig 47:412–426
Beckman JS, Beckman TW, Chen J et al (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624
Imam S, Ali SF (2000) Selenium, an antioxidant, attenuates methamphetamine-induced dopaminergic toxicity and peroxynitrite generation. Brain Res 855:186–191
Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B (1997) Glutathione transferases catalyse the detoxication of oxidized metabolite (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J 324:5–8
Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40:e331–e339
Yu SW, Wang H, Poitras MF et al (2002) Mediation of poly (ADP-ribose) polymerase-1-dependent cell death by apoptosisinducing factor. Science 297:259–263
Oliver FJ, Ménissier-de Murcia J, Nacci C et al (1999) Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J 18:4446–4454
Hassa PO, Hottiger MO (1999) A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol Chem 380:953–959
Tomasevic G, Kamme F, Stubbero DP, Wieloch M, Wieloch T (1999) The tumor suppressor p53 and its response gene p21WAF1/Cip1 are not markers of neuronal death following transient global cerebral ischemia. Neuroscience 90:781–792
Culmsee C, Mattson MP (2005) p53 in neuronal apoptosis. Biochem Biophys Res Commun 331:761–777
Graziani G, Szabo C (2005) Clinical perspectives of PARP inhibitors. Pharmacol Res 52:109–118
Zhu W, Soonpaa MH, Chen H, Shen W, Payne RM et al (2009) Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation 119:99–106
Acknowledgments
Authors are thankful to the Department of Ayurveda, Yoga and Naturalpathy, Unani, Siddha and Homeopathy (AYUSH), Ministry of Health and Family Welfare, Government of India, New Delhi for financial assistance. The authors wish to thank Mr. Dharamvir Singh for his assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, A., Khan, M.M., Hoda, M.N. et al. Quercetin Protects Against Oxidative Stress Associated Damages in a Rat Model of Transient Focal Cerebral Ischemia and Reperfusion. Neurochem Res 36, 1360–1371 (2011). https://doi.org/10.1007/s11064-011-0458-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0458-6