Abstract
Background
Most of the current knowledge on the clinical effects of stereotactic radiosurgery (SRS) on the treatment of cavernous sinus meningiomas (CSM) is based on series with limited follow-up. However, determining the role of radiation in a tumor with slow disease progression such as CSM necessitates long term follow up.
Objective
To review and pool metadata in the literature to determine the long-term outcomes of SRS with respect to clinical and radiographic tumor control of CSM.
Methods
A systematic search was conducted following MOOSE guidelines. Results were screened against predefined criteria, which excluded studies with a median follow-up less than 5 years. The incidences of each outcome were calculated using random-effects metanalysis of proportions.
Results
Seven studies met the inclusion criteria, comprising 645 patients. The median follow-up was 74 months (range 62–87). Progression-free-survival at 5, 10, and 15 years was 93.4% (95% CI 89.1–96.7%), 84.9% (95% CI 77–91.4%), and 81.3% (95% CI 74–87.7%), respectively. Clinical response to SRS at last follow-up defined as improvement of cranial nerve deficits was found in in 36.4% (95% CI 26.3–47.1%) of patients, while worsening or onset of new cranial nerve deficits was found in 11.5% (95% CI 7.9–15.7%). Radiological regression was found in 57.8% (95% CI 43–71.8%), while tumor progression was found in 8.5% (95% CI 5.2–12.6%).
Conclusion
SRS achieves excellent disease control and radiographic response in CSM. Although the risk of long-term cranial neuropathies is minimal, it is relatively higher to what has been previously reported in early series with limited follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cavernous sinus meningiomas (CSM) represent less than 0.5% of all intracranial tumors and about 10% of all skull base meningiomas [1]. CSM may arise from within the sinus itself or may originate from adjacent structures (e.g. sphenoid ridge, petroclival region) and invade the sinus secondarily [1, 2]. Although complete resection is the preferred strategy for most intracranial meningiomas, pursuing such goal in CSM assumes an elevated morbidity [1, 2]. Stereotactic radiosurgery (SRS) was introduced as an appealing alternative in the treatment of CSM, either as adjuvant therapy post operatively or as a first-line treatment [3]. Earlier studies on SRS have reported satisfactory rates of tumor control and good functional outcomes in patients with CSM [4,5,6,7,8]. Nevertheless, these studies are largely limited by a short follow up and a considerable influence of attrition bias [6, 9].
Considering the slow growth and the relatively benign behavior of meningiomas, the current medical landscape requires an up-to-date analysis of the role of SRS in the treatment CSM based on 10–20-years data. Hereby, we propose a systematic review and metanalysis of the studies reporting local tumor control and clinical outcomes at long term follow up after monofractionated radiosurgical treatment [either gamma knife (GK) RS or linear accelerator (Linac) RS], focusing our attention on three aspects:
-
(I)
Progression free survival (PFS),
-
(II)
Radiological progression/regression, and
-
(III)
Improvement/worsening/new onset of cranial neuropathies.
Methods
The literature review was performed in accordance to the recommendations by the Preferred Reporting Items for Meta-analyses Of Observational Studies in Epidemiology (MOOSE) [10]. A systematic search was performed using PubMed/Medline, SCOPUS and Cochrane databases from inception to March 2020. The literature search was performed by two independent investigators (RMP, WFP), using combinations of the following search terms: “radiosurgery”, “Gamma Knife’, “Cyber Knife”, “meningioma”, “stereotactic”, “cavernous”, “sinus”, “parasellar”, “long-term”, “outcome”.
Selection criteria
Predetermined criteria defined the following requirements to include a study in the analysis: (i) randomized controlled trial, observational study, or retrospective case series of CSM treated with any of the two types of monofractionated radiosurgical therapy [either gamma knife (GK) RS or linear accelerator (Linac) RS]; (ii) the median follow up of the study must be superior to 60 months (5 years); (iii) and studies must have reported quantitative data in regards to clinical and radiological outcomes. Studies including other treatment alternatives with data indiscernible from those treated with SRS were not selected for further analysis.
Data abstraction
Two independent and blinded reviewers (RMP and WFP) extracted data from eligible studies. Any inconsistency between both reviewers were clarified through consensus. The variables of abstraction include the following: author, years of enrollment, publication year, location, study design, number of patients, median follow-up, median age, mean or median tumor volume, median marginal radiation dose, and prior surgical resection. The primary endpoint of this metanalysis was PFS at 5, 10, and 15 years. Secondary endpoints included clinical outcomes (improvement of prior cranial neuropathies and presence of new onset or worsening of cranial neuropathies at last follow up) and radiological outcomes (reported radiological progression and regression rates). Radiological progression and regression rates were directly subtracted from each report. In cases where such rates were not explicitly reported, radiological regression was defined as any volume reduction between the pre-radiation state and the last follow up. Radiological progression was defined as any increase in size between the initial CT or MRI study prior receiving radiation therapy and the last scan performed at the end of the follow-up.
Methodological quality and bias assessment
Publication bias was evaluated across funnel plots. Methodological quality was evaluated by two investigators (RMP and WFP) using the ROBINS-I tool [11]. The quality of the evidence and certainty of assessment were evaluated through GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach [12].
Statistical analysis
Primary and secondary endpoints were pooled by metanalysis of proportions using the random-effects (RE) model [13]. PFS and clinical and radiological outcomes were calculated with Fisher’s exact test for binomial data, and then transformed using Freeman-Tukey Transformation to stabilize the variances. Heterogeneity was assessed with the Higgins I-square statistic, where an I2 greater than 50% indicated significant heterogeneity. Forest plots were used to graphically display the effect size in each study and the pooled estimates. A p-value inferior to 0.05 was considered significant. MedCalc v.19.03 (MedCalc Software Ltd, Ostend, Belgium) software was used for all analyses.
Results
Search results
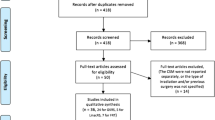
The primary search yielded 255 results. After removal of 16 duplicates, the title and abstract of 239 articles were evaluated against selection criteria. Full-text analysis was performed for 26 articles (Fig. 1). Seven studies met pre-determined eligibility criteria and were included in the meta-analysis [9, 14,15,16,17,18,19] (Table 1).
Demographic and clinical features
Six-hundred-forty-five patients with CSM that underwent SRS were reported with adequate data regarding tumor control (PFS) at 5 years, and 543 were reported with this data at 10 and 15 years. Data regarding cranial nerve deficits were detailed in 553 (Table 1). The median age included across studies was 52 (range 50–57), while the median female proportion was 77% (range 71–84). The median follow-up time was 74 months (range 62–87).
Regarding the radiosurgical parameters, the median average dose was 13.5 Gy (range 12–16), and the median tumor volume was 7.2 cc (range 5.9–14). The overall median proportion of patients who underwent a prior surgical treatment was 40% (range 29.4–60).
Tumor control
The pooled incidence of PFS at 5, 10, and 15 years was 93.4% (95% CI 89.1–96.7%; (p < 0.001, I2 = 74.5%), 84.9% (95% CI 77–91.4%; p < 0.001, I2 = 82.3%), and 81.3% (95% CI 74–87.7%; p = 0.02, I2 = 69.4%), respectively (Fig. 2).
Clinical and radiological outcomes
Clinical improvement was approximately twice more likely in patients who received SRS as a primary treatment, in comparison to those who received SRS as an adjuvant treatment after microsurgical resection, according to 4 studies included in this work [15, 17,18,19] (Table 2). Trigeminal nerve and cranial nerves involved in extraocular movements (third, fourth, and sixth nerves) are the most commonly involved (between 8–39%, and 30–62%, respectively), however, they are more likely to improve after receiving SRS (25–76% in trigeminal nerve function and 24 to 50% in oculomotor/trochlear/abducens nerve function) (Table 2). Rate of worsening or new deficits after SRS varies among 0 and 11% in oculomotor, trochlear, and abducens nerve, between 3 and 16% in trigeminal nerve, and between 3 and 9% in the optic nerve (Table 2).
The pooled incidence of improvement of cranial nerve deficit was 36.4% (95% CI 26.3–47.1%; p = 0.02, I2 = 59.8%). The pooled incidence of worsening or new cranial nerve deficit was 11.5% (95% CI 7.9–15.7%; p < 0.01, I2 = 66.3%) (Fig. 3a, b).
In the same population, the pooled incidence of radiological progression was 8.5% (95% CI 5.2–12.6%; p < 0.01, I2 = 66.3%), while the pooled incidence of radiological regression was 57.8% (95% CI 43–71.8%; p < 0.01, I2 = 93.1%) (Fig. 3c, d).
Quality and bias assessment
Funnel plots showed fair symmetry in pretty much all outcomes assessed in our study, which represent minimal publication bias (Figs. 4 and 5). Likewise, the quality of the evidence was evaluated for all endpoint against the GRADE criteria (Table 3). Certainty ranged from low to very low for all outcomes assessed, as expected given the limited quality of retrospective observational studies. The risk of bias was evaluated for all included studies using the ROBINS-I tool (Fig. 6). Overall, the risk of bias was low to moderate in 80% of the domains assessed. One of the studies [14] showed critical risk of bias in classification of interventions, as per the poor description of additional treatments (microsurgical resection) prior to receiving the radiosurgical treatment.
Discussion
The results of the present review provides evidence for the efficacy of SRS for treating CSM from a long-term perspective. Median PFS at 5, 10, and 15 years was 93% (IC 95% 89.1–96.7), 84% (IC 95% 76.9–91.3), and 81% (IC 95% 73.9–87.6), respectively. With a median follow-up of 7.5 months, radiological regression occurred in 58% (IC 95% 43–71.8) of cases, while 8.5% (IC 95% 5.2–12.6) experienced some degree of radiological progression. Our metadata also suggest that, although minimal, the risk of complications is not negligible and may include cranial neuropathy, vascular injury, and pituitary insufficiency [9, 14, 18, 19]. Incidence of these outcomes varied from rare to low and, taken together, are more infrequent than previously reported after surgical treatment [20,21,22,23,24]. Eleven percent (IC 95% 7.9–15.7) of patients with CSM experienced new onset of cranial neuropathies or worsening of prior cranial nerve deficits, while 36.4% (IC 95% 26.3–47.1) of those with prior deficits experienced some degree of improvement.
Tumor growth control and radiological outcomes
Tumor growth control rate and radiological outcomes did not significantly differ from prior studies with a shorter follow up. These medium-term series have reported PFS ranging between 84 and 100% and radiological tumor regression between 31 and 61% at 5 years [5,6,7,8,9, 25, 26]. Another study reported SRS induced tumor regression twice as frequently as that associated with fractioned radiotherapy [6] (52 vs 20% respectively), while it observed tumor progression in less than 6% of all cases.
Our results corroborate the excellent rates of disease control experienced by early series, and therefore reinforce the thesis that tumor growth, if any, usually occurs within the first two years after radiation [9]. In a cohort of 86 patients with meningiomas treated using GKRS with a prolonged follow-up, the authors observed that the time-to-recurrence occurred at a median of 5.8 years. Although the mentioned study provides equivalent conclusions about the long-term efficacy of radiosurgery for treating intracranial meningiomas, our metadata analysis suggests that CSM is a more aggressive subgroup with a shorter time to recurrence, as it has been previously described by others [23, 28]
Cranial nerve outcomes
Improvement in cranial nerve deficits occurs in about one third of cases, whereas new deficits or worsening of existing cranial nerve impairment is reported to be one in every ten patients. These numbers are consistent across the studies included in this metanalysis, although they are less encouraging than those reported by other series with a shorter follow up [6, 26, 29]. In the review by Leroy and colleagues including series with a mean follow up of 48 months, the authors observed improvement in 54% of patients with trigeminal nerve impairment, in 21% with a decreased visual acuity, and in 45% with extraocular movements deficits. Pollock et al. observed that cranial neuropathies can occur as late as 148 months after radiation [18]. These observations attest to the cumulative toxic effects of radiation on cranial nerves over time that should be considered by the practitioner when approaching a cavernous sinus lesion. Other factors that have been suggested to contribute to clinical outcomes and development of post radiosurgery neuropathies is the radiation dose and the history of a previous surgery [9, 17, 18, 30]. Clinical improvement was approximately twice more likely in patients who received SRS as a primary treatment, in comparison to those who received SRS as an adjuvant treatment after microsurgical resection, according to 4 studies included in this work [15, 17,18,19]. However, none of them demonstrated that prior surgical resection has a negative impact when other factors, such as the initial tumor volume, was included in a multivariable analysis [16]. Although the risk of recurrence is increased at a lower dose and with larger radiation volumes, it is also widely accepted that radiation dose and volume radiated are directly correlated with the risk of developing complications, including cranial neuropathies [16, 18]. Trigeminal nerve and cranial nerves involved in extraocular movements (third, fourth, and sixth nerves) are the most commonly involved (between 8–39%, and 30–62%, respectively), however, they are more likely to improve after receiving SRS (25–76% in trigeminal nerve function and 24–50% in oculomotor/trochlear/abducens nerve function) It is important to emphasize the relative resistance to radiation of the cranial nerves included in the cavernous sinus [6, 14, 16, 19, 30, 31]. Rate of worsening or new deficits after SRS varies among 0 and 11% in oculomotor, trochlear, and abducens nerve, between 3 and 16% in trigeminal nerve, and between 3 and 9% in the optic nerve. In this sense, dosage superior to 8–9 Gy has been shown to be associated with a significant risk of developing radiation induced optic neuropathy, whereas most authors have suggested that marginal doses superior to 12 Gy are needed to achieve long-term tumor control in CSM [9, 18, 30, 32,33,34]. On the other hand, marginal doses superior to 15 Gy have not been shown to provide additional benefit in terms of tumor control, while it is associated with an increased risk of radionecrosis, radiation-induced tumorigenesis, and cranial neuropathies [27, 35]. Any treatment alternative aims to achieve tumor control while causing minimal damage to neural structures and thus less impairment of neurological function. To that end, SRS should be considered a good alternative when the meningioma is confined to the cavernous sinus and secondarily affecting third, fourth, fifth, and/or sixth cranial nerves. Notwithstanding, its use should be limited when the tumor is extending beyond these limits and affects the optic nerve, as the dosage requirements for achieving tumor control barely match the safety threshold in this area.
Previous systematic reviews
To date, this is the first metanalysis assessing the safety and effectiveness of radiosurgery in a large subset of patients harboring CSM. A previous systematic review assessing the clinical outcomes of radiosurgery and fractioned radiotherapy on CSM showed similar and consistent results in terms of PFS and associated morbidity [6]. However, the authors used a non-combined analysis of the published data to draw conclusions about the role of radiosurgery in CSM [36]. Despite being the first systematic review assessing the role of SRS in CSM, the employed methodology was not sufficient to ascertain whether the positive effects of radiosurgery would be perdurable after an adequate period of observation. In 1957, Simpson reported that, even after satisfactory resections, late recurrence is not an extraordinary misfortune in patients harboring intracranial meningiomas [37]. Up to 75% of the included studies in the review by Leroy and colleagues have a follow-up inferior to 5 years [6]. Notwithstanding, the slow-growing natural history of most of meningiomas does not allow for the drawing of accurate conclusions relative to tumor control when follow up is inadequate. Spiegelman et al. [19] reported that tumor recurrence can occur as late as 84 months. Median time-to-recurrence in patients with meningiomas treated with SRS varies between 5 and 7.5 years among series. Hence, we established the threshold of 60 months as the minimum follow-up that is required to attain reliable data on the efficiency of radiosurgery on CSM.
Limitations and future directions
Despite the thorough analysis and the relatively low risk of bias, the present metanalysis has some potential limitations. First, all included studies were observational and retrospective in nature. As a result, the overall quality of evidence varied between low and very low. Beyond the apparent need for large prospective series, one of the pitfalls remains the lack of a standardized dose regimen (mean dose ranges from 12 to 16 Gy), as well as surveillance protocols (waiting time between surgery and radiation, surgery plus radiosurgery vs radiosurgery alone), which contribute to the high degree of heterogeneity observed in this metanalysis. Similarly, there is a vague definition of ‘radiological tumor regression’. While some authors have suggested using a reduction in at least 50% of the tumor volume [1], it has been defined by others as any decrease in the tumor size [16]. One way or another, most of the studies included in this analysis failed to define this variable, and data extraction is subject to authors’ interpretation [9, 14, 17,18,19]. As expected, there is a moderate number of follow-up losses in some of the included studies [14, 15]. Moreover, all except one study excluded atypical or anaplastic meningiomas from their analysis [9], while others just exclude them or simply do not provide information. Certainly, one of the major pitfalls of the studies considered in this metanalysis is the lack of information about the histopathological features of the meningiomas treated with radiosurgery. In many of the patients included, SRS is administered primarily and therefore, the histopathological grade and origin is unknown. Atypical meningiomas might represent up to one third of meningioma cases according to recently updated diagnostic cr criteria [38]. Thus, it would result reasonable to hypothesize that, at least, part of the treatment early failures is due to the fact that patients with WHO grade 2 and 3 meningiomas might be getting treated primarily with SRS. This observation is key when discussing treatment options and it should be taken into account when comparing results with surgical series, as most of them differentiates outcomes between different grades of meningiomas,
Although most of the included studies reported the rate of cranial nerve deficits shortly after receiving radiosurgery, a few reported worsening or new cranial nerve deficits during variable follow-up periods (Table 2). Considering that the effect of radiation on cranial nerves does accrue over a relatively long time even after a period of no or minimal symptoms, it is considerably likely that this complication is under reported [14, 39, 40]. In addition, there exists a large hetereogeneity among the studies when reporting individual cranial nerve outcomes after radiation and this information is commonly missing or incomplete (Table 2). Hence, until a prospective control trial can be conducted, the patient outcomes and incidence of complications following radiosurgery are highly subject to systematic bias, potentially resulting in over-statement of benefit and under-estimation of risk. Finally, the risk of publication bias is considered to be low, as per the results obtained in the funnel plots of all the analyzed outcomes. However, the high heterogeneity and the limited number of studies included in this metanalysis warrants future metanalysis in order to ascertain the validity of our results.
Conclusions
SRS achieves long term tumor control in the majority of patients, at a similar rate to preliminary series with more limited data. Nevertheless, the risk of neuropathies, although still minimal, is superior to what has been previously reported. Similarly, the rate of improvement in cranial nerve neuropathies at long term follow up is not as optimistic as was concluded in early radiosurgical retrospective reports with shorter follow up. The results of this work are a compelling evidence that SRS, either as a single treatment or as a co-adjuvant therapy, is a valid alternative in the treatment of CSM.
Data availability
This manuscript has not been previously published in whole or in part or submitted elsewhere for review.
References
Di Maio S, Ramanathan D, Garcia-Lopez R et al (2012) Evolution and future of skull base surgery: the paradigm of skull base meningiomas. World Neurosurg 78:260–275. https://doi.org/10.1016/j.wneu.2011.09.004
Martínez-Pérez R, Silveira-Bertazzo G, Rangel GG et al (2019) The historical perspective in approaches to the spheno-petro-clival meningiomas. Neurosurg Rev. https://doi.org/10.1007/s10143-019-01197-y
Lunsford LD, Flickinger J, Lindner G, Maitz A (1989) Stereotactic radiosurgery of the brain using the first United States 201 cobalt-60 source gamma knife. Neurosurgery 24:151–159. https://doi.org/10.1227/00006123-198902000-00001
Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD (2003) Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys 56:801–806. https://doi.org/10.1016/s0360-3016(03)00126-3
Lee JYK, Niranjan A, McInerney J et al (2002) Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg 97:65–72. https://doi.org/10.3171/jns.2002.97.1.0065
Leroy H-A, Tuleasca C, Reyns N, Levivier M (2018) Radiosurgery and fractionated radiotherapy for cavernous sinus meningioma: a systematic review and meta-analysis. Acta Neurochir (Wien) 160:2367–2378. https://doi.org/10.1007/s00701-018-3711-9
Litré CF, Colin P, Noudel R et al (2009) Fractionated stereotactic radiotherapy treatment of cavernous sinus meningiomas: a study of 100 cases. Int J Radiat Oncol Biol Phys 74:1012–1017. https://doi.org/10.1016/j.ijrobp.2008.09.012
Nicolato A, Foroni R, Alessandrini F et al (2002) Radiosurgical treatment of cavernous sinus meningiomas: experience with 122 treated patients. Neurosurgery 51:1153–1159. https://doi.org/10.1097/00006123-200211000-00009 (discussion 1159–1161)
Skeie BS, Enger PØ, Skeie GO et al (2010) Gamma knife surgery of meningiomas involving the cavernous sinus. Neurosurgery 66:661–669. https://doi.org/10.1227/01.NEU.0000366112.04015.E2
Stroup DF (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283:2008. https://doi.org/10.1001/jama.283.15.2008
Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. https://doi.org/10.1136/bmj.i4919
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. https://doi.org/10.1016/0197-2456(86)90046-2
dos Santos MA, de Salcedo JBP, Gutiérrez Diaz JA et al (2011) Long-term outcomes of stereotactic radiosurgery for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 81:1436–1441. https://doi.org/10.1016/j.ijrobp.2010.07.2002
Hasegawa T, Kida Y, Yoshimoto M et al (2007) Long-term outcomes of Gamma Knife surgery for cavernous sinus meningioma. J Neurosurg 107:745–751. https://doi.org/10.3171/JNS-07/10/0745
Hung Y-C, Lee C-C, Guo W-Y et al (2019) Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas: post-treatment long-term clinical outcomes, complications, and volume changes. J Neurooncol 143:261–270. https://doi.org/10.1007/s11060-019-03090-6
Metellus P, Regis J, Muracciole X et al (2005) Evaluation of fractionated radiotherapy and gamma knife radiosurgery in cavernous sinus meningiomas: treatment strategy. Neurosurgery 57:873–886. https://doi.org/10.1227/01.neu.0000179924.76551.cd (discussion 873–886)
Pollock BE, Stafford SL, Link MJ et al (2013) Single-fraction radiosurgery of benign cavernous sinus meningiomas. J Neurosurg 119:675–682. https://doi.org/10.3171/2013.5.JNS13206
Spiegelmann R, Cohen ZR, Nissim O et al (2010) Cavernous sinus meningiomas: a large LINAC radiosurgery series. J Neurooncol 98:195–202. https://doi.org/10.1007/s11060-010-0173-1
Abdel-Aziz KM, Froelich SC, Dagnew E et al (2004) Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes. Neurosurgery 54:1375–1383. https://doi.org/10.1227/01.neu.0000125542.00834.6d (discussion 1383–1384)
De Jesús O, Sekhar LN, Parikh HK et al (1996) Long-term follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery 39:915–919. https://doi.org/10.1097/00006123-199611000-00005 (discussion 919–920)
DeMonte F, Smith HK, Al-Mefty O (1994) Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg 81:245–251. https://doi.org/10.3171/jns.1994.81.2.0245
Gozal YM, Alzhrani G, Abou-Al-Shaar H et al (2019) Outcomes of decompressive surgery for cavernous sinus meningiomas: long-term follow-up in 50 patients. J Neurosurg 132:380–387. https://doi.org/10.3171/2018.10.JNS181480
O’Sullivan MG, van Loveren HR, Tew JM (1997) The surgical resectability of meningiomas of the cavernous sinus. Neurosurgery 40:238–244 (discussion 245–247)
Kimball MM, Friedman WA, Foote KD et al (2009) Linear accelerator radiosurgery for cavernous sinus meningiomas. Stereotact Funct Neurosurg 87:120–127. https://doi.org/10.1159/000204910
Roche PH, Régis J, Dufour H et al (2000) Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg 93(Suppl 3):68–73. https://doi.org/10.3171/jns.2000.93.supplement
Lippitz BE, Bartek J, Mathiesen T, Förander P (2020) Ten-year follow-up after Gamma Knife radiosurgery of meningioma and review of the literature. Acta Neurochir (Wien). https://doi.org/10.1007/s00701-020-04350-5
Mathiesen T, Lindquist C, Kihlström L, Karlsson B (1996) Recurrence of cranial base meningiomas. Neurosurgery 39:2–7. https://doi.org/10.1097/00006123-199607000-00002 (discussion 8–9)
Zeiler FA, McDonald PJ, Kaufmann AM et al (2012) Gamma Knife radiosurgery of cavernous sinus meningiomas: an institutional review. Can J Neurol Sci 39:757–762. https://doi.org/10.1017/s0317167100015572
Leavitt JA, Stafford SL, Link MJ, Pollock BE (2013) Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 87:524–527. https://doi.org/10.1016/j.ijrobp.2013.06.2047
Morita A, Coffey RJ, Foote RL et al (1999) Risk of injury to cranial nerves after Gamma knife radiosurgery for skull base meningiomas: experience in 88 patients. J Neurosurg 90:42–49. https://doi.org/10.3171/jns.1999.90.1.0042
Girkin CA, Comey CH, Lunsford LD et al (1997) Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology 104:1634–1643. https://doi.org/10.1016/s0161-6420(97)30084-0
Leber KA, Berglöff J, Langmann G et al (1995) Radiation sensitivity of visual and oculomotor pathways. Stereotact Funct Neurosurg 64(Suppl 1):233–238. https://doi.org/10.1159/000098784
Pan DH, Guo WY, Chung WY et al (1995) Early effects of Gamma knife surgery on malignant and benign intracranial tumors. Stereotact Funct Neurosurg 64(Suppl 1):19–31. https://doi.org/10.1159/000098761
Kondziolka D, Flickinger JC, Perez B (1998) Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery 43:405–413. https://doi.org/10.1097/00006123-199809000-00001 (discussion 413–414)
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012. https://doi.org/10.1016/j.jclinepi.2009.06.005
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20:22–39. https://doi.org/10.1136/jnnp.20.1.22
Pearson BE, Markert JM, Fisher WS et al (2008) Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus 24:E3. https://doi.org/10.3171/FOC/2008/24/5/E3
Ding D, Starke RM, Sheehan JP (2014) Treatment paradigms for pituitary adenomas: defining the roles of radiosurgery and radiation therapy. J Neurooncol 117:445–457. https://doi.org/10.1007/s11060-013-1262-8
Johnson S, Kano H, Faramand A et al (2019) Long term results of primary radiosurgery for vestibular schwannomas. J Neurooncol 145:247–255. https://doi.org/10.1007/s11060-019-03290-0
Funding
This study did not receive any funding relative to its elaboration.
Author information
Authors and Affiliations
Contributions
Conception and design of study: RM-P, acquisition of data: RM-P, WF-P; analysis and/or interpretation of data: RM-P, WF-P. Drafting the manuscript: RM-P, TU, LF; revising the manuscript critically for important intellectual content: SY; Approval of the version of the manuscript to be published: RM-P, WF-P, TU, LF, SY.
Corresponding authors
Ethics declarations
Conflict of interest
ASY is a consultant for Stryker Corp and has received honorarium from Mizuho America.
Ethical approval
Ethical approval was not deemed necessary by the local ethics in view of the design of the study (metanalysis).
Informed consent
Informed consent was not deemed necessary by the local ethics in view of the design of the study (metanalysis).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martinez-Perez, R., Florez-Perdomo, W., Freeman, L. et al. Long-term disease control and treatment outcomes of stereotactic radiosurgery in cavernous sinus meningiomas. J Neurooncol 152, 439–449 (2021). https://doi.org/10.1007/s11060-021-03732-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03732-8