Abstract
Ischemia/reperfusion (I/R) caused by ischemic stroke treatments leads to brain injury and its pathological mechanism is related to autophagy. The underlying mechanism of kaempferol on cerebral I/R injury needs to be explored. To establish I/R injury, we used a middle cerebral artery occlusion-reperfusion (MCAO) model in rats. MCAO rats were treated with the same amount of saline (I/R group); Treatment group rats were treated orally with kaempferol (50, 100, 200 mg/kg) for 7 days before surgery. After reperfusion for 24 h, the scores of neurological deficits and infarct volume in each group were evaluated. LC3, Beclin-1 p62, AMPK and mTOR protein expression levels were examined by TTC staining, immunofluorescence staining, qRT-PCR and western blotting assay. H&E and TTC staining showed that compared with model group, the infarction size of rats in kaempferol group was markedly reduced. Meanwhile, the results showed that kaempferol had a dose-dependent nerve function repairability. Nissl and TUNEL staining showed that kaempferol could reduce neuronal apoptosis and ameliorate neuronal impairment after I/R. Western blotting and qRT-PCR results showed that kaempferol could protect the brain from ischemia reperfusion by activating autophagy. In addition, add AMPK inhibitor, western blotting and immumohistochemical staining showed that kaempferol mediated AMPK/mTOR signal pathway in MCAO rats. Kaempferol could mediate the AMPK signal pathway to regulate autophagy and inhibit apoptosis to protect brain against I/R injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to a WHO report [1], ischemic stroke is the leading cause of death and disability worldwide. Due to the high rate of morbidity and mortality of the disease, strategies to promote recovery following the ischemic stroke are extremely urgent. To some extent, timely thrombolytic therapy could attenuate the local brain injury caused by ischemia. However, blood reperfusion, which follows cerebral ischemia, could accelerate and lead to more serious brain tissue damage, accompanied by oxidative stress [2], mitochondrial dysfunction [3], neuroinflammation, apoptosis [4], autophagy [5] and other pathological reactions, which is well known as ischemia–reperfusion injury (I/R). Wu et al. believed that protecting the morphological and functional integrity of mitochondria should be the key mechanism for its neuroprotection [6]. Therefore, it has become the focus of research to find neuroprotective drugs and methods against the pathological reaction of cerebral ischemia in recent decades.
Autophagy, as a basic biological process of the degradation and regeneration of damaged organelles and intracellular molecules, is involved in a variety of pathophysiological processes [7]. The mechanism of autophagy mainly depends on the members of the autophagy-related (ATG) protein family. It is well known that autophagy is associated with the progression of different kinds of disease, such as cancer [8], diabetes, ischemic cardiovascular disease [9]. Recently, it was reported that autophagy played a vital role in the pathological processes during cerebral ischemia–reperfusion [10]. Numerous researches have shown that autophagy is a double-edged sword, which has both beneficial and adverse effects on neurons during cerebral ischemia [11]. Zeng et al. [12] found that paracetamol significantly promoted autophagy during ischemia, accompanied by the activation of AMPK/mTOR signaling pathway and alleviated ischemic damage. Whereas, Shen-mai San (Rb1, Rg1, schizandrin, and DT-13; 6:9:5:4) could mitigate I/R injury by mediating AMPK/mTOR signaling pathway to inhibit autophagy [13].
Kaempferol, a natural flavonol in many eatable and medical plants, has caught much attention for its various effects on anti-inflammatory, anti-oxidant and anti-apoptotic activities [14]. Suchal et al. [15] found kaempferol attenuated myocardial ischemia–reperfusion injury in diabetic rats by reducing AGE-RAGE/mitogen-activated protein kinase (MAPK) induced oxidative stress and inflammation. Previous studies have showed that kaempferol acted as an anti-inflammatory agent in LPS-induced neuroinflammation in vitro and in vivo. In addition, Li et al. [16] demonstrated that kaempferol alleviated neuroinflammation by mediating NF-κB pathway to improve neurological deficits in cerebral I/R rats. Moreover, Wu et al. [6] demonstrated that kaempferol enhanced the effect of autophagy during oxygen and glucose deprivation, conducing to protecting neuron survival from succinate-induced injury. Nevertheless, the regulation mechanism of neuroprotection-related autophagy in cerebral ischemic injury has not yet been revealed.
In this study, the neuroprotective effect and its possible mechanism of kaempferol related to autophagy were studied in the rat model of cerebral ischemia–reperfusion. In addition, we investigate whether kaemferol plays a brain-protective role by modulating AMPK/mTOR signaling pathway to activate autophagy.
Materials and Methods
Animals
A total of 50 healthy male Sprague–Dawley rats (specific pathogen-free[SPF]), weighing 220 ± 10 g were purchased from Beijing Vital river company. Under the condition of constant temperature (25 °C) and constant humidity (60%), the rats were placed in a controlled environment with a light/dark cycle for 12 h, and they could get water and food freely. All animal procedures were carried out in accordance with the guidelines of the Chinese Society for Laboratory Animals Science to minimize animal suffering.
Middle Cerebral Artery Occlusion (MCAO) Model
SD rats were free of food and water for 1 week before experiments. Rats were anesthetized with intraperitoneal injection of pentobarbital sodium (40 mg/kg) and subjected to middle cerebral artery occlusion (MCAO) as we described previously [17]. Two hours after cerebral ischemia, the nylon flament was gently withdrawn to restore blood flow, which is called reperfusion. The sham-operated controls underwent similar surgical procedures without occlusion of the middle cerebral artery.
Treatment and Animal Group
Rats were randomly divided into 5 groups: animals were treated with sham surgery and the same amount of saline (sham group); MCAO rats were treated with the same amount of saline (I/R group); Treatment group rats were treated orally with kaempferol (50, 100, 200 mg/kg) for 7 days before surgery.
Neurological Deficits
As described in the study by Longa [18], neurological deficits were measured after 24 h of reperfusion using the following scale: Normal, 0 = no motor deficits; mild, 1 = torso turning to the ipsilateral side when held by the tail and exhibiting forelimb weakness; moderate, 2 = circling to the contralateral side, but normal posture at rest; severe, 3 = the affected side is unable to bear weight at rest; critical, 4 = no spontaneous locomotor activity or barrel rolling.
TTC Staining
Rat brain infarct size was assessed by staining brain slices with 2% 2,3,5-triphenylterzolium chloride (TTC, sigma, USA) as described previously [19]. After staining, all the sections were fixed in 4% formaldehyde for 12 h. Then infarct volume was calculated by Image J software (NIH) and analyzed statistically.
H&E Staining
The rats were transcardially perfused with normal saline followed by 4% PFA (Sigma, US) at 24 h after the MCAO. Hippocampus were removed and post-fixed in 4% PFA for 24 h and embedded in paraffin. Four-micrometer-thick sections were cut in the microtome (KEDEE, Jinhua, ZJ, China) and stained with hematoxylin and eosin (H&E). The pyramidal neurons of the CA1 region were observed, as they are the most vulnerable to I/R injury.
Immunohistochemistry Assay
Hippocampus tissues were fixed with 4% paraformaldehyde (sigma, USA) fixed at 4 °C for 24 h. They were washed with PBS, 30%, 50% and 70% alcohol in turn. Then hippocampus tissues were dehydrated and then embedded in paraffin. Tissues Sections (4-μm) were cut by microtome (KEDEE, Jinhua, ZJ, China). The paraffin samples were removed from the sections with xylene and rehydrated with graded alcohol gradually. The slides were washed with 1% Triton-100 in PBS for 15 min and then blocked with 5% goat serum, the slides were incubated with primary antibodies (LC3, p-AMPK and p-mTOR) at 4 °C overnight. Next, the slides were washed twice with 1% Triton-100 in PBS and incubated with biotinylated horse anti-mouse IgG in the blocking buffer. The reaction was amplified for 30 min with avidin–biotin complex, using an ABC kit. The slides were treated with DAB, dehydrated through a graded series of alcohol and sealed with the neutral gum. At last, the stained images were viewed under a light microscope (Leica, Germany).
Nissl Staining
The procedure of tissue section preparation is consistent with that of immunohistochemical staining. After dewaxing, paraffin sections were immersed in the Nissl staining reagent for 5 min. Slides were dehydrated with gradient alcohol, washed with xylene and sealed with neutral gum (Solarbio, Beijing, China). Histological changes in the hippocampal CA1 subfield were observed by the microscope (Olympus, Tokyo, Japan) to assess the neuronal damage [20].
Terminal Deoxynucleotidyl-Transferase-Mediated dUTP Nick End Labeling (TUNEL) Staining
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) kit (Beyotime, Beijing, China) was used to detect the apoptosis index in each group. The 6-μm tissue sections were prepared as before. TUNEL staining was performed with a TUNEL kit according to the manufacturer’s protocol. Randomly, five fields were observed under a ×400 magnification light microscope and the images were collected.
Quantitative Real-Time PCR
Total RNA of hippocampus tissue was isolated using the RNeasy Mini Kit (Qiagen, Duesseldorf, Germany) according to the manufacturer’s instructions and cDNA was reversed using transcriptase. Quantitative real-time PCR was performed using Transtart Top Green qPCR SuperMix according to the experiment guidelines. The mRNA expressions were normalized to the β-actin, and then the relative mRNA expression levels were calculated using the 2−ΔΔCT method.
Western Blotting Assay
The proteins were isolated from hippocampus tissue using lysis buffer (Beyotime, Shanghai, China), and protein concentrations were examined using a Bicinchoninic Acid (BCA) protein assay kit (Beyotime, Shanghai, China). The proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% skimmed milk, the membranes were incubated with primary antibodies for p-AMPK, AMPK, p-mTOR, mTOR, LC3, Bax, Bcl-2, and cleaved-caspase-3 for 12 h at 4 °C. The membranes were incubated with secondary antibodies (1:20000, ZSGB-BIO, Beijing, China) at room temperature for 2 h. Then the enhanced chemiluminescent (ECL) reagent (Thermo Fisher, Waltham, MA, US) and Image J software were used to detect the expression levels.
Data Analysis
Data was showed as mean ± standard (SD) of three independent samples. Student’s unpaired t-test was used to evaluate the significant difference in two groups, and one-way ANOVA was involved in multiple comparisons. P value less than 0.05 is considered statistically significant.
Results
Kaempferol Reduced MCAO-Induced Brain Injury
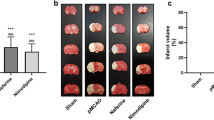
To investigate the effect of kaempferol on MCAO-induced brain injury, we first established the rat MCAO model. Rats were divided into the sham group, MCAO group, and MCAO group treated with kaempferol (50 mg/kg, 100 mg/kg, 200 mg/kg). Generally, higher neurological deficit scores were associated with more severe motor impairment. The neurobehavioral tests revealed no neurobehavioral dysfunction symptoms in the sham group; therefore, the rats had a neurological score of 0. As shown in Fig. 1A, compared with I/R group, the scores of treatment group were lower significantly. Notably, kaempferol could markedly improve neurological function at dose of 100 mg/kg. TTC staining results (Fig. 1B) showed that compared with I/R group, the infarct volume of rats in the treatment group was significantly reduced. Meanwhile, it showed that the reduction of infarct volume was most obvious in the medium dose of kaempferol group (100 mg/kg). Taken together, the results revealed that kaempferol promoted neurofunctional repair and attenuated the brain damage.
Kaempferol reduced MCAO-induced brain injury. Neurological deficits were assessed using Longa’s score, and brain infarct volume was assessed using TTC staining. A Neurological scores. B Representative images of TTC stained brain sections. Red areas indicated non-infarcted tissue, and white areas indicate infarcted tissue. The quantification of infarct volume was shown in (B). Data were represented as mean ± SD, *P < 0.05, **P < 0.01, vs sham; #P < 0.05, ##P < 0.01, ###P < 0.001, vs I/R; n = 10
Kaempferol Ameliorated Neuronal Impairment and Apoptosis of Hippocampus Tissues in I/R Model Rats
In order to further study the effects of kaempferol on the improvement of neuronal injury, H&E staining and Nissl staining were performed to assess the neuronal damage. As shown in Fig. 2A, H &E staining showed I/R caused remarkable cell death in the infracted area, which was mitigated by kaempferol. Consistent with the Nissl staining (Fig. 2B), kaempferol could repair neuronal impairment. Then, to evaluate the apoptosis of hippocampus tissue, TUNEL staining was used to detect the positive cells in hippocampus tissue. As shown in Fig. 2C, the results showed that compared with the sham group, the positive cells were significantly increased in the I/R group with the statistical difference. After treatment with kaempferol, the TUNEL positive cells were lower than that in the I/R group. In addition, western blotting assay was performed to detect the expression of Bax, Bcl-2, cleaved-caspase-3 to study the effect of autophagy and apoptosis after treatment with kaempferol in MCAO rats. The level of cleaved-caspase-3 and Bax were markedly increased in the I/R group compared with the sham group. Accordingly, compared with the I/R group, the expression of Bax and cleaved-caspase-3 in the treatment group was significantly down-regulated. (Fig. 2D). Whereas, the expression of Bcl-2 was significantly reduced in the I/R group compared with the sham group. After treatment with kaempferol, the expression of Bcl-2 was significantly upregulated. Based on the results, we suggest that kaempferol treatment may reduce neuronal apoptosis and ameliorate neuronal impairment after cerebral I/R.
The effect of kaempferol on neuronal injury and the apoptosis in MCAO rats. A H&E staining was used to detect MCAO induced brain injury. Representative images of different groups were shown in (A). B Nissl staining was performed to detect brain neuronal damage. Representative images and the quantification of infract neurons were shown in (B). TUNEL staining was used to detect the apoptosis of brain tissue in MCAO rats. Representative images and the quantification of TUNEL were shown in (C). Western blotting assay was used to measure the expression of Bax, Bcl-2 and cleaved-caspase-3 in the hippocampus. Representative bands showed the expression and the protein expression was normalized to β-actin. The quantification of the protein levels was shown in (D). Data were represented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, vs sham; #P < 0.05, ##P < 0.01, vs I/R; n = 10
Kaempferol Activated Autophagy During I/R in Rat Hippocampus Tissue
Next we tested whether kaempferol activates autophagy during I/R in rat hippocampus tissue. Firstly, immunohistochemistry staining was used to detect the expression of LC3. As shown in Fig. 3A, compared with the sham group, the expression of LC3 was increased significantly. After treatment with kaempferol, the expression of LC3 was increased with a significantly statistical difference. Then qRT-PCR assay was performed to detect the mRNA expression of Atg4, Atg5 and Atg7 in different groups. As shown in Fig. 3B, it showed that the expression of Atg4, Atg5 and Atg7 in the I/R group was significantly up-regulated compared with the sham group. Compared with I/R group, the expression of Atg4, Atg5 and Atg7 was increased significantly in the kaempferol group. To investigate the effect of kaempferol on autophagy activity after I/R, we assessed the expression of Beclin-1 and p62 and the ratio of LC3II/LC3I via western blotting. As shown in Fig. 3C, the expression of Beclin-1 proteins and the ratio of LC3II/LC3I in the hippocampus were significantly increased in the I/R group compared with the sham group, whereas the expression of p62 was reduced significantly. After treatment with kaempferol, the expression of Beclin-1 and the ratio of LC3II/LC3I were increased and the expression of p62 was reduced significantly compared with I/R group. The results suggested that kaempferol activated autophagy and played a protective role during the I/R.
The effect of kaempferol on autophagy in MCAO rats. A The expression of LC3 in the hippocampus was detected by immunohistochemical staining. Representative images and the quantification of the LC3 levels were shown in (A). QRT-PCR assay was performed to detect the mRNA expression of ATG4, ATG5 and ATG7 in the hippocampus (B). Western blotting assay was used to measure the protein expression of Beclin1, LC3I/II and p62 in the hippocampus. Representative bands showed the expression and the protein expression was normalized to β-actin. Quantification of the protein levels was shown in (C). Data were represented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, vs sham; #P < 0.05, ##P < 0.01, vs I/R; n = 10
The Protective Effect of Kaempferol on Cerebral Ischemia–Reperfusion in Rats Was Achieved by Activating Autophagy
In order to further explore the effect of kaempferol on cerebral ischemia–reperfusion in rats, we treated rats with kaempferol (100 mg/kg), 3-MA (a phosphatidylinositol-3 kinase inhibitor that blocks autophagy), or kaempferol+3-MA. LC3I/II is an important autophagy biomarker, and the ratio of LC3II/LC3I increases when autophagy occurs in injured tissues. As shown in Fig. 4A, B, both immunohistochemistry and western blot results showed significant changes in the ratio of LC3II/LC3I in different groups. The ratio of LC3II/LC3I in the kaempferol group is increased compared with I/R group, but the ratio of LC3II/LC3I is lower in I/R+3-MA group. Additionally, western blot results showed that the expression of Bax and cleaved-caspase-3 in the kaempferol group is increased compared with I/R group. Whereas the expression of Bax and cleaved-caspase-3 were lower in I/R+3-MA group. Similarly the expression of Bcl-2 showed the opposite trend. Besides, TUNEL staining results (Fig. 4C) confirmed that kaempferol could significantly reduce the apoptosis of cells in hippocampus tissue compared with the I/R group, and the kaempferol+3-MA group can increase the apoptotic cells which were statistically significant. We found that kaempferol could inhibit apoptosis during cerebral ischemia reperfusion by activating autophagy.
The effect of kaempferol on autophagy and apoptosis in MCAO rats. A The expression of LC3 in the hippocampus was detected by immunohistochemical staining. Representative images and the quantification of the LC3 levels were shown in (A). Western blotting assay was performed to detect the expression of Bax, Bcl-2, cleaved-caspase-3 and LC3I/II in the hippocampus. Representative bands showed the expression of Bax, Bcl-2, cleaved-caspase-3, LC3I/II and the expression was normalized to β-actin. Quantification of the protein levels is shown in (B). TUNEL staining assay was used to measure the apoptosis in hippocampus. Representative images and the quantification of the apoptosis levels were shown in (C). Data were represented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, vs sham; #P < 0.05, ##P < 0.01, ###P < 0.001, vs I/R; &P < 0.05, &&P < 0.01, &&&P < 0.001, vs I/R+Kae100, n = 10
Kaempferol Mediated AMPK/mTOR Signal Pathway in MCAO Rats
To investigate the effect of kaempferol on the AMPK/mTOR signal pathway in the hippocampus of MCAO rats, the expression of AMPK, p-AMPK, mTOR and p-mTOR were determined by western blotting assay (Fig. 5A). As a result, compared with the sham group, AMPK phosphorylation was increased significantly, whereas mTOR phosphorylation was reduced obviously in I/R group. After treatment with kaempferol, compared with the I/R group, AMPK phosphorylation was increased significantly, whereas mTOR phosphorylation was reduced with a significant difference. Furthermore, the levels of mTOR and AMPK phosphorylation were determined by immunohistochemical staining. As shown in Fig. 5B, C, the data showed a similar trend to western blotting results. The data revealed that kaempferol mediated AMPK/mTOR signal pathway in MCAO rats.
Kaempferol mediated AMPK/mTOR signal pathway in MCAO rats. A Western blotting assay was performed to detect the expression of AMPK, p-AMPK, mTOR and p-mTOR in the hippocampus. Representative bands showed the expression of p-AMPK and p-mTOR. Quantification of the protein levels was shown in (A). The expression of p-AMPK and p-mTOR was detected by immunohistochemical staining. Representative images and the quantification of the p-AMPK and p-mTOR levels were shown in (B and C). Data were represented as mean ± SD, *P < 0.05, **P < 0.01, vs sham; #P < 0.05, ##P < 0.01, vs I/R; n = 10
Antagonistic Effects of Dorsomorphin (AMPK Inhibitor) on the Protective Effect of Kaempferol in MCAO Rats
To investigate the mechanism by which kaempferol activates the AMPK signaling pathway, the expression of p-AMPK, AMPK, mTOR, p-mTOR and LC3I/II in the hippocampus was detected by western blotting assay. As shown in Fig. 6A, the results showed that compared with the sham group, the AMPK phosphorylation and the ratio of LC3II/LC3I in the I/R group was increased significantly, whereas the mTOR phosphorylation was reduced significantly. Compared with I/R group, the AMPK phosphorylation and the ratio of LC3II/LC3I was reduced significantly in the I/R+Dor group, accordingly the mTOR phosphorylation was increased with significant difference. In addition, the expression of LC3 was also detected using immumohistochemical staining. As shown in Fig. 6B, it showed the same trend to the western blot results. Western blotting assay was performed to measure the expression of Bax, Bcl-2 and cleaved-caspase-3 in hippocampus tissue. As shown in Fig. 6C, compared with the sham group, the expression of Bax and cleaved-caspase-3 was increased significantly in I/R group, whereas the expression of Bcl-2 was reduced with significant difference. The expression of Bax and cleaved-caspase-3 was upregulated significantly in the I/R+Dor group compared with I/R group, while the expression of Bcl-2 was downregulated. Taken together, it revealed that the effect on the protective effect of kaempferol in MCAO rats.
Dorsomorphin (AMPK inhibitor) can reverse the protective effect of kaempferol. Western blotting assay was performed to detect the expression of AMPK, p-AMPK, mTOR, p-mTOR and LC3I/II in the hippocampus. Representative bands showed the protein levels of AMPK, p-AMPK, mTOR, p-mTOR and LC3I/II. The expression was normalized to β-actin. Quantification of the protein levels was shown in (A). The expression of LC3 was detected by immunohistochemical staining. Representative images and the quantification of the LC3 levels were shown in (B). Western blotting assay was used to detect the expression of Bax, Bcl-2 and caspase-3 in the hippocampus. The expression was normalized to β-actin. Quantification of the protein levels was shown in (C). Data were represented as mean ± SD, *P < 0.05, **P < 0.01, vs sham; #P < 0.05, ##P < 0.01, vs I/R; &P < 0.05, &&P < 0.01, vs I/R+Kae100, n = 10
Discussion
Thrombolytic therapy for stroke is widely used in clinical treatment, but the associated reperfusion injury still greatly affects the clinical prognosis [21]. Therefore, it is crucial to seek alternative medicines or methods, which can inhibit disease progression, and which can protect the brain against ischemia reperfusion damage. In recent decades, traditional Chinese medicine has been widely used for the prevention or treatment of ischemic stroke. The present study examined the mechanism of kaempferol induced autophagy to protect the brain in vivo. Consistent with previous research [22], kaempferol could significantly attenuate brain injury and reduce the volume of cerebral infarction in rats. Compared with kae 100 group, kae 200 group significantly increased the infarct volume, possibly because of the toxicity of kaempferol. In order to promote the clinical application, the toxicological experiments of kaempferol and the effect of combination with of tPA and kaemperol still need further study.
Our results suggest that induction of autophagy may be involved in the protective mechanism of kaempferol against cerebral I/R injury. The pathological mechanism of ischemic stroke has been further studied, and some studies have proved that regulating autophagy can inhibit neuron apoptosis and reduce brain injury [23]. Autophagy, as one hotpot of biomedical research, is a process of cell self-degradation mediated by lysosomes. When the number of abnormal organelles increases or accumulates, autophagic vesicles will form in the cell and then combine with lysosomes to form autophagosomes [24]. The research by Shao et al. demonstrated that artesunate has a protective effect on ischemic cerebral infarction, and the protective effect may increase autophagy by inhibiting the activity of mTOR [25]. In their study, it was found that ischemic hypoxia stimulation promoted the expression of p-mTOR, while artesunate decreased its expression. Moreover, the protective effect of artesunate on ischemic brain infarction is reversed by 3-MA (autophagy inhibitor) and LEU (p-mTOR agonist leucine), which revealed the activation of autophagy plays a key role in the protection of MCAO induced brain injury. LncRNA SNHG12-induced autophagy activation alleviated cerebral I/R injury, which was partially reversed by an autophagy inhibitor 3-MA [26]. MiR-202-5p attenuates cerebral ischemia reperfusion injury in MCAO rats by targeting eIF4E-mediated induction of autophagy [27]. More and more studies have revealed that autophagy plays neuroprotective effects during cerebral I/R injury. Zhang et al. confirmed SIRT3 protected a rotenone-induced PD cell model through the regulation of autophagy, which, in part, is mediated by activation of the AMPK/mTOR pathway [28]. On the contrary, Wang et al. [29] demonstrated that autophagy activation during cerebral ischemia reperfusion injury is harmful. Their results showed that MCAO-induced neuronal cell death is mediated by the activation of autophagy, while silibinin exerts its neuroprotective effect by inhibiting autophagy. Furthermore, Wang et al. found that LncRNA H19 inhibited autophagy by regulating DUSp5-ERK1/2 axis and protected brain injury [30]. In brief, the role of autophagy in cell death/survival in cerebral I/R injury remains controversial. In the present study, autophagy-related genes ATG4, ATG5, ATG7, autophagy-related proteins Beclin1 and p62 in the MCAO group were significantly up-regulated, and autophagy was further activated after kaempferol treatment. In addition, compared with I/R+kaempferol group, the apoptotic level was significantly increased after the combination of autophagy inhibitor 3-MA (Fig. 4B, C). We demonstrated that kaempferol could activate autophagy, inhibit neuronal apoptosis, protect the brain from injury and improve neurological deficits during I/R injury.
Autophagy and apoptosis have been shown to be correlated in cerebral ischemia–reperfusion injury. Some studies have confirmed that p62-induced autophagy is activated, the apoptosis mechanism can be triggered during I/R injury [31]. Montero suggested that DHA plays a neuroprotective role through inhibiting apoptosis via the activation of autophagy [32]. Previous studies have indicated that ezetimibe attenuates neuronal apoptosis through AMPK dependent autophagy activation after MCAO in rats [33]. Consistent with previous studies, our results revealed the inhibitory effect of kaempferol against MCAO induced apoptosis was promoted by rapamycin, while rescued by 3-MA. The findings suggested the new mechanism of kaempferol against brain injury, which activates autophagy and inhibits neuron apoptosis.
Additionally, the signaling pathway of kaempferol activating autophagy in cerebral ischemia–reperfusion injury has been further explored. AMPK/mTOR has been considered as a pivotal signal pathway in the regulation of autophagy [34]. Autophagy is a pathophysiological response during ischemia/reperfusion injury, which mainly exists in myocardial ischemia/reperfusion injury and cerebral ischemia/reperfusion injury. Jia et al. found galectins induced autophagy by controlling master regulators of metabolism, mTOR and AMPK, in response to lysosomal damage [35]. Some research reported that TIGAR protected against neuronal injury partly through inhibiting autophagy by regulating the mTOR/S6KP70 signaling pathway [36]. Some researchers suggested that melatonin could protect against myocardial injury by activating AMPK mediated autophagy [37]. Ren et al. confirmed that the potential mechanism of Yangxinkang tablets in the treatment of myocardial injury is to inhibit autophagy through AMPK/mTOR signaling pathway [38]. Sun et al. [39] suggested regulation of autophagy via the AMPK/mTOR/P70S6K signaling pathway may be the protective mechanism against ischemic stroke. Kaempferol plays a protective role in palmitic acid-induced pancreatic β-cell death through modulation of autophagy via AMPK/mTOR signaling pathway [40]. Similarly, as a monomer of traditional Chinese medicine, kaempferol could increase the ratio of p-AMPK and p-mTOR in MCAO rats. In addition, after treatment with dorsomorphin (an inhibitor of AMPK), it reversed kaempferol-induced autophagy. Taken together, we found that kaempferol can activate the AMPK/mTOR signaling pathway to induce autophagy and protect the brain against ischemic-reperfusion injury.
In summary, we confirmed that kaempferol can minimize cerebral injury in MCAO rats, activate autophagy, and inhibit apoptosis. Our study demonstrated autophagy plays a vital role in cerebral ischemia–reperfusion injury, and kaempferol could regulate AMPK/mTOR signaling pathway to activate autophagy to protect the brain against ischemic injury. These results have partially revealed the molecular neuroprotective mechanisms of kaempferol. Our work not only provided novel insight into the neuroprotective effects of kaempferol but also suggested clarification of the mechanism of kaempferol may provide the theoretical basis for the clinical treatment of stroke.
Data Availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- MCAO:
-
Middle cerebral artery occlusion-reperfusion
- I/R:
-
Ischemia/reperfusion
- MAPK:
-
Mitogen activated protein kinase
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
- H&E:
-
Hematoxylin and eosin
- TUNEL:
-
Terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling
- PVDF:
-
Polyvinylidene difluoride
- BCA:
-
Bicinchoninic Acid
- Dor:
-
Dorsomorphin
- SDS-PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
References
Wang H et al (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053):1459–1544
Guo QQ et al (2020) ATM-CHK2-beclin 1 axis promotes autophagy to maintain ROS homeostasis under oxidative stress. EMBO J 39(10):e103111
Youn DH et al (2020) Extracellular mitochondrial dysfunction in cerebrospinal fluid of patients with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neurocrit Care 33(2):422–428
Lai Y et al (2020) Restoration of L-OPA1 alleviates acute ischemic stroke injury in rats via inhibiting neuronal apoptosis and preserving mitochondrial function. Redox Biol 34:101503
Chen C et al (2020) Electroacupuncture pretreatment prevents ischemic stroke and inhibits Wnt signaling-mediated autophagy through the regulation of GSK-3β phosphorylation. Brain Res Bull 158:90–98
Wu B et al (2017) Succinate-induced neuronal mitochondrial fission and hexokinase II malfunction in ischemic stroke: therapeutical effects of kaempferol. Biochim Biophys Acta Mol Basis Dis 1863(9):2307–2318
Mishra SK et al (2020) Emerging roles for human glycolipid transfer protein superfamily members in the regulation of autophagy, inflammation, and cell death. Prog Lipid Res 78:101031
Lin Z et al (2020) RNA m(6) a methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J 39(12):e103181
Wu D, Zhang K, Hu P (2019) The role of autophagy in acute myocardial infarction. Front Pharmacol 10:551
Wang M et al (2019) Homocysteine enhances neural stem cell autophagy in in vivo and in vitro model of ischemic stroke. Cell Death Dis 10(8):561
Mo Y, Sun YY, Liu KY (2020) Autophagy and inflammation in ischemic stroke. Neural Regen Res 15(8):1388–1396
Zeng C et al (2016) Crocin-elicited autophagy rescues myocardial ischemia/reperfusion injury via paradoxical mechanisms. Am J Chin Med 44(3):515–530
Guo Z et al (2014) A combination of four active compounds alleviates cerebral ischemia-reperfusion injury in correlation with inhibition of autophagy and modulation of AMPK/mTOR and JNK pathways. J Neurosci Res 92(10):1295–1306
Lagoa R et al (2009) Kaempferol protects against rat striatal degeneration induced by 3-nitropropionic acid. J Neurochem 111(2):473–487
Suchal K et al (2017) Molecular pathways involved in the amelioration of myocardial injury in diabetic rats by kaempferol. Int J Mol Sci 18(5):1001
Li WH et al (2019) Kaempferol attenuates neuroinflammation and blood brain barrier dysfunction to improve neurological deficits in cerebral ischemia/reperfusion rats. Brain Res 1722:146361
Zhang K, Zhang Q (2019) ALK5 signaling pathway mediates neurogenesis and functional recovery after cerebral ischemia/reperfusion in rats via Gadd45b. Cell Death Dis 10(5):360
Longa EZ et al (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91
Guang HM, Du GH (2006) Protections of pinocembrin on brain mitochondria contribute to cognitive improvement in chronic cerebral hypoperfused rats. Eur J Pharmacol 542(1–3):77–83
Gao M et al (2008) Acute neurovascular unit protective action of pinocembrin against permanent cerebral ischemia in rats. J Asian Nat Prod Res 10(5–6):551–558
Stamatovic SM et al (2020) A novel approach to treatment of thromboembolic stroke in mice: redirecting neutrophils toward a peripherally implanted CXCL1-soaked sponge. Exp Neurol 330:113336
Zannad F et al (2020) SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 396(10254):819–829
Aimo A et al (2021) Relative efficacy of Sacubitril-Valsartan, Vericiguat, and SGLT2 inhibitors in heart failure with reduced ejection fraction: a systematic review and network meta-analysis. Cardiovasc Drugs Ther 35(5):1067–1076
Sica V et al (2015) Organelle-specific initiation of autophagy. Mol Cell 59(4):522–539
Shao M et al (2018) Protectiveness of artesunate given prior ischemic cerebral infarction is mediated by increased autophagy. Front Neurol 9:634
Yao X et al (2019) LncRNA SNHG12 as a potent autophagy inducer exerts neuroprotective effects against cerebral ischemia/reperfusion injury. Biochem Biophys Res Commun 514(2):490–496
Li B et al (2020) MiR-202-5p attenuates neurological deficits and neuronal injury in MCAO model rats and OGD-induced injury in neuro-2a cells by targeting eIF4E-mediated induction of autophagy and inhibition of Akt/GSK-3β pathway. Mol Cell Probes 51:101497
Zhang M et al (2018) SIRT3 protects rotenone-induced injury in SH-SY5Y cells by promoting autophagy through the LKB1-AMPK-mTOR pathway. Aging Dis 9(2):273–286
Wang M et al (2016) Silibinin prevents autophagic cell death upon oxidative stress in cortical neurons and cerebral ischemia-reperfusion injury. Mol Neurobiol 53(2):932–943
Wang J et al (2017) Long non-coding RNA H19 induces cerebral ischemia reperfusion injury via activation of autophagy. Aging Dis 8(1):71–84
Zhang Y et al (2019) The role of astragaloside IV against cerebral ischemia/reperfusion injury: suppression of apoptosis via promotion of P62-LC3-autophagy. Molecules 24(9):1838
Montero ML et al (2020) Docosahexaenoic acid protection against palmitic acid-induced lipotoxicity in NGF-differentiated PC12 cells involves enhancement of autophagy and inhibition of apoptosis and necroptosis. J Neurochem 155(5):559–576
Dai J et al (2018) Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int Immunopharmacol 54:177–187
Kim J et al (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141
Jia J et al (2019) Galectins control MTOR and AMPK in response to lysosomal damage to induce autophagy. Autophagy 15(1):169–171
Zhang DM et al (2019) TIGAR alleviates ischemia/reperfusion-induced autophagy and ischemic brain injury. Free Radic Biol Med 137:13–23
Di S et al (2020) The protective effects of melatonin against LPS-induced septic myocardial injury: a potential role of AMPK-mediated autophagy. Front Endocrinol (Lausanne) 11:162
Ren PH et al (2020) Yangxinkang tablet protects against cardiac dysfunction and remodelling after myocardial infarction in rats through inhibition of AMPK/mTOR-mediated autophagy. Pharm Biol 58(1):321–327
Sun X et al (2020) Eugenol attenuates cerebral ischemia-reperfusion injury by enhancing autophagy via AMPK-mTOR-P70S6K pathway. Front Pharmacol 11:84
Varshney R, Gupta S, Roy P (2017) Cytoprotective effect of kaempferol against palmitic acid-induced pancreatic β-cell death through modulation of autophagy via AMPK/mTOR signaling pathway. Mol Cell Endocrinol 448:1–20
Acknowledgements
We are grateful to all participates for their contributions for the present study. The supporting funding projects of the manuscript are as follows: National Natural Science Foundation of China (Grants No. 81501140). National Natural Science Foundation of China (Grants No. 81502656)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, Y., Xia, F., Gao, R. et al. Kaempferol Mediated AMPK/mTOR Signal Pathway Has a Protective Effect on Cerebral Ischemic-Reperfusion Injury in Rats by Inducing Autophagy. Neurochem Res 47, 2187–2197 (2022). https://doi.org/10.1007/s11064-022-03604-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03604-1