Abstract
Our previous studies have shown that pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β) in red nucleus (RN) are involved in the development of neuropathic pain and play facilitated roles on the mechanical allodynia induced by peripheral nerve injury. The current study was designed to evaluate the expression and effect of IL-10, an anti-inflammatory cytokine, in the RN of rats with spared nerve injury (SNI). Immunohistochemical staining results demonstrated when 3 weeks after SNI, the expression level of IL-10 in the contralateral RN of SNI rats was apparently higher than those of sham-operated and normal rats. To further study the effect of IL-10 in the development of neuropathic pain, different doses of IL-10 (1.0, 0.5 and 0.1 μg/μl) were microinjected respectively into the RN contralateral to the nerve injury side of SNI rats. Results demonstrated that higher doses of IL-10 (1.0 and 0.5 μg/μl) significantly attenuated the mechanical allodynia of neuropathic rats, while 0.1 μg/μl of IL-10 did not show any analgesic effect. These results suggest that IL-10 of RN participates in the development of neuropathic pain and plays inhibitory roles on the mechanical allodynia induced by SNI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence has shown that peripheral nerve injury can stimulate the central nervous system (CNS) to express both pro-inflammatory and anti-inflammatory cytokines, which play crucial roles in the establishment and maintenance of neuropathic pain [1–3]. Pro-inflammatory cytokines, such as interleukin-1 beta (IL-1β), IL-6 and tumor necrosis factor-alpha (TNF-α), usually induce or facilitate neuropathic pain [1], while blockade of pro-inflammatory cytokines and/or administration of anti-inflammatory cytokines, such as IL-10, reduce neuropathic pain in animal models [4, 5].
IL-10 is one of the most important regulators in the immune system and secreted by a variety of immune cells including activated type 2 T helper cells (TH2), B cells and monocytes. Recent studies have shown that IL-10 is also synthesized in the CNS and implicated in the development of neuropathic pain [3, 6–8]. Peripheral nerve injury results in the increased expression of IL-10 in both injured and contralateral noninjured peripheral nerves and their DRG [3, 6, 7]. Some analgesic drugs, such as betamethasone, mirtazapine and adenosine 2A receptor agonist ATL313 can relieve the neuropathic pain through increasing the expression of IL-10, and the analgesic effect can be abolished by administration of neutralizing IL-10 antibodies [9–11]. Moreover, systemic administration or intrathecal (i.t.) injection of IL-10 could dose-dependently reduce the dynorphin-induced allodynia, quisqualic acid (QUIS)-induced spontaneous pain-like behaviors, osteoma-induced pain, Leishmania major-induced hyperalgesia and ultraviolet radiation-induced hyperalgesia [12–16]. In rodent experiments, Mahoney, Watkins and co-workers have demonstrated that intrathecal injection of PEGylated IL-10, polymer-based IL-10, viral vectors or non-viral vectors encoding IL-10 could prevent the development of pain or reverse established pain induced by nerve constriction or injection of pain-causing substances into the nerve sheath [17–23]. All of these studies suggest that IL-10 is involved in the development of pain and plays an analgesic effect.
The roles of IL-10 in the peripheral and spinal level of neuropathic pain models seem to be well defined, but in the supraspinal level they remain obscure. Previous studies have demonstrated that TNF-α and IL-1β are up-regulated in the contralateral red nucleus (RN) of spared nerve injury (SNI) rats, and microinjection of their corresponding antibodies could alleviate the mechanical allodynia induced by SNI [24–26], suggesting that TNF-α and IL-β in RN participate in the development of neuropathic pain and play facilitated roles on the mechanical allodynia induced by peripheral nerve injury. In this study, we detected the expression of anti-inflammatory cytokine IL-10 in the RN of SNI rats by immunohistochemistry and an increased IL-10 immunoreactivity was observed in the contralateral RN of SNI rats as compared with those of sham-operated and normal rats. To further explore the effects of IL-10 in RN, different doses of IL-10 were microinjected into the contralateral RN of SNI rats and the results showed that higher doses of IL-10 significantly attenuated the mechanical allodynia of neuropathic rats.
Materials and Methods
Animals
Male Sprague-Dawly rats weighing 200–230 g were used for the study, all of which were purchased from the Experimental Animal Center of Shaanxi Province, China. All animals were housed with ad libitum access to food and water and maintained on a 12/12 light/dark cycle. All experiments were approved by the Institutional Animal Care Committee of Xi’an Jiaotong University in accordance with the ethical guidelines of the International Association for the Study of Pain [27].
Spared Nerve Injury
The neuropathic pain was induced by tightly ligating the tibial and common peroneal nerve with leaving the sural nerve intact as reported previously [28]. Briefly, after rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally), the sciatic nerve and its three terminal branches were exposed by directly incising through the biceps femoris muscle of the right hind limb. The tibial and common peroneal branches were tightly ligated by 5–0 silk sutures and sectioned distal to the ligation, removing 2–4 mm of the distal nerve stump. Great care was taken to avoid contacting or stretching the intact sural nerve. Muscle and skin were closed in two layers. In sham-operated group, rats were treated in the same way but the nerve was neither ligated nor sectioned. After surgery, rats were allowed to recover from anaesthesia in an observation chamber with a warming light. In normal control group, rats were free of any treatment. Rats of SNI group were used for further experiments only when the withdrawal threshold of right hind paw was less than 4.0 g in response to von Frey filaments stimulation.
Immunohistochemistry
Three weeks after surgery, 24 rats including SNI group (n = 8), sham-operated group (n = 8) and normal control group (n = 8) were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg). Each rat was fixed by perfusion through the aortic arch with 250 ml ice-cold heparinized normal saline, followed by 420 ml Bouin’s fluid (300 ml saturate nitroxanthic acid solution, 100 ml 40 % formaldehyde and 20 ml glacial acetic acid). The brain region containing red nucleus was harvested, postfixed in Bouin’s fluid for 2 days and then dehydrated by 30 % sucrose.
All brain tissues were embedded in OCT and sectioned coronally into 20 μm thick sections using a LEICACM 1850 ultramicrotome. One slice from 100 μm was picked and three slides were used for analyzing one animal. After routine treatments of acetone and 3 % hydrogen peroxide, slides were blocked with 5 % goat serum in PBS for 1 h and then incubated overnight with rabbit anti-rat IL-10 polyclonal antibody (working dilution 1:100, Boster Bio-Engineering Limited Co., Wuhan, China) at 4 °C. Horseradish peroxidase (HRP) labeled goat anti-rabbit IgG for 30 min and DAB was used for staining. As a control, the primary antibody or secondary antibody was omitted and isotypic antibody (normal rabbit IgG, Boster Bio-Engineering Limited Co., Wuhan, China) was used to confirm the immunospecificity of the IL-10 reaction.
Histological sections were viewed with Olympus DP70 microscope and the images were captured with Olympus BX-51 camera. The area ratio of IL-10 positive cells (area of positive signal/area of interesting) and the integrated optical density (IOD) were analyzed using Image pro-plus (IPP) and Motic Med (version 6.0) software. Each slide was analyzed with the same size of arbitrary areas (600 μm × 800 μm) and total 24 blinded slides (3 arbitrary slides/rat) for each experimental group were calculated. All data were analyzed by an assistant who was unaware of the treatment groups.
Catheterization and Drug Administration
Two weeks after SNI, total 32 rats with SNI were anesthetized intraperitoneally with sodium pentobarbital (50 mg/kg) for surgery. The rat skull was exposed and a stainless steel guide cannula (0.8 mm in diameter) was stereotaxically implanted at a position 2.0 mm dorsal to the left RN, where an increased IL-10 immunoreactivity was observed after SNI according to the results of immunohistochemistry, at the following coordinates: 5.2–6.7 mm to bregma, 0.6–1.4 mm lateral, 4.4–5.4 mm below cortical surface [29]. The guide cannula was fixed on the skull with four microscrews and dental cement. A stainless steel plug was inserted into the guide cannula and kept in place until the intracerebral injection. After surgery, each rat was injected intraperitoneally with penicillin 0.2 million units per day for consecutive 4 days. During this period, rats were housed individually in cage with free access to water and food.
One week after catheterization, a 1.0 μl microsyringe (0.4 mm in diameter) with its tip extending 2.0 mm beyond the end of the guide cannula was inserted into RN through the guide cannula after the plug was removed. Drugs dissolved in normal saline (0.5 μl) were then slowly infused into RN within 60 s and the microsyringe was left in place for an additional 30 s to minimize the drug solution flowing back into the injector track. Drugs used in this study included different doses of recombinant rat IL-10 (1.0, 0.5 and 0.1 μg/μl; PeproTech Inc., USA). The same volume of 0.9 % normal saline was injected in control rats. At the end of experiments, pontamine sky blue was injected into RN and the microinjection sites were histologically verified.
Behavioral Measures
One week after catheterization (i.e., 3 weeks after SNI), the mechanical withdrawal threshold of SNI rats were measured blindly by an experimenter before and 10, 20, 30, 40, 50, 60, 70 min after drug administration by using the up-down method [30]. The rat was placed in transparent plastic box (280 × 250 × 210 mm) with a metal wire mesh floor that allowed full access to the paws from underneath. Ten von Frey filaments (Stoelting Company, Wood Dale, IL, USA) ranged from 0.4 to 15.0 g were used to measure the mechanical allodynia. Starting with filament 4.31 (0.2 g) which is one of the middle of the series of filaments, von Frey filaments were applied from underneath perpendicularly to the right hind paw with sufficient force to cause slight bending and held for 6–8 s. The pattern of positive and negative responses was converted into a 50 % withdrawal threshold using the formula given by Dixon [31]: 50 % withdrawal threshold = 10(X+kd)/104, where X is the value of the final von Frey hair used (in log units), k is the tabular value for the pattern of positive/negative responses modified from Dixon [30], and d is the mean difference between stimuli in log units (0.17). In the cases where continuous positive or negative responses were observed all the way out to the end of the stimulus spectrum, values of 0.25 or 15.0 g were assigned, respectively.
Data Analysis
Statistical analyses were performed by using SigmaStat 2.03 and all data were presented as mean ± standard deviation. Linear regression was used to assess the correlation between the effects and doses of IL-10. Differences in the area ratio of IL-10 positive cells and IOD were tested statistically by one-way analysis of variance (one-way ANOVA). Differences in drug effect among groups were tested statistically by two-way repeated measures of analysis of variance (two-way RM ANOVA) with a multiple comparison for analysis of the differences in entire observation time or at each time point among different groups. P < 0.05 was considered to be statistically significant.
Results
General
Three weeks after SNI, the mechanical withdrawal threshold of hind paw ipsilateral to the nerve injury (1.35 ± 0.64 g, n = 8) was significantly decreased (P < 0.001) as compared with those from the sham-operated group (12.21 ± 2.47 g, n = 8) and normal control group (11.95 ± 4.1 g, n = 8), while no difference of withdrawal threshold was observed in the contralateral hind paw. These results suggest that the neuropathic pain model with monolateral mechanical allodynia has been created successfully and is consistent with previous report [32].
Increased Expression of IL-10 Protein in Red Nucleus
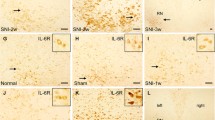
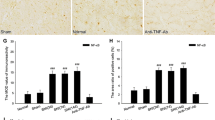
Three weeks after SNI, a stronger immunoreactivity of IL-10 was observed in the contralateral red nucleus of SNI rats (Fig. 1a, d, g) and a weaker immunoreactivity of IL-10 was observed in the ipsilateral RN of SNI rats and sham-operated rats (Fig. 1b, e, h). In normal rats, no obvious immunoreactivity of IL-10 was found in the both sides of RN (Fig. 1c, f, i). The control experiments, in which the primary antibody or secondary antibody was omitted and normal rabbit IgG was used, did not find any positive staining, suggesting that the immunoreactivity of IL-10 in red nucleus was specific (Fig. 1j, k, l). The area ratio of IL-10 positive cells and IOD values of sham and SNI groups above those of control group were analyzed. In SNI group, the area ratio of IL-10 positive cells and the IOD values in the contralateral RN were 0.08 ± 0.02 and 10.98 ± 3.46, respectively. While in the sham-operated group, the area ratio of IL-10 positive cells and the IOD values in the contralateral RN were 0.08 ± 0.02 and 2.65 ± 1.23, respectively (Fig. 2). After statistic analysis, no significant difference (P > 0.05) was found in the area ratio of IL-10 positive cells between SNI and sham-operated groups (Fig. 2a), but a significantly increased IOD level (P < 0.01) was observed in the contralateral RN of SNI group (Fig. 2b). These results indicated that SNI rats and sham-operated rats had the same amount of IL-10 positive cells in their contralateral RN, while the protein expression of IL-10 in positive cells was up-regulated significantly in the SNI rats as compared with that of sham-operated rats.
Immunohistochemical staining of IL-10 in the red nucleus (RN) at 3 weeks following spared nerve injury (SNI). Three weeks after SNI, a stronger immunoreactivity of IL-10 was observed in the contralateral red nucleus of SNI rats (a, d and g) and a weaker immunoreactivity of IL-10 was observed in the ipsilateral RN of SNI rats and sham-operated rats (b, e and h). In normal rats, no obvious immunoreactivity of IL-10 was found in the both sides of RN (c, f and i). The control experiments, in which the primary antibody or secondary antibody was omitted and normal rabbit IgG was used, did not find any positive staining, suggesting that the immunoreactivity of IL-10 in red nucleus was specific (j, k and l). L left, R right. Scale bars a, b and c: 200 μm; d, e and f: 100 μm; g, h and i: 10 μm; j, k and l: 50 μm
Quantitative analysis of IL-10 immunoreactive intensity in the contralateral red nucleus (RN) of spared nerve injury (SNI) and sham-operated rats. The area ratio of IL-10 positive cells (a) in the RN did not show any significant difference between SNI and sham-operated groups (P > 0.05), while the integrated optical density (IOD) level (b) in the RN of SNI rats was significantly higher than that of sham-operated rats (P < 0.01), suggesting that SNI rats and sham-operated rats have the same amount of IL-10 positive cells in their contralateral RN, while the protein expression of IL-10 in positive cells was up-regulated significantly in the SNI rats as compared with that of sham-operated rats. ** P < 0.01, compared with sham-operated group (one-way ANOVA)
Effect of IL-10 on the Allodynia Induced by SNI
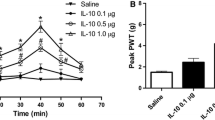
Three weeks after SNI, total 32 rats with mechanical allodynia (the withdrawal thresholds before and after SNI were 11.73 ± 2.59 g and 0.89 ± 0.10 g, respectively) was divided into four groups randomly. After microinjection of different doses of IL-10 (1.0, 0.5, 0.1 μg/μl) and normal saline into the RN contralateral to the nerve injury paw, the mechanical allodynia induced by SNI was depressed in a dose-dependent manner (r = 0.999, P < 0.001). The mean withdrawal thresholds during the 60 min (10–70 min) observation period were 2.31 ± 1.26 g (n = 8) for 1.0 μg/μl of IL-10, 1.61 ± 0.58 g (n = 8) for 0.5 μg/μl of IL-10, 1.19 ± 0.30 g (n = 8) for 0.1 μg/μl of IL-10 and 1.06 ± 0.09 g (n = 8) for normal saline, respectively. As shown in Fig. 3, the time course curves (i.e., normal saline treated group and three different doses of IL-10 treated groups) were significantly different among treatments (F(3,126) = 5.046, P = 0.009), across times (F(6,126) = 47.864, P < 0.001) and for their interaction (F(18,126) = 8.807, P < 0.001). Further analyses indicated that 1.0 μg/μl of IL-10 microinjected into RN significantly increased the withdrawal threshold of SNI rats (t = 3.54, P = 0.012), the peak analgesic effect occurred at 50 min and thereafter gradually reduced to the baseline at 70 min. IL-10 at dose of 0.5 μg/μl also increased the withdrawal threshold of SNI rats and displayed obvious analgesic effect at 50 min (t = 3.678, P = 0.004). However, 0.1 μg/μl of IL-10 did not show any effect on the mechanical allodynia (P > 0.05) as compared with the normal saline treated group. The detailed comparisons at each time point among groups and the microinjection sites of IL-10 and normal saline in RN region are shown in Fig. 3.
Time course curve graph showing the anti-allodynia effect of different doses (1.0, 0.5 and 0.1 μg/μl) of IL-10 microinjected into the contralateral red nucleus (RN) of spared nerve injury (SNI) rats. During 60 min (10–70 min) observation period, 1.0 μg/μl of IL-10 microinjected into RN significantly increased the withdrawal threshold (P = 0.012) of SNI rats. 0.5 μg/μl of IL-10 also increased the withdrawal threshold of SNI rats and displayed obvious analgesic effect at 50 min (P = 0.004). However, 0.1 μg/μl of IL-10 did not show any effect on the mechanical allodynia (P > 0.05) as compared with normal saline treated group. * P < 0.05, ** P < 0.01 and *** P < 0.001, compared with normal saline group at those time points (two-way RM ANOVA). a The locations of 1.0 μg/μl of IL-10 microinjection sites in RN region. b The locations of 0.5 μg/μl of IL-10 microinjection sites in RN region. c The locations of 0.1 μg/μl of IL-10 microinjection sites in RN region. d The locations of normal saline microinjection sites in RN region. RN red nucleus, PAG periaqueductal gray, Aq aqueduct, PaR pararubral nucleus, DpMe deep mesencephalic nucleus
Discussion
Red nucleus is an important nucleus of extracorticospinal tract, and comprises an important subcortical relay station of a massive descending motor tract (rubrospinal tract). Previous studies have suggested that RN is involved in regulating muscle tension, motor learning, triggering conditioned motor responses, postural corrections, modification of jaw movements and the recovery of movement after spinal injury [33–38]. Most neurons in the RN in the intact and decerebrate cat exhibit phasic discharge preferentially in the swing phase of locomotion, during which they influence the activity of flexor muscles [34]. Unilateral lesions of the RN in rats give rise to a characteristic asymmetry in which abnormal braking and propulsive forces are produced during locomotion [35]. Apart from its well established roles in motor system, recent studies suggest that RN is involved in pain processing and aversive events. The studies have shown that stimulation of peripheral nerve or limbs could cause the changes of electrical activities of neuron in RN and chemical or electrical stimulation of RN increases the pain threshold and produces analgesic effect assessed during nociceptive pain experiments [39–41]. Furthermore, RN receives the fibers from the sensorimotor cortex and it has been suggested that cortex employs the rubrospinal tract to suppress the nociceptive transmission from the spine.
Our previous studies have demonstrated that the expression of TNF-α, IL-1β and nerve growth factor (NGF) are up-regulated in the contralateral RN of SNI rats, and microinjection of their corresponding antibodies could alleviate the mechanical allodynia induced by SNI [24, 25, 42]. These results suggest that TNF-α, IL-β and NGF in RN are involved in the development of neuropathic pain and play facilitated roles on the mechanical allodynia induced by SNI. In the current study, we observed an increased expression of IL-10 in the contralateral RN of rats at 3 weeks following SNI, suggesting that not only the pro-inflammatory cytokines but also anti-inflammatory cytokine IL-10 may be involved in the pain modulation mediated by RN. This is consistent with previous studies that IL-10 is up-regulated in CNS during inflammatory or neuropathic pain [3, 6, 7].
To further study the roles of IL-10 in the development of neuropathic pain, different doses of IL-10 were microinjected into the RN contralateral to the nerve injury side of SNI rats and the changes of mechanical withdrawal threshold were measured dynamically. Results indicate that IL-10 could dose-dependently decrease the mechanical allodynia induced by SNI, suggesting that IL-10 of RN is involved in the development of neuropathic allodynia and plays inhibitory roles in SNI rats. This is consistent with studies that systemic or intrathecal administration of IL-10 protein or IL-10 gene therapy can attenuate the pain-related behaviors [12–23]. Combining with our previous studies [24, 25, 42], we conclude that RN is involved in the modulation of neuropathic pain and plays both facilitated roles through pro-inflammatory cytokines and inhibitory roles through anti-inflammatory cytokine IL-10.
Previous studies have identified that IL-10 receptors express on the membrane surface of only astrocytes and microglia, but not spinal cord neurons [8, 43]. That is, IL-10 can not play the analgesic effect by directly acting on neurons, but by acting on activated astrocytes and microglia and then indirectly affects the activation of neurons. Further studies indicate that IL-10 plays the analgesic effect mainly by suppressing the production of some chemokines and cell adhesion molecules, and further inhibiting the recruitment and activation of immune cells [4]; reducing the production of pro-inflammatory cytokines (e.g., IL-1, IL-6 and TNF-α) at multiple levels, including transcription, translation and release [4, 15, 44–46]; interrupting the pro-inflammatory cytokines signaling by down-regulating the expression of pro-inflammatory cytokine receptors [44, 45]. In addition, IL-10 can inhibit the production of reactive oxygen and nitrogen intermediates [46]. Although various ways are involved in IL-10 mediated analgesic effect, which ways are involved in the analgesic effect of red nucleus IL-10 on earth still need further studies to verify.
References
Wieseler-Frank J, Maier SF, Watkins LR (2005) Central proinflammatory cytokines and pain enhancement. Neurosignals 14(4):166–174. doi:10.1159/000087655
Sawada M, Suzumura A, Marunouchi T (1995) Cytokine network in the central nervous system and its roles in growth and differentiation of glial and neuronal cells. Int J Dev Neurosci 13(3–4):253–264. doi:10.1016/0736-5748(94)00076-F
Jancálek R, Dubový P, Svízenská I, Klusáková I (2010) Bilateral changes of TNF-α and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J Neuroinflammation 7:11. doi:10.1186/1742-2094-7-11
Wagner R, Janjigian M, Myers RR (1998) Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-α expression. Pain 74(1):35–42. doi:10.1016/S0304-3959(97)00148-6
Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, Wei F, Dubner R, Ren K (2009) Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol Pain 5:75. doi:10.1186/1744-8069-5-75
Jancalek R, Svizenska I, Klusakova I, Dubovy P (2011) Bilateral changes of IL-10 protein in lumbar and cervical dorsal root ganglia following proximal and distal chronic constriction injury of peripheral nerve. Neurosci Lett 501(2):86–91. doi:10.1016/j.neulet.2011.06.052
Ruohonen S, Jagodi M, Khademi M, Taskinen HS, Ojala P, Olsson T, Röyttä M (2002) Contralateral non-operated nerve to transected rat sciatic nerve shows increased expression of IL-1beta, TGF-beta1, TNF-alpha, and IL-10. J Neuroimmunol 132(1–2):11–17. doi:10.1016/S0165-5728(02)00281-3
Mizuno T, Sawada M, Marunouchi T, Suzumura A (1993) Production of interleukin-10 by mouse glial cells in culture. Biochem Biophys Res Commun 205(3):1907–1915. doi:10.1006/bbrc.1994.2893
Xie W, Luo S, Xuan H, Chou C, Song G, Lv R, Jin Y, Li W, Xu J (2006) Betamethasone affects cerebral expressions of NF-kappaB and cytokines that correlate with pain behavior in a rat model of neuropathy. Ann Clin Lab Sci 36(1):39–46
Zhu J, Wei X, Feng X, Song J, Hu Y, Xu J (2008) Repeated administration of mirtazapine inhibits development of hyperalgesia/allodynia and activation of NF-κB in a rat model of neuropathic pain. Neurosci Lett 433(1):33–37. doi:10.1016/j.neulet.2007.12.037
Loram LC, Harrison JA, Sloane EM, Hutchinson MR, Sholar P, Taylor FR, Berkelhammer D, Coats BD, Poole S, Milligan ED, Maier SF, Rieger J, Watkins LR (2009) Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J Neurosci 29(44):14015–14025. doi:10.1523/JNEUROSCI.3447-09.2009
Laughlin TM, Bethea JR, Yezierski RP, Wilcox GL (2000) Cytokine involvement in dynorphin-induced allodynia. Pain 84(2–3):159–167. doi:10.1016/S0304-3959(99)00195-5
Yu CG, Fairbanks CA, Wilcox GL, Yezierski RP (2003) Effects of agmatine, interleukin-10, and cyclosporin on spontaneous pain behavior after excitotoxic spinal cord injury in rats. J Pain 4(3):129–140. doi:10.1054/jpai.2003.11
Kim WM, Jeong CW, Lee SH, Kim YO, Cui JH, Yoon MH (2011) The intrathecally administered Kappa-2 opioid agonist GR89696 and interleukin-10 attenuate bone cancer–induced pain through synergistic interaction. Anesth Analg 113(4):934–940. doi:10.1213/ANE.0b013e318227824e
Karam MC, Hamdan HG, Abi Chedid NA, Bodman-Smith KB, Baroody GM (2007) Interleukin-10 reduces hyperalgesia and the level of Interleukin-1beta in BALB/c mice infected with Leishmania major with no major effect on the level of interleukin-6. J Neuroimmunol 183(1–2):43–49. doi:10.1016/j.jneuroim.2006.11.003
Saadé NE, Nasr IW, Massaad CA, Safieh-Garabedian B, Jabbur SJ, Kanaan SA (2000) Modulation of ultraviolet-induced hyperalgesia and cytokine upregulation by interleukins 10 and 13. Br J Pharmacol 131(7):1317–1324. doi:10.1038/sj.bjp.0703699
Soderquist RG, Milligan ED, Harrison JA, Chavez RA, Johnson KW, Watkins LR, Mahoney MJ (2010) PEGylation of interleukin-10 for the mitigation of enhanced pain states. J Biomed Mater Res A 93(3):1169–1179. doi:10.1002/jbm.a.32611
Milligan ED, Soderquist RG, Malone SM, Mahoney JH, Hughes TS, Langer SJ, Sloane EM, Maier SF, Leinwand LA, Watkins LR, Mahoney MJ (2006) Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biol 2(4):293–308. doi:10.1017/S1740925X07000488
Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, Watkins LR (2007) Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 21(5):686–698. doi:10.1016/j.bbi.2006.10.012
Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O’Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, Chavez RA, Leinwand LA, Watkins LR (2005) Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 21(8):2136–2148. doi:10.1111/j.1460-9568.2005.04057.x
Milligan ED, Sloane EM, Langer SJ, Cruz PE, Chacur M, Spataro L, Wieseler-Frank J, Hammack SE, Maier SF, Flotte TR, Forsayeth JR, Leinwand LA, Chavez R, Watkins LR (2005) Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain 1:9. doi:10.1186/1744-8069-1-9
Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, Flotte TR, Johnson KW, Mahoney MM, Chavez RA, Leinwand LA, Watkins LR (2006) Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain 126(1–3):294–308. doi:10.1016/j.pain.2006.07.009
Sloane EM, Soderquist RG, Maier SF, Mahoney MJ, Watkins LR, Milligan ED (2009) Long-term control of neuropathic pain in a non-viral gene therapy paradigm. Gene Ther 16(4):470–475. doi:10.1038/gt.2009.21
Li X, Wang J, Wang Z, Dong C, Dong X, Jing Y, Yuan Y, Fan G (2008) Tumor necrosis factor-alpha of red nucleus involved in the development of neuropathic allodynia. Brain Res Bull 77(5):233–236. doi:10.1016/j.brainresbull.2008.08.025
Wang Z, Wang J, Li X, Yuan Y, Fan G (2008) Interleukin-1 beta of red nucleus involved in the development of allodynia in spared nerve injury rats. Exp Brain Res 188(3):379–384. doi:10.1007/s00221-008-1365-1
Jaggi AS, Singh N (2011) Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res 1381:187–201. doi:10.1016/j.brainres.2011.01.002
Zimmermann M (1983) Ethical guideline for investigations of experimental pain in conscious animals. Pain 16(2):109–110
Bourquin AF, Süveges M, Pertin M, Gilliard N, Sardy S, Davison AC, Spahn DR, Decosterd I (2006) Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain 122(1–2):14.e1–14.e14. doi:10.1016/j.pain.2005.10.036
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, Sydney
Chaplan SR, Bach FW, Pogrel JW, Chung JW, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. Neurosci Methods 53:55–63. doi:10.1016/0165-0270(94)90144-9
Dixon WJ (1980) Efficient analysis of experimental observation. Ann Rev Pharmacol Toxicol 20:441–462
del Rey A, Yau HJ, Randolf A, Centeno MV, Wildmann J, Martina M, Besedovsky HO, Apkarian AV (2011) Chronic neuropathic pain-like behavior correlates with IL-1β expression and disrupts cytokine interactions in the hippocampus. Pain 152(12):2827–2835. doi:10.1016/j.pain.2011.09.013
Küchler M, Fouad K, Weinmann O, Schwab ME, Raineteau O (2002) Red nucleus projections to distinct motor neuron pools in the rat spinal cord. J Comp Neurol 448(4):349–359. doi:10.1002/cne.10259
Lavoie S, Drew T (2002) Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol 88(4):1791–1814
Muir GD, Whishaw IQ (2000) Red nucleus lesions impair overground locomotion in rats: a kinetic analysis. Eur J Neurosci 12(3):1113–1122. doi:10.1046/j.1460-9568.2000.00987.x
Zelenin PV, Beloozerova IN, Sirota MG, Orlovsky GN, Deliagina TG (2010) Activity of red nucleus neurons in the cat during postural corrections. J Neurosci 30(43):14533–14542. doi:10.1523/JNEUROSCI.2991-10.2010
Satoh Y, Ishizuka K, Murakami T (2007) Changes in cortically induced rhythmic jaw movements after lesioning of the red nucleus in rats. Brain Res 1165:60–70. doi:10.1016/j.brainres.2007.06.008
Basso DM, Beattie MS, Bresnahan JC (2002) Descending systems contributing to locomotor recovery after mild or moderate spinal cord injury in rats: experimental evidence and a review of literature. Restor Neurol Neurosci 20:189–218
Steffens H, Rathelot JA, Padel Y (2000) Effects of noxious skin heating on spontaneous cell activity in the magnocellular red nucleus of the cat. Exp Brain Res 131(2):215–224. doi:10.1007/s002219900279
Liu M, Liu X, Liu B (1991) The analgesia effect of red nucleus and strengthening effect thereof to the acupuncture analgesia. Zhen Ci Yan Jiu 1:48–53
Huang M, Liu M, Li X (1992) The analgesic effect of red nucleus and preliminary research on its mechanism. Zhen Ci Yan Jiu 17:166–170
Jing YY, Wang JY, Li XL, Wang ZH, Pei L, Pan MM, Dong XP, Fan GX, Yuan YK (2009) Nerve growth factor of red nucleus involvement in pain induced by spared nerve injury of the rat sciatic nerve. Neurochem Res 34(9):1612–1618. doi:10.1007/s11064-009-9950-7
Ledeboer A, Wierinckx A, Bol JG, Floris S, Renardel de Lavalette C, De Vries HE, van den Berg TK, Dijkstra CD, Tilders FJ, van Dam AM (2003) Regional and temporal expression patterns of interleukin-10, interleukin-10 receptor and adhesion molecules in the rat spinal cord during chronic relapsing EAE. J Neuroimmunol 136(1–2):94–103. doi:10.1016/S0165-5728(03)00031-6
Sawada M, Suzumura A, Hosoya H, Marunouchi T, Nagatsu T (1999) Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J Neurochem 72(4):1466–1471. doi:10.1046/j.1471-4159.1999.721466.x
Zhou Z, Peng X, Hao S, Fink DJ, Mata M (2008) HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor α in spinal cord microglia. Gene Ther 15(3):183–190. doi:10.1038/sj.gt.3303054
Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP (2001) Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol 168(1):144–154. doi:10.1006/exnr.2000.7604
Acknowledgments
The authors wish to thank Prof. Yanhong Ji and Shemin Lu for their expert help in reviewing the manuscript. The project was sponsored by the National Natural Science Foundation of China (No. 31070979) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhi-Hong Wang, Xiao-Yan Zeng are co-first author.
Rights and permissions
About this article
Cite this article
Wang, ZH., Zeng, XY., Han, SP. et al. Interleukin-10 of Red Nucleus Plays Anti-Allodynia Effect in Neuropathic Pain Rats with Spared Nerve Injury. Neurochem Res 37, 1811–1819 (2012). https://doi.org/10.1007/s11064-012-0795-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0795-0