Abstract
Oxidative stress is believed to contribute to neurodegeneration following ischemic injury. The present study was undertaken to evaluate the possible antioxidant neuroprotective effect of curcumin (Cur) on neuronal death of hippocampal CA1 neurons following transient forebrain ischemia in rat. Treatment of Cur (200 mg/kg/day, i.p.) at three different times (immediately, 3 h and 24 h after ischemia) significantly (P<0.01) reduced neuronal damage 7 days after ischemia. Also, treatment of ischemic rats with Cur decreased the elevated levels of MDA and increased GSH contents, catalase and SOD activities to normal levels. In the in vitro, Cur was as potent as antioxidant (IC50 = 1 μM) as butylated hydroxytoluene. The present study demonstrates that curcumin treatment attenuates forebrain ischemia-induced neuronal injury and oxidative stress in hippocampal tissue. Thus treatment with curcumin immediately or even delayed until 24 h may have the potential to be used as a protective agent in forebrain ischemic insult in human.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a non-communicable disease of increasing socioeconomic importance in ageing populations [1]. Transient global cerebral ischemia (forebrain ischemia) is one of the major causes of stroke. In humans, transient forebrain ischemia occurs as a result of cardiovascular disorders such as cardiac arrest and cardiac embolism. It is potentially very devastating since it affects the whole brain [2]. In humans and animals, a forebrain ischemia attack induces selective delayed death in hippocampal cornu ammonis 1 (CA1) pyramidal neurons which is observed 3–7 days after forebrain ischemia in the untreated rat and gerbil [2–5].
Investigations into the mechanisms contributing to the selective and delayed hippocampal neuronal death in different animal models of cerebral ischemia have implicated several factors such as increase in glutamate activity, increase in intracellular calcium (Ca2+) concentration [3], and over-expression of pro-inflammatory genes such as cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) [6]. Many of these changes are associated with increased reactive oxygen species (ROS) production such as superoxide anion (O ·−2 ), hydroxyl radical (OH·), hydrogen peroxide (H2O2) and nitric oxide (NO) that can occur both during ischemia and at reperfusion [7, 8]. The role of oxidative stress becomes much greater in the case where cerebral blood flow is restored, because reflow to previous ischemic brain results in an increase in oxygen level, and consequently causes severe oxidative injury to the tissue by massive production of ROS [9]. It has been demonstrated in numerous studies that ROS are directly involved in oxidative damage with cellular macromolecules such as lipid, proteins and nucleic acids in ischemic tissues [7, 8].
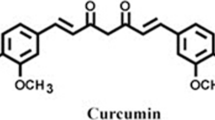
Curcumin is the main active ingredient in turmeric, extracted from the powdered dry rhizome of Curcuma longa Linn which belongs to the Zingiberaceae family. Curcumin is well known to be a potent antioxidant in various in vivo and in vitro models. Curcumin inhibits lipid peroxidation in rat liver, erythrocyte membrane and brain homogenates [10, 11]. Curcumin can lower lipid peroxidation by maintaining the activities of endogenous antioxidant enzymes such as superoxide dismutase, catalase and other antioxidants such as glutathione at higher levels [10, 11]. Several authors demonstrated that curcumin is a powerful scavenger of ROS such as O ·−2 , OH·, and NO [12, 13]. Curcumin could prevent or reduce oxidative stress-induced progression of age-related neurodegenerative disorders such as Alzheimer’s disease [14, 15]. Additionally, several studies have shown that curcumin at relatively low concentration exhibits remarkable anti-inflammatory effects [16–18]. This study was designed to investigate the possible protective effect of curcumin against transient global cerebral ischemia induced-hippocampal CA1 neuronal damage after 7 days of reperfusion using in vivo forebrain ischemia model and histological technique to count the number of intact neurons within the hippocampal CA1 subfield and by assessing oxidative stress-related biochemical parameters in the hippocampus tissue in the rat.
Materials and methods
All experimental procedures were approved by Research Ethic Committee of College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
Animals
Adult male Wistar albino rats weighing 220–250 g were used in this study. These animals were obtained from the Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. Animals were housed in suitable metabolic polypropylene cages under controlled hygienic environmental conditions. Rats were maintained at room temperature (25°C) and a normal light cycle (12 h light and 12 h dark) during the periods of the experiments. Animals were allowed free access to pulverized standard rat pellet food and tap water throughout the study period.
Drugs and chemicals
Curcumin and thiobarbituric acid (TBA) used in this study were purchased from Sigma Chemical Co. (St. Louis, MO, USA), while Ellman’s reagent was purchased from Fluka Chemical Company (Switzeland). Biochemical kits for measurement of superoxide dismutase activity and total protein concentration were purchased from Randox Laboratories Ltd. (UK).
Experimental protocol
Rats were randomly divided into 7 groups of 15 animals each. The first and second groups were received drinking water and curcumin (200 mg/kg i.p. once daily) respectively. The 3rd and 4th groups were subjected to sham-operated ischemia and 10 min forebrain ischemia, respectively. In the 5th, 6th and 7th groups, rats were subjected to10 min forebrain ischemia and received curcumin at a dose of 200 mg/kg i.p. immediately, 3 h, and 24 h after reperfusion respectively. Curcumin was administered for seven consecutive days (perfusion time).
Transient forebrain ischemia model
Transient forebrain ischemia was induced by a combination of bilateral carotid artery clamping and hypotension (2-vessel occlusion model) according to the method of Smith et al. [19]. Briefly, rats under general anesthesia (sodium pentobarbital; 30 mg/kg, i.p.), the common carotid arteries were exposed by means of a ventral midline neck incision. Both common carotid arteries were exposed, separated from the vagus nerve and occluded for 10 min with microaneurysmal clips (Dieffenbach Bulldog Clamp, 25 mm straight, Germany) which consistently resulted in delayed neuronal death in the CA1 region of the hippocampus [20, 21]. At the end of the occlusion period, the clamps were released; allowing restoration of carotid blood flow, and the incision was sutured with 2-0 silk sutures. Hypotension was achieved by withdrawal of blood from the jugular vein into heparinized syringe. Blood pressure was maintained at 50 mmHg during the ischemic period. In sham-operated animals, the arteries were freed from connective tissue but were not occluded. Body temperature was kept at 37°C by using controlled heating pad and heating lamps throughout the entire period of ischemia and postischemic recovery under anesthesia. A rectal thermometer was used to monitor body temperature (Apelex Rectal Thermometer, Panlab, Bagneux, France).
Histological analysis of hippocampal CA1 subregion
Seven days after ischemia, 5 rats from each group were anesthetized with sodium pentobarbital (100 mg/kg). Rats were then transcardially perfused with cold saline followed by 4% formalin in phosphate-buffered saline (0.1 M; pH 7.4). The brains were removed from the skull and fixed in same fixative for 24 h. Thereafter, the brains were embedded in paraffin, then 5 μm thick sections were coronally cut at the level of the dorsal hippocampus by a rotatory microtome (Leica CM3050S, Leica Microsystems, Bensheim, Germany). The segments of the hippocampal CA1 region per 1000-μm lengths from bregma −3.3, −3.8, and −4.3 were counted for viable cells. Tissue sections were stained with hematoxylin and eosin. The hippocampal damage was determined by counting the number of intact neurons in the stratum pyramidal within the CA1 subfield at a magnification of 20× (Nikkon E 600, digital camera DXM1200F, Nikon Corporation, Tokyo, Japan). Only neurons with normal visible nuclei were counted. The mean number of CA1 neurons per millimeter linear length for both hemispheres in sections of dorsal hippocampus was calculated for each group of animals. An observer who was unaware of the drug treatment for each rat made all assessments of histological sections.
Biochemical analysis
Seven days after ischemia, 10 rats from each group were decapitated and brains were quickly removed and hippocampus was harvested on a cold stage. Hippocampus was washed with saline, blotted dry on a filter paper, weighed and then 10% (w/v) homogenates was made in ice-cold saline.
Assay of lipid peroxidation and reduced glutathione
The degree of lipid peroxidation in the hippocampal neuronal tissue was determined by measuring thiobarbituric acid reactive substance (TBARS) in the tissue homogenate [22]. The absorbance was measured spectrophotometrically at 532 nm and the concentrations were expressed as nmol MDA/mg protein. The tissue levels of the acid soluble thiols, mainly GSH, were assayed colorimetrically at 412 nm [23]. The contents of GSH were expressed as μmol/g wet tissue.
Assay of catalase and superoxide dismutase
The catalase activity was determined spectrophotometrically by the method of Higgins et al [24]. The decrease in absorbance of hydrogen peroxide (H2O2) at 240 nm was followed. The activity was expressed as μmol/min/mg protein using the molar absorbance of 43.6 for H2O2. The superoxide dismutase assay was a slight modification of the indirect inhibition assay developed by McCord and Fridovich [25]. In this method, xanthine-oxidase (XOD) was utilized to generate a superoxide flux, which reacts with 2-(4-iodophenyl)-3(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. The absorbance obtained from INT reduction to red formazon by superoxide was measured at 505 nm.
Measurement of protein
Protein concentration was estimated by the method of Lowry et al. [26] using bovine serum albumin as a standard.
Assay of the in vitro non-enzymatic lipid peroxidation induced by iron-ascorbate in the hippocampus homogenate
Lipid peroxidation in the hippocampal tissue was determined by measuring TBARS induced by iron-ascorbate [27]. The reaction mixture contained varying amount of curcumin or butylated hydroxytoluene (BHT), 0.75 ml phosphate buffer (50 mM, pH, 7.4), 50 μl hippocampus homogenate, 0.1 ml of 1 mM ferric chloride and 0.1 ml of 1 mM ascorbic acid. The tubes were incubated at 37°C for 30 min and the extent of peroxidation was measured by thiobarbituric acid (TBA) test. To the above test tubes, the following were added, 0.1 ml butylated hydroxytoloune (2% w/v) to stop further lipid peroxidation, 1.0 ml TBA (1% w/v in 0.05 M NaOH) and 1.0 ml TCA acid (2.8%) and placed in a water bath at 90°C for 20 min. At the end of the incubation time, the tubes were cooled and centrifuged for 5 min at 5,000 g. The chromogen was extracted with 2.0 ml n-butanol and absorbance was read at 532 nm. Tubes containing reagent blank, homogenate were subjected to TBA test served as controls. Data were plotted as a percentage of inhibition.
Statistical analysis
Differences between obtained values (mean±SD) were carried out by one way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparison test. A P value of 0.05 or less was taken as a criterion for a statistically significant difference.
Results
Histopathological observations
The effects of curcumin treatment on the numbers of intact neurons in CA1 region per 1 mm of hippocampus at 7 days post 10 min of forebrain ischemia in rats are shown in Figures 1 and 2. The control and sham-operated groups revealed healthy neuronal cells (Fig. 1) amounted by ~164±2 intact neurons/1 mm (Fig. 2). Forebrain ischemia resulted in massive neuronal damage manifested as a significant (P<0.01) 77% decrease in the number of normal-appearing neurons (Figs. 1, 2). Animals treated with curcumin immediately, 3 h, and 24 h after reperfusion and continued for seven successive days resulted in a significant 3.2, 3.2, and 2.7-fold increase in the number of intact neurons, respectively as compared to ischemic group (Figs. 1, 2). However, still a significant (P<0.05) 25% decrease in the number of intact neurons when compared to the control group. The comparison of normal-appearing neurons counts between animals treated with curcumin at different times at and after reperfusion confirmed no significant difference was measured. In addition, when control animals were compared with sham-operated, and curcumin alone groups there were no significant differences (P>0.05) were observed (Figs. 1, 2).
Photomicrographs illustrate neurons within the CA1 region of the hippocampus stained with hematoxylin and eosin at magnification of 20× 7 days after transient forebrain ischemia showing the protective effect of curcumin administered at three different intervals against ischemia-mediated cell loss in the CA1 hippocampal area. (a) Coronal sections showing intact neurons in the hippocampal CA1 region of control rats; (b) Coronal sections showing intact neurons in the hippocampal CA1 region of sham-operated rats; (c) Most pyramidal cell died in the CA1 subfield 7 days following reperfusion in rats subjected to 10 min forebrain ischemia. In contrast, curcumin treatment immediately, 3 h, and 24 h after reperfusion conferred neuroprotection by markedly reducing the number of damaged pyramidal cells in the CA1 subfield (d, e, and f respectively; scale bar 50 μm)
Effects of forebrain ischemia and curcumin on the number of intact neurons in rat hippocampal CA1 region. Results are expressed as mean±SD of five rats and data were analyzed by one-way ANOVA followed by Tukey–Kramer multiple comparisons test. *Significantly different from control, sham, and ischemia + curcumin groups
Biochemical observations
Effects of curcumin treatment on oxidative stress-related biochemical changes in the hippocampus tissue after 7 days of forebrain ischemia insult
Seven days after transient forebrain ischemia insult, there was 33% decrease in hippocampal GSH level (Fig. 3), a 2-fold increase in MDA concentration (Fig. 4), a significant (P<0.05) 42% decrease in SOD activity (Fig. 5), as well as a significant (P<0.05) 37% decrease in catalase activity (Fig. 6) in comparison to control or sham-operated groups. Administration of curcumin immediately, 3 h, and 24 h after reperfusion significantly (P<0.05) returned hippocampal GSH level of ischemic rats to that of control group (Fig. 3) while increase in MDA concentration was significantly (P<0.05) prevented in animals treated with curcumin even when the treatment was delayed till 24 h after reperfusion (Fig. 4). Treatment with curcumin immediately, 3 h, and 24 h after reperfusion and continued for seven successive days resulted in a complete (P<0.05) prevention of the decrease in antioxidant enzymes (SOD and catalase) activities in the animals subjected to ischemia (Figs. 5, 6). One the other hand, there was no significant difference in oxidative stress-related biochemical parameters namely GSH, MDA, SOD and catalase activities between control and sham-operated, and curcumin alone groups.
Effect of curcumin treatments after 10 min forebrain ischemia on reduced glutathione (GSH) level in rat hippocampus. Data are presented as means±SD, (n = 10), and data were analyzed by one-way ANOVA followed by Tukey–Kramer multiple comparisons test. *Significantly different from control, sham, and ischemia + curcumin groups
Effect of curcumin treatments on malondialdehyde levels in rat hippocampus after forebrain ischemia. Results are expressed as mean±SD of 10 rats and data were analyzed by one-way ANOVA followed by Tukey–Kramer multiple comparisons test. *Significantly different from control, sham, and ischemia + curcumin groups
Effect of curcumin against Fe2+-induced lipid peroxidation in vitro
Curcumin was found to be equally potent antioxidant as BHT (standard antioxidant) in vitro. Figure 7 shows the effect of different concentrations of curcumin and BHT against lipid peroxidation induced by FeCl3-ascorbate. Both curcumin and BHT inhibited lipid peroxidation in a concentration dependent manner. The IC50 (concentration that inhibits 50% of lipid peroxidation) for curcumin and BHT were similar and calculated to be 1 μM.
Discussion
The data of present study indicated that a 10 min of forebrain ischemia insult in rat resulted in a massive damage to CA1 hippocampal neurons (greater than 75% of the neurons died) when assessed at 7 days after reperfusion (Figs. 1, 2). This degree of cell loss was similar to most published studies in rat and gerbil models of forebrain ischemia (for example [3, 8, 20, 28–32]).
Also the data of this study confirmed that massive tissue damage is directly associated with the induction of oxidative stress. In biological systems, lipid peroxidation is found to be an important cause of cell membrane destruction and neuronal death. MDA, one of the major products of lipid peroxidation, has been extensively studied and measured as an index of lipid peroxidation [33]. In the present study, during forebrain ischemia and reperfusion, there is a dramatic increase in hippocampal MDA level and was noted to be accompanied by a depletion of hippocampal GSH. This suggested that forebrain ischemia and reperfusion induced overproduction of ROS which caused hippocampal oxidative stress. These results are in harmony with several others reports [34, 35].
The current study also demonstrated that the activities of the enzymatic antioxidant (SOD and catalase) in rat hippocampal tissues were decreased dramatically after forebrain ischemia insult (Figs. 5, 6). The decrease in antioxidant enzyme activities may be explained as result of an attack of ROS to the active site of the enzymes or consumption of antioxidant enzyme by ROS. In fact, O ·−2 inhibits catalase activity [36], and H2O2 may inactivate SOD through a modification in histidine residue located in the active site of the enzyme [37]. Inactivation or decrease SOD and catalase activities due to ROS after forebrain ischemia insult may lead to extensive and later neuronal damage that occurs in the hippocampal CA1. These results are completely consistent with previously reported studies [34, 35, 38]. For example, Homi et al. [38] observed that after brief periods of transient global cerebral ischemia and reperfusion, the activities of both SOD and catalase were significantly decreased. These results suggested that the reduction of SOD and catalase activities is involved in the delayed neuronal degeneration observed in the next 48–72 h after global cerebral ischemia in hippocampal CA1 neurons.
There were two previous studies that investigated the effect of curcumin on cerebral ischemia. The first study was reported by Ghoneim et al. [39]. In this study the model of forebrain ischemia was produced by bilateral common carotid artery occlusion for 1 h, followed by reperfusion for only another 1 h. The effects of curcumin against forebrain ischemia induced neuronal damage were only investigated by assessing oxidative stress-related biochemical parameters in the rat forebrain (hippocampus CA1 which is the morst susceptible to damage after forebrain ischemia was not investigated). The period of reperfusion in this study was only 1 h, which is not enough to see any hippocampal damage after forebrain ischemia. It is well documented by several studies as mentioned in the introduction, that hippocampal injury is not observed until 3–7 days after forebrain ischemia [2–5]. Furthermore, oxidative stress-related biochemical parameters that used in this study are not enough to indicate or to exclude the damage or protection of neuron in absence of histological assessment, which is a reliable way to confirm neuronal damage or survival after ischemia. The second study was reported by Thiyagarajan and Sharma [40]. In this study, focal cerebral ischemia was induced in rat by middle cerebral artery occlusion (MCAO) for as long as 2 h followed by only 22 h of reperfusion. Focal cerebral ischemia was used in this study but not global cerebral ischemia to evaluate the neuroprotection of curcumin after 22 h of reperfusion. Also it did not investigate the selective delayed death in hippocampal CA1 pyramidal neurons. Again the period of reperfusion in this study (22 h) was not enough to see a significant hippocampal CA1 neuronal damage as mentioned before.
In the current study, curcumin administered (200 mg/kg i.p.) immediately after ischemia or at 24 h of reperfusion and continued to be administered for 7 days, resulted in a dramatic protection against forebrain ischemia-induced neuronal death of the CA1 pyramidal neurons in rats (Figs. 1, 2). This was evidenced by the fact that curcumin rescued most of CA1 pyramidal neurons from ischemic death. This is consistent with recent study that indicated that curcumin reduced oxidative stress damage and prevented changes in locomotor activity after cerebral ischemia [41]. Curcumin has been found to prevent or reduce oxidative stress-induced progression of Alzheimer’s disease [14, 15]. Also, several previous studies have demonstrated the activity of curcumin against cytotoxicity in vitro [42], and in vivo [43]. Naik et al. [41] observed that curcumin provides protection against cytological changes and lipid peroxidation induced by ethanol induced oxidative stress in the liver slices. In vivo, Shoskes [43] demonstrated that curcumin resulted in preservation of histological integrity, with a decrease in tubular damage and interstitial inflammation after renal ischemia and reperfusion injury in rats.
In our study, when ischemic rats were treated with curcumin administered (200 mg/kg i.p.) immediately after ischemia or at 24 h of reperfusion and continued to be administered for 7 days, the depletion of hippocampal GSH (Fig. 3) and increase of MDA (Fig. 4) level were prevented. This protection of curcumin may be due to the antioxidant property of curcumin. This is supported by the in vitro experiment in this study that indicated that curcumin protected rat’s hippocampal tissue against iron-induced lipid peroxidation at very low concentration. This antioxidant activity of curcumin against iron-induced lipid peroxidation was similar to BHT (standard antioxidant). This result indicated that curcumin is a powerful antioxidant against lipid peroxidation induced by iron in hippocampal tissue. Also, similar effects of curcumin against elevation of MDA level and depletion of GSH were observed in myocardial ischemia in rats [44]. Curcumin was able to decrease the levels of xanthine oxidase, O ·−2 and lipid peroxides and protect rat myocardium damage-induced by ischemic insult.
In biological systems, O ·−2 is inactivated mainly by SOD while H2O2 is decomposed to water by catalase. GSH also participates in the reductive detoxification of H2O2. During the ischemia and reperfusion, ROS cannot be readily scavenged because of low activities of SOD and catalase as well as the low level of GSH in the brain [45]. In the present study, the treatment with curcumin, immediately or delayed after 24 h of forebrain ischemia, markedly suppressed the declined SOD and catalase activities in the hippocampal tissue. The protection of curcumin against oxidative stress caused by forebrain ischemia insult could be carried out by enhancing the cerebral activity of SOD, catalase, GSH, and perhaps by scavenging and preventing ROS generation.
Our results indicated that no significant modification in hippocampal GSH, lipid peroxidation, and enzymatic antioxidant activities (SOD and catalase) were observed in rats treated with curcumin alone. These results suggest that administration of curcumin dose not modify the basal GSH contents, basal level of lipid peroxidation as well as the activities of both SOD and catalase in the rat hippocampus.
Finally, histological and biochemical assessment showed that curcumin with a dose of 200 mg/kg attenuated hippocampal neuronal damage induced by transient forebrain ischemia. This dose in rats is equivalent to approximately a total dose of 2.5 g in adult man weighing 70 kg taking in consideration the differences in surface areas and weights between the two species [46]. Curcumin is relatively safe in human. Indeed, it was administered in human at a dose of 8 g/day for 3 months without noticeable side effects [47].
The results of the present study indicated that curcumin confers protection against transient forebrain ischemia insult by attenuating forebrain ischemia-induced neuronal injury and oxidative stress in hippocampal tissue even when the treatment was delayed by 24 h. It is concluded that curcumin with the protective time window up to 24 h after reperfusion, is clinically beneficial in the in cases of transient forebrain ischemia that result from resuscitated cases of cardiac arrest with resuscitation. If curcumin provides neuroprotection against forebrain ischemia in humans, as seen in rats, curcumin treatment would act to save a number of patients from forebrain ischemia damage.
References
Feigin VL, Lawes CMM, Bennett DA, Anderson CS (2003) Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. The Lancet Neurol 2:43–53
Wolfe CD (2000) The impact of stroke. Br Med Bull 56:275–286
Colbourne F, Li H, Buchan AM, Clemens JA (1999) Continuing postischemic neuronal death in CA1: influence of ischemia duration and cytoprotective doses of NBQX and SNX-111 in rats. Stroke 30:662–668
Rami A, Agarwal R, Botez G, Winckler J (2000) mu-Calpain activation, DNA fragmentation, and synergistic effects of caspase and calpain inhibitors in protecting hippocampal neurons from ischemic damage. Brain Res 866:299–312
Barber PA, Auer RN, Buchan AM, Sutherland GR (2001) Understanding and managing ischemic stroke. Can J Physiol Pharmacol 79:283–296
Candelario-Jalil E, Ajamieh HH, Sam S, Martínez G, Leon OS (2000) Nimesulide limits kainate-induced oxidative damage in the rat hippocampus. Eur J Pharmacol 39:295–298
Liu PK, Grossman RG, Hsu CY, Robertson CS (2001) Ischemic injury and faulty gene transcripts in the brain. Trends Neurosci 24:581–588
Candelario-Jalil E, Mhadu NH, Ai-Dalain SM, Martínez G, Leon OS (2001) Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci Res 41:233–241
Chan PH (1996) Role of oxidants in ischemic brain damage. Stroke 27:1124–1129
Reddy AP, Lokesh BR (1994) Effect of dietary turmeric (Curcuma longa) on iron-induced lipid peroxidation in the rat liver. Food Chem Toxicol 32:279–283
Araujo CC, Leon LL (2001) Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz 96:723–728
Zhao BL, Li XJ, He RG, Cheng SJ, Xin WJ (1989) Scavenging effect of extracts of green tea and natural antioxidants on active oxygen radicals. Cell Biophys 14:175–185
Li JK, Lin-shia SY (2001) Mechanisms of cancer chemoprevention by curcumin. Proc Natl Sci Counc Repub China B 25:59–66
Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JLA (2005) Potential role of the curry spice curcumin in Alzheimer’s disease. Curr Alzheimer Res 2:131–136
Cole GM, Lim GP, Yang F, Teter B, Begum A, Ma Q, Harris-White ME, Frautschy SA (2005) Prevention of Alzheimer’s disease: omega-3 fatty acid and phenolic anti-oxidant interventions. Neurobiol Aging 2:133–136
Huang MT, Newmark HL, Frenkel K (1997) Inhibitory effects of curcumin on tumorigenesis in mice. J Cell Biochem 27:26–34
Plummer SM, Holloway KA, Manson MM, Munks RJL, Kaptein A, Farrow S, Howells L (1999) Inhibition of cyclo-oxygenase-2 expression in colon cells by the chemoprevention agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signaling complex. Oncogene 18:6013–6020
Skrzypczak-Jankun E, McCabe NP, Selman SH, Jankun J (2000) Curcumin inhibits lipoxygenase by binding to its central cavity: theoretical and X-ray evidence. Int J Mol Med 6:521–526
Smith ML, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjo BK (1984) Models for studying long-term recovery following forebrain ischemia in the rat, II: a 2-vessel occlusion model. Acta Neurol Scand 69:385–401
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–69
Henrich-Noack P, Prehn JHM, Krieglstein J (1996) TGF-β1 protects hippocampal neurons against degeneration caused by transient global ischemia: dose response relationship and potential neuroprotective mechanisms. Stroke 27:1609–1615
Ohkawa H, Ohish N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid. Anal Biochem 95:351–358
Ellman GL (1959) Tissue sulfahydryl groups. Arch Biochem Biophys 82:70–77
Higgins C, Bachner R, McCallster J, Boxer L (1978) Polymorphonucler leukocyte species differences in the disposal of hydrogen peroxide. Proc Soc Exp Biol Med 158:478–481
McCord J, Fridovich I (1969) An enzymatic function for erythrocuprein (Hemocuprein). J Biol Chem 244:6049–6055
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Houghton PJ, Zarka R, de las Heras B, Hoult JR (1995) Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med 61:33–36
Knuckey NW, Palm D, Primiano M, Epstein MH, Johanson CE (1995) N-acetylcysteine enhances hippocampal neuronal survival after transient forebrain ischemia in rats. Stroke 26:305–311
Al Nita D, Nita V, Spulber S, Moldovan M, Popa DP, Zagrean AM, Zagrean L (2001) Oxidative damage following cerebral ischemia depends on reperfusion-a biochemical study in rat. J Cell Mol Med 5:163–170
Nanri K, Montecot C, Springhetti V, Seylaz J, Pinard E (1998) The selective inhibitor of neuronal nitric oxide synthase, 7-nitroindazole, reduces the delayed neuronal damage due to forebrain ischemia in rats. Stroke 29:1248–1254
Chandrasekaran K, Mehrabian Z, Spinnewyn B, Drieuand K, Fiskum G (2001) Neuroprotective effects of bilobalide, a component of the Ginkgo biloba extract (EGb 761), in gerbil global brain ischemia. Brain Res 922:282–292
Shen H, Zhang L, Yuen D, Logan R, Jung BP, Zhang G, Eubanks JH (2002) Expression and function of A1 adenosine receptors in the rat hippocampus following transient forebrain ischemia. Neuroscience 114:547–556
Fuh KC, Meneshian A, Patel CB, Takiar V, Bulkley GB (2002) Signal transduction by reactive oxygen species: alternative paradigms for signaling specificity. Surgery 131:601–612
Candelario-Jalil E, Alvarez D, Merino N, Sonia Leon O (2003) Delayed treatment with nimesulide reduces oxidative stress following global ischemic brain injury in gerbils. Neurosci Res 47:245–253
Al-Majed AA (2004) Aminoguanidine prevents oxidative stress insult following transient forebrain ischemia in the rat hippocampus. Saudi Pharmaceutic J 12:150–156
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 25:5751–5754
Tokuda Y, Uozumi T, Kawassaki T (1993) The superoxide dismutase activities of cerebral tissues, assayed by the chemiluminescence method, in the gerbil focal ischemia/reperfusion and global ischemia models. Neurochem Int 23:107–114
Homi HM, Freitas JJ, Curi R, Velasco IT, Junior BA (2002) Changes in superoxide dismutase and catalase activities of rat brain regions during early global cerebral transient ischemia/reperfusion. Neurosci Lett 333:37–40
Ghoneim AI, Abdel-naim AB, Khalifa AE, El-denshary ES (2002) Protective effects of curcumin against ischemia/reperfusion insult in rat forebrain. Pharmacol Res 46:273–279
Thiyagarajan M, Sharma SS (2004) Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci 74:969–985
Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY (2005) Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res 82:138–148
Naik RS, Mujumdar AM, Ghaskadbi S (2004) Protection of liver cells from ethanol cytotoxicity by curcumin in liver slice culture in vitro. J Eethanopharmacol 95:31–37
Shoskes DA (1998) Effect of bioflavonoids quercetin and curcumin on ischemic renal injury: a new class of renoprotective agents. Transplantation 27:147–152
Manikandan P, Sumitra M, Aishwarya S, Manohar BM, Lokanadam B, Puvanakrishnan R (2004) Curcumin modulates free radical quenching in myocardial ischaemia in rats. Int J Biochem Cell Biol 36:1967–1980
Sinet PM, Heikkila RE, Cohen G (1980) Hydrogen peroxide production by rat brain in vivo. J Neurochem 34:1421–1428
Paget GE, Barnes JM (eds) (1964) In evaluation of drug activities: pharmacometrics. Academic press, New York and London
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY (2001) Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21:2895–2900
Acknowledgements
This work was supported by operating grant from King Abdulaziz City for Science & Technology (KACST; LP-8-49).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Omar, F.A., Nagi, M.N., Abdulgadir, M.M. et al. Immediate and Delayed Treatments with Curcumin Prevents Forebrain Ischemia-Induced Neuronal Damage and Oxidative Insult in the Rat Hippocampus. Neurochem Res 31, 611–618 (2006). https://doi.org/10.1007/s11064-006-9059-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9059-1