Abstract
Sepsis-associated encephalopathy (SAE) is associated with increased mortality, morbidity, and long-term cognitive impairments. Its pathophysiology remains to be determined and an effective pharmacologic treatment is lacking. The goal of this study was to investigate the effects of the mitochondria-targeted peptide SS-31 on mitochondrial function and cognitive deficits in SAE mice. C57BL/6 male mice were randomly divided into sham, sham + SS-31, cecal ligation and puncture (CLP), and CLP + SS-31 groups. Peptide SS-31 (5 mg/kg) was intraperitoneally administrated immediately after operation and afterwards once daily for six consecutive days. Surviving mice were subjected to behavioral tests and the hippocampus was collected for biochemical analysis 7 days after operation. The results showed that CLP resulted in high mortality rate and cognitive deficits, representative characteristics of SAE. A physiological mechanistic investigation revealed that mitochondrial function of hippocampus was severely impaired, coupled with reactive oxygen species (ROS) generation, triggering neuronal apoptosis and inflammation. Notably, administration of peptide SS-31 protected the integrity of mitochondria, reversed the mitochondrial dysfunction, inhibited the apoptosis resulting from the release of cytochrome c, diminished the response of inflammation, and ultimately reversed the behavior deficits in the SAE mice. In conclusion, our data demonstrate that daily treatment with mitochondria-targeted peptide SS-31 reduces mortality rate and ameliorates cognitive deficits, which is possibly through a mechanism of reversing mitochondrial dysfunction and partial inhibition of neuronal apoptosis and inflammation in the hippocampus of the SAE mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe sepsis is associated with multiple organ dysfunctions including acute brain dysfunction, termed here sepsis-associated encephalopathy (SAE) in the present study, with long-term cognitive impairment and functional disability among survivors [1–6]. Plenty of evidence points to the role of mitochondrial dysfunction in the pathogenesis of SAE. The roles of mitochondria reach far beyond energy production and involve diverse metabolic pathways, which can have remarkable consequences for cells and the health of the organism [7]. Moreover, the available literature based on clinical studies and experimental sepsis in animal models of SAE is controversial regarding the involvement of mitochondrial functions [8–12]. Despite the importance of mitochondrial dysfunction, the pathophysiology of SAE remains poorly understood. Since much scientific knowledge on SAE is relatively new, proposed sepsis treatments focus on prompt antibiotic therapy, control of the infectious source, and global hemodynamic goals. Brain dysfunction, or SAE, has been neglected until recently because there are no precise, well-established clinical or biological markers of damage to the brain during sepsis [2, 13]. As a result, there is no report concerning the effective treatment to rapidly reverse mitochondrial dysfunctions and high mortality rate in SAE [13, 14].
SS-31 is a new and innovative mitochondria-targeted antioxidant and can readily cross the blood–brain barrier and cell membrane. It then concentrates >1,000-fold in the mitochondrial inner membrane, independent of mitochondrial membrane potential (MMP) [15–18]. It can scavenge various ROS (i.e., hydrogen peroxide [H2O2], superoxide [·O2 −], and hydroxyl radicals [·OH]), improve ATP production, and maintain MMP in many animal models of neurodegenerative diseases [15–18]. These mitochondria-protective effects are the basis of a new strategy to reverse cognitive deficits and reduce mortality rate in the SAE mice. The goal of this study was to investigate if a mitochondria-targeted peptide SS-31 may improve mitochondrial functions, ameliorate cognitive deficits, and reduce mortality rate in a SAE mouse model. We also explored the potentially mechanistic connections to apoptosis and inflammation.
Materials and Methods
Animals
C57BL/6 male mice (26–32 g) were purchased from the Animal Center of Jinling Hospital, Nanjing, China. All experimental procedures and protocols used in the present study were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals from the National Institutes of Health, USA. The animals were housed under a 12-h light/dark cycle in a temperature-controlled room of 22–24 °C with free access to food and water.
Surgical Procedure and Experimental Protocol
The mice were subjected to cecal ligation and puncture (CLP) as previously described [19]. Each mouse was intraperitoneally anesthetized with 2 % sodium pentobarbital (40 mg/kg, Sigma) in saline. The cecum was isolated carefully and then ligated with 4.0 silk below the ileocecal junction, approximately 1.2 cm from the distal end. The cecum was then perforated twice with a sterile 22-gauge needle and was gently squeezed to extrude the fecal contents into the peritoneal cavity. The cecum was then returned to the peritoneal cavity and the laparotomy was closed with 4.0 silk sutures. For mice that served as sham operations, the cecum was exposed in the same manner as CLP but was neither ligated nor punctured. All mice were then resuscitated with subcutaneous lactated Ringers (30 ml/kg) immediately after surgery. The entire procedure was completed within 8 min. All animals were returned to their cages with free access to food and water. Because this study was designed to assess endogenous immune response in mice as well as ROS scavenging, anti-apoptotic, and anti-inflammatory effects of SS-31, antibiotics were not given.
The animals were randomly divided into the following four groups: sham group (n = 30), sham + SS-31 group (n = 30), CLP group (n = 60), and CLP + SS-31 group (n = 60). Mice received injection of normal saline or SS-31 (5 mg/kg, China Peptides Co. Ltd., China) immediately after operation and once daily for six consecutive days thereafter. Injections (20 ml/kg) were intraperitoneally given and alternated daily between the left and right side of the abdomen. The dose of SS-31 was selected based on previous studies showing that repeated administration of SS-31 at 5 mg/kg presented maximum neuroprotective effects without any side effect in mice models [16, 18]. Seven days after operation, surviving animals were subjected to behavioral tests and the hippocampus was dissected for biochemical analysis. Each animal performed only one single behavioral test. All behavioral results and 7-day survival rates were recorded with blind assignments.
Open Field Test
On the seventh day, mice were gently placed in the center of a white plastic chamber (40 cm × 40 cm × 40 cm) for 5 min while exploratory behavior was automatically recorded by a video tracking system (XR-XZ301, Shanghai Xin Soft Information Technology Co. Ltd., Shanghai, China). Travel speed lower than 0.25 mm/s was defined as immobility. The total distance and total time traveled in the open field were recorded. After each test, the arena was cleaned with 75 % alcohol to avoid the presence of olfactory cues.
Fear Conditioning Test (FCT)
The FCT was performed as described by Zhang et al. [20, 21] with modifications. The training in FCT (XR-XC404, Shanghai Xin Soft Information Technology Co. Ltd.) was performed as follows. Each mouse was allowed to explore the FCT chamber for 390 s followed by a 2-Hz pulsating tone (80 dB, 3,600 Hz) that persisted for 60 s and a mild electric foot shock (1 s, 0.75 mA). To test for short-term memory (STM) and long-term memory (LTM), all mice were returned to the training chamber 2 and 24 h later. A context test to evaluate hippocampus-dependent memory was performed by placing mice back in the same test chamber for a total of 390 s without any stimulation. After the context test, each mouse was placed for 390 s in a novel chamber altered in shape, color, and smell. The same tone was presented for another 180 s without the foot shock to evaluate hippocampus-independent memory [20, 22]. Cognitive deficits in the test was assessed by measuring the amount of time the mouse demonstrated “freezing behavior,” which is defined as a completely immobile posture except for respiratory efforts. Freezing behavior was automatically recorded by a video tracking system.
Hippocampus Mitochondria
The hippocampal tissues were harvested 7 days after operation and homogenized in ice-chilled Dounce homogenizers (1:10, w/v) using isolation buffer (Beyotime Institute of Biotechnology, Shanghai, China) and centrifuged at 1,000×g for 5 min at 4 °C. Supernatants were transferred into new tubes and centrifuged at 8,000×g for 10 min at 4 °C. Supernatants were removed and centrifuged at 12,000×g to obtain pure cytosol fractions, and mitochondria-enriched pellets were gently resuspended and washed again with isolation buffer, then re-pelleted by centrifugation at 1,000 and 8,000×g for 5 and 10 min, respectively. The cytosol fraction was isolated to determine cytosolic cytochrome c (Cyt C) levels. The concentration of mitochondrial protein was determined using the Micro BCA protein assay kit (Beyotime Institute of Biotechnology) with bovine serum albumin as standard.
Electron Transport Chain (ETC) Enzymes Activities
ETC enzyme activity was assayed spectrophotometrically as specific donor-acceptor oxidoreductase activity according to the ETC assay kit (Genmed Scientifics Inc., Shanghai, China) following the manufacturer’s protocols. Purified mitochondria samples were freeze-thawed three times to fully expose the enzymes to substrates and achieve maximal activities.
Complex I (NADH-ubiquinone oxidoreductase) activity was evaluated by measuring the decrease in absorbance due to the oxidation of NADH at 340 nm. Complex II (succinate decylubiquinone 2,6-dichloroindophenol [DCIP] oxidoreductase) activity was determined by measuring the reduction of the absorbance of DCIP at 600 nm. Complex III (ubiquinol-cytochrome c reductase) activity was determined by measuring the reduction of the absorbance of cytochrome c at 550 nm. Complex IV (cytochrome c oxidase) activity was determined by measuring the absorbance at 550 nm due to the oxidation of ferrocytochrome c.
Mitochondrial Membrane Potential (MMP) Level
The MMP level was determined following the manufacturer’s protocols of a 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolylcarbocyanine iodide (JC-1) mitochondrial membrane potential detection kit (Genmed Scientifics Inc.) by measuring fluorescence intensity (exCitation 490 nm, emission 520 nm) in a spectrofluorometer.
Mitochondrial Permeability Transition Pore (mPTP) Opening
mPTP opening was determined by a colorimetric assay kit (Genmed Scientifics Inc.). Opening of the mPTP causes mitochondrial swelling, which can be conveniently detected spectrophotometrically as a decrease in light scattering (and thus absorbance) at 540 nm.
ROS and ATP Content
Hippocampal tissues were harvested 7 days after the operation and homogenized to quantify the amount of ROS and ATP. Intracellular ROS was detected using a ROS assay kit (Genmed Scientifics Inc.) by means of an oxidation-sensitive fluorescent probe (DCFH-DA) in a spectrofluorometer (excitation 490 nm, emission 520 nm). Assessment of relative ATP content based on the reaction of ATP with recombinant firefly luciferase and its substrate luciferin was performed using the ATP bioluminescence assay kit (Beyotime Institute of Biotechnology) following the manufacturer’s instructions.
Western Blotting
The hippocampus was harvested 7 days after the operation for the determination of total Cyt C (1:5,000; Abcam), caspase 3 (1:1,000; Cell Signaling Technology), and Nlrp3 (1:500; Thermo Scientific) levels. Cytosolic Cyt C levels were also determined. Bands were visualized by enhanced chemiluminescence and quantitated with the Image Quant Software (Syngene).
Enzyme-Linked Immunosorbent Assay (ELISA)
We detected interleukin-1β and neuron-specific enolase (NSE) levels of the hippocampus by ELISA kits (Abcam) following the protocols provided by the manufacturer. Readings were normalized to the amount of a standard protein.
Data Analysis
Data are presented as mean ± SEM and analyzed by the Statistical Product for Social Sciences (SPSS; version 17.0, IL). The difference among the groups was determined by one-way analysis of variance followed by the Bonferroni test. The survival rate was estimated using the Kaplan-Meier method and compared using the log-rank test. A p value <0.05 was regarded as statistical significance.
Results
SS-31 Improved Survival Rate in CLP Mice
Our previous study demonstrated that CLP mice showed high mortality rate within 7 days [19]. Therefore, the rapid therapeutic effect of SS-31 on the survival rate of CLP mice was evaluated within 7 days in the present study. When SS-31 was immediately administrated after CLP for six consecutive days, there was a significant increase in survival rate in the SS-31-treated CLP (CLP + SS-31) group compared to the normal saline-treated CLP group (71.67 versus 53.33 %) (Fig. 1).
SS-31 treatment improved survival rate within 7 days after CLP. The mice were randomly divided into sham, sham + SS-31, CLP, and CLP + SS-31 groups to receive normal saline or SS-31 (5 mg/kg) immediately after operation and afterwards once daily for six consecutive days. Survival rate was evaluated within 7 days
SS-31 Attenuated Cognitive Deficits in SAE Mice
SAE is characterized by functional disabilities and long-term cognitive impairment among survivors [1–5]. We checked whether the improved survival rate after the administration of SS-31 was associated with ameliorations in functional disabilities and cognitive impairment.
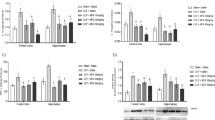
First, we used an open field test to evaluate general locomotors activities. The results showed that the two CLP groups (+/− SS-31) displayed significant decreases in total distance compared to the two sham groups (+/− SS-31), while no difference was observed between the two sham groups. However, the CLP + SS-31 group showed a remarkable increase in total distance compared to the CLP-only group (Fig. 2a). Interestingly, there is no difference in total traveling time among the four groups (Fig. 2b), which was probably the remaining effect of systemic organ dysfunction besides the cognitive impairment 7 days post CLP operation.
SS-31 treatment ameliorated the behavior performance of CLP-induced SAE mice revealed by the open field test. a Total traveling distance. b Total traveling time. Grouping and SS-31 treatment were defined the same as in Fig. 1. Data were presented as mean ± SEM (n = 8). * p < 0.05, versus the sham group; # p < 0.05 versus the CLP group. For the procedure of the open field test, see “Materials and Methods”
Next, a fear conditioning test was performed to assess whether SS-31 could improve the ability of mice to learn and remember an association between environmental cues and aversive experiences. The freezing time in a 24-h (but not a 2-h) context test was significantly shorter in the CLP group than in the sham groups, indicating that CLP induces hippocampus-dependent long-term memory deficit in the SAE mice, which could be attenuated by SS-31 treatment (Fig. 3c). There was no significant difference in freezing time in the 2-h context (Fig. 3a), 2-h tone (Fig. 3b), and 24-h tone tests among the four groups (Fig. 3d).
Effect of SS-31 treatment on the freezing time of SAE mice revealed by the fear conditioning test. SS-31 treatment only increased the freezing time in 24 h context test (c). There was no significant difference in freezing time in the 2-h context test (a), 2-h tone test (b), and 24-h tone test (d) among the four groups. Data were presented as mean ± SEM (n = 10). * p < 0.05, versus the sham group; # p < 0.05, versus the CLP group. For the procedure of the fear conditioning test, see “Materials and Methods”
SS-31 Protected Activities of Complexes I and III in the Hippocampus of SAE Mice
We hypothesized that sepsis-induced mouse cognitive deficits in learning and memory result from hippocampus injury. Mitochondrial dysfunctions in different tissues associated with the severity of sepsis and septic shock were proposed to be the pathological mechanism [8–12]. In the current study, complexes I, II, III, and IV activities of the ETC in mouse hippocampal cells were measured. The results showed that the activities of complexes I and III decreased in the two CLP groups compared to the two sham groups (Fig. 4a, c), while there were no significant differences in complexes II and IV activities among the four groups (Fig. 4b, d). However, SS-31 treatment reversed the decreases in complexes I and III activities compared to the CLP group (Fig. 4a, c). Combining the results above, we conclude that the protective effect of the mitochondria-targeted antioxidant SS-31 is based on the recovery of complexes I and III activities in hippocampus.
SS-31 treatment reversed the decreases of enzyme activities of ETC in SAE mice. The CLP group showed decreases in complexes I and III activities (a, c) compared to the sham groups, while there was no significant difference in complexes II and IV activities among the four groups (b, d). Data were presented as mean ± SEM (n = 6). * p < 0.05, versus the sham group; # p < 0.05, versus the CLP group
SS-31 Prevented ROS Generation and Protected the Integrity of Mitochondria
Complexes I and III are the major sources in mitochondria for ROS [23, 24], which may further damage the integrity and structure of the mitochondria. We then measured the effects of SS-31 on ROS levels and mitochondrial function-related indices. The results showed that CLP-induced increases in ROS levels were repressed, and ATP levels were elevated, by the administration of SS-31 (Fig. 5a, b), suggesting recovery of mitochondrial functions.
To test whether SS-31 could protect the integrity of mitochondria after CLP, we measured MMP and the opening of mPTP, two important parameters of mitochondria membrane. The administration of SS-31 restored MMP and prevented the opening of mPTP, counteracting the effect of CLP (Fig. 6a, b).
SS-31 treatment protected the integrity of mitochondrial membrane from damage. a Mitochondrial membrane potential (MMP) in the hippocampal place cells. b Mitochondrial permeability transition pore (mPTP) opening in the hippocampal place cells. Data were presented as mean ± SEM (n = 6). * p < 0.05, versus the sham group; # p < 0.05, versus the CLP group
SS-31 Inhibited the Occurrence of Apoptosis and Neuronal Injury in the Hippocampus of SAE Mice
Injured membrane of mitochondria would leak Cyt C from the intermembrane space to initiate apoptosis. In our current SAE model, CLP triggered the leakage of Cyt C from mitochondria to the cytosol, which in turn induced the cleavage of caspase 3, a major apoptotic biomarker (Fig. 7a, b). SS-31 treatment inhibited the release of Cyt C and cleavage of caspase 3 (Fig. 7a, b). We then checked a specific biomarker for neuronal injury, NSE, which exists in mature neurons and cells of neuronal origin and increases following neuronal injuries [25]. The results showed that CLP induced a significant increase of NSE level, which was reduced by the administration of SS-31 (Fig. 7c). Taken together, the results suggest that SS-31 has protective effects against apoptosis and neuronal injury in the hippocampus of SAE mice.
SS-31 treatment prevented apoptosis and neuronal damage. a The levels of total Cyt C, cytosolic Cyt C, and activated caspase 3 in the hippocampus revealed by Western blotting. b Quantitative data of a. c The levels of NSE in the hippocampus revealed by ELISA. Data were presented as mean ± SEM (n = 6). * p < 0.05, versus the sham group; # p < 0.05, versus the CLP group
SS-31 Reduced Inflammatory Response in the Hippocampus of SAE Mice
The process of inflammation, occurring in response of a variety of injuries, is orchestrated by an array of molecules produced locally, among of which Nlrp3 and IL-1β are members of the inflammasome and proinflammatory factors, respectively. We checked the level of Nlrp3 by Western blot and that of IL-1β by ELISA to assess whether the protective effect of SS-31 on mitochondrial injury would diminish inflammatory responses in the hippocampus area. The result showed that the levels of Nlrp3 and IL-1β increased significantly in the CLP group compared to the sham groups, and SS-31 treatment inhibited the activation of Nlrp3 and IL-1β (Fig. 8a, b). Therefore, administration of SS-31 reduced the inflammatory response.
Discussion
We have previously demonstrated that CLP mice showed high mortality rate in the first 7 days after operation and survivors exhibited significant cognitive deficits [19]. Here, we show that daily treatment of the mitochondria-targeted peptide SS-31 produced effective therapeutic outcomes by reducing the 7-day mortality rate and improving cognitive deficits of CLP-induced SAE mice. The protective effect of SS-31 is achieved through reversing mitochondrial dysfunction, inhibiting apoptosis, and suppressing inflammation in mouse hippocampus. To the best of our knowledge, the present study is the first report on the protective effect of SS-31 in CLP-induced SAE mouse model.
Although it has been proposed that severe sepsis induces brain mitochondrial dysfunction in experimental models, the affected brain functional area has not been accurately identified [9, 11]. This study points to the hippocampus, an important tissue for learning and memory as an affected area. The hippocampus, together with the cortex, was also found by magnetic resonance imaging to exhibit tissue-specific early damage [26]. Although SS-31 may target mitochondria in other tissues, our data definitively support that SS-31 protects hippocampus from damage in the SAE mice. Interestingly, when hippocampus-dependent and hippocampus-independent memory was measured by the context test and tone test, respectively, we found that CLP could decrease freezing time in the context test 24 h after training, but not in the tone test, suggesting that CLP impairs hippocampus-dependent memory and SS-31 does act on the hippocampus area to reverse the effect.
The mitochondria do not only function in energy production, but are also involved in diverse metabolic pathways. It is possible that some mitochondria functions are tissue-specific. For example, it was observed that rats submitted to CLP presented decreased ETC activity in complex I, but not in complexes II, III, and IV, 24, 48, and 96 h post CLP in cerebellum, hippocampus, striatum, and cortex [9], while others found a reduction in complex IV activity only in the brain tissue 24 h after CLP [11]. Selective dysfunctions of the ETC complexes were also demonstrated in the liver, heart, skeletal muscle, and ileum [27–29]. Here, we showed significant decreases in ETC activities for complexes I and III in hippocampus 7 days after CLP, but not for complexes II and IV. The difference may also depend on the animal model, tissue, and duration of experiments [9, 11, 27–29]. Regardless of the complex impaired, the downstream effect on ATP production, ROS generation, MMP, and opening of mPTP are quite similar [30–32]. From this point of view, the mitochondria-targeted peptide SS-31 maintains mitochondrial functions by acting more than a simple antioxidant to scavenge mitochondrial ROS [15–18].
The stimulation in ROS generation due to defects in mitochondrial energy metabolism can also modulate mitochondrial dynamics. The reciprocal interactions between ETC impairment-induced ROS and ROS modulation on ETC function may cause a feed forward, self-amplifying loop that creates cellular damages far beyond direct ROS-induced effects. For instance, mitochondrial dysfunction triggers cell death pathways and inflammatory signaling pathways [33, 34]. Consistent with this scenario, our data demonstrated the involvement of mitochondria in apoptosis and inflammation, with typical features such as the release of cytochrome c, cleavage of caspase 3, and increases of Nlrp3 and IL-1β. Hence, dysfunctions in the ETC, increases of the mPTP, ROS generation, apoptosis, and inflammation in hippocampus could be a network (rather than disconnected individual events) that causes encephalopathy, leading to cognitive deficits.
In conclusion, CLP impairs hippocampus-dependent cognition, induces mitochondrial dysfunction, and triggers mitochondria-associated apoptosis and inflammation. All these effects are reversed by daily treatment of the mitochondria-targeted peptide SS-31. Our results could lead to a new strategy for the early treatment of SAE.
References
Bleck TP, Smith MC, Pierre-Louis SJ, Jares JJ, Murray J, Hansen CA (1993) Neurologic complications of critical medical illnesses. Crit Care Med 21:98–103
Gofton TE, Young GB (2012) Sepsis-associated encephalopathy. Nat Rev Neurol 8:557–566. doi:10.1038/nrneurol.2012.183
Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304:1787–1794. doi:10.1001/jama.2010.1553
Michels M, Vieira AS, Vuolo F, Zapelini HG, Mendonca B, Mina F, Dominguini D, Steckert A, Schuck PF, Quevedo J, Petronilho F, Dal-Pizzol F (2014) The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav Immun. doi:10.1016/j.bbi.2014.07.002
Mina F, Comim CM, Dominguini D, Cassol-Jr OJ, Dall Igna DM, Ferreira GK, Silva MC, Galant LS, Streck EL, Quevedo J, Dal-Pizzol F (2014) Il1-beta involvement in cognitive impairment after sepsis. Mol Neurobiol 49:1069–1076. doi:10.1007/s12035-013-8581-9
Schwalm MT, Pasquali M, Miguel SP, Dos Santos JP, Vuolo F, Comim CM, Petronilho F, Quevedo J, Gelain DP, Moreira JC, Ritter C, Dal-Pizzol F (2014) Acute brain inflammation and oxidative damage are related to long-term cognitive deficits and markers of neurodegeneration in sepsis-survivor rats. Mol Neurobiol 49:380–385. doi:10.1007/s12035-013-8526-3
Zampieri FG, Park M, Machado FS, Azevedo LC (2011) Sepsis-associated encephalopathy: not just delirium. Clinics (Sao Paulo) 66:1825–1831
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360:219–223. doi:10.1016/S0140-6736(02)09459-X
Comim CM, Rezin GT, Scaini G, Di-Pietro PB, Cardoso MR, Petronilho FC, Ritter C, Streck EL, Quevedo J, Dal-Pizzol F (2008) Mitochondrial respiratory chain and creatine kinase activities in rat brain after sepsis induced by cecal ligation and perforation. Mitochondrion 8:313–318. doi:10.1016/j.mito.2008.07.002
Crouser ED (2004) Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 4:729–741. doi:10.1016/j.mito.2004.07.023
d’Avila JC, Santiago AP, Amancio RT, Galina A, Oliveira MF, Bozza FA (2008) Sepsis induces brain mitochondrial dysfunction. Crit Care Med 36:1925–1932. doi:10.1097/CCM.0b013e3181760c4b
Fredriksson K, Tjader I, Keller P, Petrovic N, Ahlman B, Scheele C, Wernerman J, Timmons JA, Rooyackers O (2008) Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS One 3:e3686. doi:10.1371/journal.pone.0003686
Iacobone E, Bailly-Salin J, Polito A, Friedman D, Stevens RD, Sharshar T (2009) Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med 37:S331–S336. doi:10.1097/CCM.0b013e3181b6ed58
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric S (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi:10.1097/CCM.0b013e31827e83af
Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH (2010) Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis 20(Suppl 2):S609–S631. doi:10.3233/JAD-2010-100564
Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF (2006) Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem 98:1141–1148. doi:10.1111/j.1471-4159.2006.04018.x
Szeto HH (2008) Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal 10:601–619. doi:10.1089/ars.2007.1892
Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF (2009) Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal 11:2095–2104. doi:10.1089/ARS.2009.2445
Wu J, Dong L, Zhang M, Jia M, Zhang G, Qiu L, Ji M, Yang J (2013) Class I histone deacetylase inhibitor valproic acid reverses cognitive deficits in a mouse model of septic encephalopathy. Neurochem Res 38:2440–2449. doi:10.1007/s11064-013-1159-0
Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z (2012) Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 71:687–698. doi:10.1002/ana.23536
Ji M, Dong L, Jia M, Liu W, Zhang M, Ju L, Yang J, Xie Z, Yang J (2014) Epigenetic enhancement of brain-derived neurotrophic factor signaling pathway improves cognitive impairments induced by isoflurane exposure in aged rats. Mol Neurobiol. doi:10.1007/s12035-014-8659-z
Saab BJ, Maclean AJ, Kanisek M, Zurek AA, Martin LJ, Roder JC, Orser BA (2010) Short-term memory impairment after isoflurane in mice is prevented by the alpha5 gamma-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology 113:1061–1071. doi:10.1097/ALN.0b013e3181f56228
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344. doi:10.1113/jphysiol.2003.049478
Liu Y, Fiskum G, Schubert D (2002) Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80:780–787
Hsu AA, Fenton K, Weinstein S, Carpenter J, Dalton H, Bell MJ (2008) Neurological injury markers in children with septic shock. Pediatr Crit Care Med 9:245–251. doi:10.1097/PCC.0b013e3181727b22
Bozza FA, Garteiser P, Oliveira MF, Doblas S, Cranford R, Saunders D, Jones I, Towner RA, Castro-Faria-Neto HC (2010) Sepsis-associated encephalopathy: a magnetic resonance imaging and spectroscopy study. J Cereb Blood Flow Metab 30:440–448. doi:10.1038/jcbfm.2009.215
Kozlov AV, Staniek K, Haindl S, Piskernik C, Ohlinger W, Gille L, Nohl H, Bahrami S, Redl H (2006) Different effects of endotoxic shock on the respiratory function of liver and heart mitochondria in rats. Am J Physiol Gastrointest Liver Physiol 290:G543–G549. doi:10.1152/ajpgi.00331.2005
Peruchi BB, Petronilho F, Rojas HA, Constantino L, Mina F, Vuolo F, Cardoso MR, Goncalves CL, Rezin GT, Streck EL, Dal-Pizzol F (2011) Skeletal muscle electron transport chain dysfunction after sepsis in rats. J Surg Res 167:e333–e338. doi:10.1016/j.jss.2010.11.893
Zapelini PH, Rezin GT, Cardoso MR, Ritter C, Klamt F, Moreira JC, Streck EL, Dal-Pizzol F (2008) Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion 8:211–218. doi:10.1016/j.mito.2008.03.002
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163. doi:10.1152/physrev.00013.2006
Ramsden DB, Ho PW, Ho JW, Liu HF, So DH, Tse HM, Chan KH, Ho SL (2012) Human neuronal uncoupling proteins 4 and 5 (UCP4 and UCP5): structural properties, regulation, and physiological role in protection against oxidative stress and mitochondrial dysfunction. Brain Behav 2:468–478. doi:10.1002/brb3.55
Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE (2005) Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res 79:231–239. doi:10.1002/jnr.20292
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489
Vieira HL, Belzacq AS, Haouzi D, Bernassola F, Cohen I, Jacotot E, Ferri KF, El Hamel C, Bartle LM, Melino G, Brenner C, Goldmacher V, Kroemer G (2001) The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite, and 4-hydroxynonenal. Oncogene 20:4305–4316
Acknowledgments
This work was supported by the National Nature Science Foundation of China; Grant numbers: 31071085, 31371060, 81271216, 81300946.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, J., Zhang, M., Hao, S. et al. Mitochondria-Targeted Peptide Reverses Mitochondrial Dysfunction and Cognitive Deficits in Sepsis-Associated Encephalopathy. Mol Neurobiol 52, 783–791 (2015). https://doi.org/10.1007/s12035-014-8918-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8918-z