Abstract
Purpose
Stereotactic radiosurgery (SRS) is an effective and less invasive therapeutic option for cavernous sinus (CS) tumors. However, its long-term effectiveness and neurological outcomes have yet to be fully elucidated. We aimed to examine the long-term outcomes of SRS for CS tumors.
Methods
Overall, a cohort of 113 patients with benign CS tumors, including 91 with meningioma, 14 with trigeminal schwannoma (TS), and eight with cavernous hemangioma, treated with SRS at our institution from 1990 to 2018, was included. Tumor control and functional preservation/recovery were evaluated in detail.
Results
The median post-SRS follow-up period was 77 months (interquartile range, 39–177). Progression-free survival (PFS) was 97% at 5 years, 89% at 10 years, and 87% at 15 years for the entire cohort; 96% at 5 years and 87% at 10 years for meningiomas; and 100% at 10 years for the other tumors. No significant difference was observed between meningiomas and non-meningiomas (log-rank test, p = 0.107). Improvement in cranial nerve (CN) function was observed in 35 (27%) patients. TSs tended to show CN improvements more often than meningiomas did (total improvements, 62% vs. 23%; p = 0.004; eye movement function, 100% vs. 20%; p = 0.002). CN deterioration or development of new CN deficits was observed in 11 (10%) patients.
Conclusion
SRS provides good tumor control and acceptable long-term outcome with sufficient preservation of CN function in patients with benign CS tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cavernous sinus (CS) is an important anatomical structure containing the internal carotid artery (ICA) and the third, fourth, fifth, and sixth cranial nerves (CNs). A variety of benign tumors, such as meningiomas, trigeminal schwannomas (TS), and cavernous hemangiomas (CH), can arise within or extend into this structure, causing impairment of visual function, extraocular movement, facial sensory function, and other CN functions [1,2,3]. Surgical resection is the standard primary treatment to achieve immediate mass reduction for large tumors and also obtain a histopathological diagnosis although it is possible to make an accurate diagnosis based only on the characteristics of advanced neuroimaging findings in most cases. Despite well-established microscopic and endoscopic skull base techniques, surgical interventions for such tumors remain challenging due to their deep skull base location and proximity to the ICA, CNs, visual pathways, and pituitary gland. Preservation of a CN may require surgeons to leave tumor remnants behind, and tumor recurrence/regrowth is possible [4,5,6]. Radiotherapy plays an important role in balancing tumor control and functional preservation, but whether functional recovery is achievable following radiotherapy remains questionable, especially in cases of large symptomatic tumors, since immediate mass reduction is not achievable [5].
Stereotactic radiosurgery (SRS) is a less invasive treatment option utilizing head fixation and highly focused narrow beam radiation that enables precise targeting with a steep dose fall-off. Given the structural features of the CS in which the CNs run along its outer wall, it is theoretically feasible to intensely irradiate tumors while minimizing irradiation to the CNs. Previous literature has reported favorable short-term to mid-term outcomes following either SRS alone or in combination with surgery (5-years tumor control rates in CS meningiomas ranging 94–98%) [7,8,9,10]. CS-specific radiation-induced adverse events (RAE), deterioration of CN III-VI functions, and ICA stenosis/occlusion, albeit rare, have been reported [11,12,13,14,15,16,17]. However, there remains a paucity of data on its long-term outcomes.
This study aimed to clarify the long-term outcomes of tumor control and CN functioning following SRS for benign CS tumors.
Methods
Patient and tumor characteristics
The data of 190 patients with CS tumors, treated with SRS from June 1990 to June 2018 at our institution, were collected from the institutional gamma knife database. Both intra-CS tumors and para-CS tumors extending into the CS were defined as CS tumors. The exclusion criteria were: (1) functioning (n = 22) and non-functioning (n = 14) pituitary adenomas with CS extensions, (2) pathologically confirmed non-benign tumors, including World Health Organization (WHO) grade II/III meningiomas (n = 12), hemangiopericytomas (n = 1), chordomas (n = 18), chondrosarcomas (n = 9), and metastatic tumors (n = 1). As a result, data on 113 patients with benign CS tumors, including 91 meningiomas, 14 TSs, and 8 CHs, were included in the analysis.
Most diagnoses were based on histopathological findings from prior surgery (n = 75). The timing of SRS for postoperative recurrent (n = 31) or residual tumor (n = 44) was determined using either the judgment of the surgeon, a referring physician, or by patient request, without any arbitrary selection. Radiographic diagnosis, without prior surgery, was used in 38 cases (19 meningiomas, 12 TSs, and 7 CHs). All radiographic images were reviewed by two independent neuroradiologists and attending neurosurgeons. The study was approved by the Institutional Review Board of our institution (#2231) and conducted in compliance with the principles of the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. All patients provided written informed consent for study participation.
The procedures and techniques of SRS
Leksell Gamma Knife (Elekta Instruments, Stockholm, Sweden) was used for all SRS treatments. The detailed treatment process is reported in a previous paper [18, 19]. After head fixation using a Leksell frame (Elekta Instruments, Stockholm, Sweden), stereotactic imaging (computed tomography [CT] before July 1996; magnetic resonance imaging [MRI] between August 1996 and January 2018, followed by cone-beam CT) was performed to obtain precise tumor data. Neurosurgeons and radiation oncologists performed radiosurgical planning using commercially available software (KULA planning system) until 1998 and Leksell Gamma Plan thereafter (Elekta Instruments). In principle, 14–16 Gy was prescribed to the tumor margin using a 50 ± 5% isodose line. If the tumor was suspected to be aggressive and was sufficiently distant from radiosensitive cerebral structures, such as the optic apparatus or the brainstem, the tumor margin dose was increased to 16 Gy to achieve long-term tumor control.
Follow-up and treatment outcomes
After SRS, MRI was regularly performed every 6 months for the first couple of years and annually thereafter. Radiographic findings were independently assessed by neuroradiologists and neurosurgeons. Tumor progression or shrinkage were defined by the Response Assessment in Neuro-Oncology (RANO) criteria [20]. Transient expansion, typically occurring in schwannomas due to radiation-induced tumor swelling at around 6 months after SRS, followed by shrinkage at approximately 18 months, was meticulously distinguished from actual tumor progression by evaluating consecutive MRIs [21, 22]. The neurological status of the patients and their responses to treatment were prospectively collected at each hospital visit, and a Common Terminology Criteria for Adverse Events (CTCAE, version 5.0) grade was retrospectively assigned on the basis of the respective descriptions. Data on patients who dropped out of regular follow-ups or returned to referring physicians were collected via telephone conversation, and follow-up radiographic images were obtained for our independent review. Radiosurgical plans and follow-up images of typical cases are shown in Fig. 1.
Diagnostic radiological imaging using post-contrast T1-weighted magnetic resonance imaging (MRI) of two demonstrative cases with cavernous sinus tumor. (A1) Radiosurgical plans for a 64-year-old female patient with the right cavernous sinus meningioma who had a prior partial resection. Targeted tumor is 31 × 45 × 25 mm and 14.7 mL. The yellow line indicates the 45% isodose line of the prescribed treatment dose of 14 Gy. Green lines indicate the isodose lines (18, 13, and 11 Gy). (A2) Follow-up MRI at 129 months after the radiosurgery showing the well-controlled and shrinking tumor. (B1) Radiosurgical plans for a 57-year-old female patient with the left cavernous sinus hemangioma. Targeted tumor is 21 × 26 × 14 mm and 4.5 mL. The yellow line indicates the 50% isodose line of the prescribed treatment dose of 16 Gy. Green lines indicate the isodose lines (20, 14, and 10 Gy). (B2) Follow-up MRI at 199 months after the radiosurgery showing the well-controlled and shrinking tumor
Statistical methods
Baseline characteristics of the patients were compared using the chi-square test for categorical variables and the Mann–Whitney U test for continuous variables. Progression-free survival (PFS) rates were calculated using the Kaplan–Meier method and compared among tumor types using the log-rank test. Factors associated with PFS were examined using bivariate and multivariable Cox proportional hazard analyses. Continuous variables were entered into models after being dichotomized using their median values. Where post-SRS recurrence/regrowth was confirmed, recurrence patterns and features were examined in more detail. Post-SRS CN outcomes were summarized, and factors associated with functional improvement, deterioration, and new deficits were examined with logistic regression analysis, and these rates were calculated and compared using the Kaplan–Meier method. Statistical analyses were performed using JMP Pro 15 software (SAS Institute Inc., Cary, NC, USA).
Results
Participant characteristics
Patient baseline characteristics are shown in Table 1 and Online Resource Supplementary Table 1. The median post-SRS follow-up period was 77 months (interquartile range [IQR], 39–177). When comparing the baseline characteristics between tumor types, the maximum diameter (29 mm vs. 24 mm, p = 0.029) was significantly larger, and the prescription dose (16 vs. 14 Gy, p = 0.012) and central dose (32 vs. 28 Gy, p = 0.039) were significantly higher in meningiomas than in TS. Patients underwent prior surgery significantly more often in the meningioma group than the TS and CH groups (meningioma, 79%; TS, 14% [p = 0.001]; and CH, 13% [p = 0.001]). Of those, 21 patients with meningioma (23%) had undergone surgery two or more times before SRS.
Tumor control
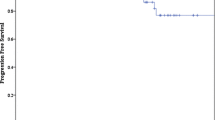
Of all the patients, 112 (99%) were alive at the final follow-up visit, and the single (1%) death was due to suicide, unrelated to the tumor and associated treatment. At the last follow-up, 49 (43%) tumors had decreased in size, 54 (48%) remained unchanged, and 10 (9%) increased in size. Tumor shrinkage was observed in 32 patients (35%) with meningioma, 10 (71%) with TS, and seven (81%) with CH. Tumor progression was not observed except for 10 patients with meningioma. In the entire cohort, the PFS was 97% at 5 years, 89% at 10 years, and 87% at 15 years (Fig. 2A). The tumor specific PFS was 96% at 5 years, 87% at 10 years for meningioma, and 100% at 10 years for the other tumors. There were no significant differences between two cohorts of meningioma and non-meningioma in PFS (log-rank test, p = 0.107; Fig. 2B). PFS was 100% at 5 years and 90% at 10 years with SRS alone and 96% at 5 years and 87% at 10 years for SRS with prior surgery (log-rank test, p = 0.056; Fig. 2C).
Kaplan–Meier curves for (A) progression-free survival rates for the entire cohort, B progression-free survival rates comparing meningiomas and non-meningiomas, C progression-free survival rates comparing stereotactic radiosurgery (SRS) alone and SRS with prior surgery, and D progression-free survival rates in meningioma comparing SRS alone and SRS with prior surgery
Since tumor recurrence was only observed in meningiomas, the analysis of potential risk factors for tumor recurrence was performed for meningiomas. No significant factors were found in the bivariate and multivariable analyses (Table 2). Baseline characteristics of patients with post-SRS recurrence are summarized in Online Resource Supplementary Table 2. All recurrences were noted for meningiomas at a median period of 87 months (IQR, 48–160 months) after SRS. Nine (90%) tumors were post-surgical recurrence, and three (30%) tumors were treated with suboptimal radiation coverage because of proximity to the optic apparatus or brainstem structure. The patterns of recurrence were intra-field in four (40%) patients and marginal (recurrence occurred out of the radiation field but within 20% isodose line) in six (60%). PFS in meningioma was 100% at 5 years and 89% at 10 years for SRS alone and 96% at 5 years and 87% at 10 years for SRS with prior surgery (log-rank test, p = 0.207; Fig. 2D).
Improvement of cranial nerve deficits
The details of CN function are summarized in Table 3 and Online Resource Supplementary Table 3. 83 patients (73%) had 128 CN deficits before SRS. The rate of pre-SRS CN deficits was the highest in meningiomas (69/91, 76%), followed by 12 (86%) in TSs, and two (25%) in CHs.
In the entire cohort, CN improvement was observed in 35 (27%) CNs, including four (18%) with visual deficits, 11 (24%) with third, fourth, and sixth CN deficits, 16 (36%) with trigeminal neuropathy, and 1 (50%) with ptosis. Among tumor types, improvement of third, fourth, and sixth CN deficits was significantly more common in patients with TS (100%) than in meningioma (20%, p = 0.002), whereas no significant difference was observed in improvements in the other CN deficits. The cumulative rates of post-SRS improvement of CN functions are shown in Fig. 3A. The post-SRS CN improvement was observed at a median period of 13 months (IQR, 6–24 months). More significant improvements were observed in non-meningiomas than in meningiomas (log-rank test, p = 0.002; Fig. 3B). The improvement rates of CN V (57% vs. 26%, p = 0.042) and all CN (41% vs. 22%, p = 0.035) were significantly higher in SRS alone than in SRS with prior surgery (Online Resource Supplementary Table 4). Additionally, 101 CN deficits caused by tumor compression were relieved in 32 CNs (32%) after SRS.
A Kaplan–Meier curves for cranial nerve function improvement rates after radiosurgery in all tumors and B improvement rates comparing meningiomas and non-meningioma. C Kaplan–Meier curves of cranial nerve function preservation rates after radiosurgery in all tumors and D preservation rates comparing meningiomas and non-meningioma
Radiation-induced adverse events
Details of new or worsened CN deficits following SRS are shown in Table 3 and Online Resource Supplementary Table 3. 11 (10%) CN deficits had deteriorated or newly developed at a median period of 4 months (IQR, 3–13 months) following SRS, including 1 (1%) visual deficit, four (4%) extraocular movement disorders, and six (5%) trigeminal neuropathies. All were mild or transient (CTCAE grade 1–2). The cumulative rates of post-SRS deteriorated/newly developed CN functions are shown in Fig. 3C. There were no significant differences between meningioma and other tumors (Fig. 3D). Aside from the CN deficits, hydrocephalus was observed in one patient (0.9%) with well-controlled meningioma who underwent ventriculoperitoneal shunting 100 months after SRS. In this case, the association between the tumor and hydrocephalus was unclear. No temporal lobe necrosis was observed in the entire cohort. Post-SRS signal change, as a high-intensity signal change on T2-weighted imaging in the temporal lobe, was identified in two patients with meningioma (1.6%) 3‒6 months after SRS. Both were asymptomatic and the signal change diminished at 13‒24 months. Hypopituitarism was observed in one patient (0.9%) with meningioma 47 months after SRS, and asymptomatic ICA stenosis was observed in one patient (0.9%) with meningioma 169 months after SRS.
Discussion
In this study, we analyzed the long-term outcomes of SRS for CS tumors. Using a similar radiosurgical strategy, we defined the tumor margins and prescribed conformally and selectively a marginal dose of 14–16 Gy. The 10-year PFS was 89%, with the post-SRS CN improvement rate reaching 27%, while maintaining a low CN deterioration rate (10%). These results suggest that SRS would be a reasonable treatment option, providing long-term tumor control with favorable neurological outcomes.
The results showed that the tumor control rate is excellent, especially in non-meningioma CS tumors. Most baseline characteristics of tumors in this cohort were similar, with differences in tumor size and the history of prior surgery. The differences in PFSs between meningioma and non-meningioma tumors possibly reflected differences in tumor biology, which might be due to selection bias in that most meningiomas are postoperative cases. In the literature, tumor control rates were reported to be 77–100% in TS at median observation periods of 27–91 months, and 100% in CH at 30–40 months [12,13,14,15,16,17, 23,24,25,26,27,28]. Our data are comparable to these studies. The PFS in the SRS with prior surgery group showed lower values than that of the SRS alone group. The inter-group differences were not statistically significant, but this may be due to the lack of statistical power and should be treated with caution.
The post-SRS CN improvements were also excellent, likely the result of the high tumor shrinkage rate. Regarding RAEs, our study demonstrated excellent functional preservation rates, which might be due to the highly selective high dose irradiation which SRS can provide. Therefore, non-meningioma CS tumors are an excellent indication for SRS.
Treatment strategies need to be formulated according to the characteristics of individual tumors. SRS plays an adjunctive role for recurrent/residual tumors and can be a primary treatment modality in TS and CH.
The PFS of meningioma was slightly lower than that of non-meningiomas but was still satisfactory at 96% at 5 years and 87% at 10 years. Most previous retrospective studies and the International Stereotactic Radiosurgery Society Practice Guidelines reported excellent tumor control rates of 86–99% at 5 years and 69–97% at 10 years with favorable functional preservation rates of 80–100%, consistent with our results [18, 29,30,31,32,33,34,35,36,37,38,39,40]. We found that six (60%) recurrent meningiomas presented with marginal recurrences after a mid-to-long period of tumor control (range, 67–208 months). Recurrences can be explained by the intrinsic features of meningiomas. They easily blend into the meninges, making it challenging to accurately define tumor margins. It is important to meticulously pursue dural tails using thin-slice MRIs. In post-surgical recurrence/regrowth cases, surgical scar tissue might obscure the true tumor margins. Nine (90%) recurrences were postoperative cases, and in situ comparisons to pre-operative images may highlight the true extent of the tumor. Although the role of surgery remains debatable, and immediate mass reduction may be necessary in certain cases, primary SRS may be reasonable for selective cases with small-sized to medium-sized CS tumors, unless the tumor has atypical radiographic features. Four (40%) cases had in-field recurrence, and three were likely explained by suboptimal coverage because of the proximity of the optic apparatus and brainstem or incomplete coverage of a tumor part extending into the tentorial edge. The remaining case had a WHO grade I tumor with biologically aggressive features, including a Ki-67 index of 15%.
CN dysfunction as post-SRS RAEs has occurred in 1–23% of patients in the past [7,8,9, 13,14,15,16,17,18, 23, 24, 26, 27, 30,31,32,33,34, 37,38,39,40,41,42]. In our study, 11 of all 113 patients (10%) experienced deterioration or emergence of new CN dysfunction after SRS, which was lower than previously reported (Online Resource Supplementary Table 5). Notably, 10 of 11 (91%) of these occurred in patients with CS meningioma. There was no significant difference among tumor types, and all of the dysfunctions were either CTCAE grade 1 or 2. Comparing surgery and SRS, a meta-analysis of 2065 CS meningiomas showed that the incidence of neurological complications was significantly lower with SRS alone (25.7%) compared with SRS after surgery (59.6%) [43]. We failed to find a similar pattern, which could be explained by our relatively small patient number, although it might be due to our lower RAE rate. A certain portion of RAEs may be avoidable by meticulously defining tumor margins and reducing direct irradiation to the CNs using thin-slice MRIs. Based on these results, SRS would be a safe modality for preserving CN function in CS tumors. Aside from CN deficits, we also identified one carotid artery stenosis (0.8%). In previous reports on SRS for pituitary adenoma and CH, the incidence of radiation-induced ICA stenosis/occlusion range was 0.4%‒2.8% [44, 45]. Although ICA stenosis/occlusion is rare and rarely becomes symptomatic, long-term follow-up is needed.
This study has several limitations. First, it was a retrospective, single-institution study with potential selection bias. In addition, the clinical practice standards specific to the institution were used, thereby impairing the generalizability of the findings. Second, 38 tumors in this cohort were radiographically diagnosed, therefore the certainty of these radiological diagnoses could be less reliable than those with histological confirmation. Finally, a larger sample size for each tumor type would be desirable for future studies to confirm our findings.
Conclusion
We re-confirmed that SRS achieved excellent treatment efficacy for benign skull base tumors invading the CS. It could achieve a valid tumor control and an acceptable long-term outcome with sufficient preservation of CN function.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
McCormick PC, Bello JA, Post KD (1988) Trigeminal schwannoma. Surgical series of 14 cases with review of the literature. J Neurosurg 69:850–860. https://doi.org/10.3171/jns.1988.69.6.0850
Tagle P, Huete I, Méndez J, del Villar S (1986) Intracranial cavernous angioma: presentation and management. J Neurosurg 64:720–723. https://doi.org/10.3171/jns.1986.64.5.0720
Adegbite AB, Khan MI, Paine KW, Tan LK (1983) The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg 58:51–56. https://doi.org/10.3171/jns.1983.58.1.0051
Rennert RC, Ravina K, Strickland BA, Bakhsheshian J, Fredrickson VL, Russin JJ (2018) Complete cavernous sinus resection: an analysis of complications. World Neurosurg 119:89–96. https://doi.org/10.1016/j.wneu.2018.07.206
Nanda A, Thakur JD, Sonig A, Missios S (2016) Microsurgical resectability, outcomes, and tumor control in meningiomas occupying the cavernous sinus. J Neurosurg 125:378–392. https://doi.org/10.3171/2015.3.JNS142494
O’Sullivan MG, van Loveren HR, Tew JM, Jr (1997) The surgical resectability of meningiomas of the cavernous sinus. Neurosurgery 40:238–244; discussion 245–237. https://doi.org/10.1097/00006123-199702000-00003
Tishler RB, Loeffler JS, Lunsford LD, Duma C, Alexander E 3rd, Kooy HM, Flickinger JC (1993) Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys 27:215–221. https://doi.org/10.1016/0360-3016(93)90230-s
Leber KA, Berglöff J, Pendl G (1998) Dose–response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg 88:43–50. https://doi.org/10.3171/jns.1998.88.1.0043
Liu AL, Wang C, Sun S, Wang M, Liu P (2005) Gamma knife radiosurgery for tumors involving the cavernous sinus. Stereotact Funct Neurosurg 83:45–51. https://doi.org/10.1159/000085544
Tripathi M, Batish A, Kumar N, Ahuja CK, Oinam AS, Kaur R, Narayanan R, Gurnaani J, Kaur A (2020) Safety and efficacy of single-fraction gamma knife radiosurgery for benign confined cavernous sinus tumors: our experience and literature review. Neurosurg Rev 43:27–40. https://doi.org/10.1007/s10143-018-0975-8
Starke RM, Williams BJ, Hiles C, Nguyen JH, Elsharkawy MY, Sheehan JP (2012) Gamma knife surgery for skull base meningiomas. J Neurosurg 116:588–597. https://doi.org/10.3171/2011.11.JNS11530
Huang CF, Kondziolka D, Flickinger JC, Lunsford LD (1999) Stereotactic radiosurgery for trigeminal schwannomas. Neurosurgery 45:11–16; discussion 16. https://doi.org/10.1097/00006123-199907000-00002
Sheehan J, Yen CP, Arkha Y, Schlesinger D, Steiner L (2007) Gamma Knife surgery for trigeminal schwannoma. J Neurosurg 106:839–845. https://doi.org/10.3171/jns.2007.106.5.839
Sun J, Zhang J, Yu X, Qi S, Du Y, Ni W, Hu Y, Tian Z (2013) Stereotactic radiosurgery for trigeminal schwannoma: a clinical retrospective study in 52 cases. Stereotact Funct Neurosurg 91:236–242. https://doi.org/10.1159/000345258
Ryu J, Lee SH, Choi SK, Lim YJ (2018) Gamma knife radiosurgery for trigeminal schwannoma: a 20-year experience with long-term treatment outcome. J Neurooncol 140:89–97. https://doi.org/10.1007/s11060-018-2934-1
Snyder MH, Shepard MJ, Chen CJ, Sheehan JP (2018) Stereotactic radiosurgery for trigeminal schwannomas: a 28-year single-center experience and review of the literature. World Neurosurg 119:e874–e881. https://doi.org/10.1016/j.wneu.2018.07.289
Pomeraniec IJ, Taylor DG, Cohen-Inbar O, Xu Z, Lee Vance M, Sheehan JP (2019) Radiation dose to neuroanatomical structures of pituitary adenomas and the effect of gamma knife radiosurgery on pituitary function. J Neurosurg 132:1499–1506. https://doi.org/10.3171/2019.1.JNS182296
Shin M, Kurita H, Sasaki T, Kawamoto S, Tago M, Kawahara N, Morita A, Ueki K, Kirino T (2001) Analysis of treatment outcome after stereotactic radiosurgery for cavernous sinus meningiomas. J Neurosurg 95:435–439. https://doi.org/10.3171/jns.2001.95.3.0435
Shinya Y, Hasegawa H, Shin M, Sugiyama T, Kawashima M, Takahashi W, Iwasaki S, Kashio A, Nakatomi H, Saito N (2019) Long-term outcomes of stereotactic radiosurgery for vestibular schwannoma associated with neurofibromatosis Type 2 in comparison to sporadic schwannoma. Cancers 11:1498. https://doi.org/10.3390/cancers11101498
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Nagano O, Higuchi Y, Serizawa T, Ono J, Matsuda S, Yamakami I, Saeki N (2008) Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg 109:811–816. https://doi.org/10.3171/JNS/2008/109/11/0811
Mindermann T, Schlegel I (2014) How to distinguish tumor growth from transient expansion of vestibular schwannomas following gamma knife radiosurgery. Acta Neurochir 156:1121–1123. https://doi.org/10.1007/s00701-014-2063-3
Kano H, Niranjan A, Kondziolka D, Flickinger JC, Dade Lunsford L (2009) Stereotactic radiosurgery for trigeminal schwannoma: tumor control and functional preservation Clinical article. J Neurosurg 110:553–558. https://doi.org/10.3171/2008.7.jns0812
Albano L, Losa M, Nadin F, Barzaghi LR, Parisi V, Del Vecchio A, Bolognesi A, Mortini P (2019) Safety and efficacy of multisession gamma knife radiosurgery for residual or recurrent pituitary adenomas. Endocrine 64:639–647. https://doi.org/10.1007/s12020-019-01876-2
Lee WJ, Cho KR, Choi JW, Kong DS, Seol HJ, Nam DH, Lee JI (2020) Gamma knife radiosurgery as a primary treatment for nonfunctioning pituitary adenoma invading the cavernous sinus. Stereotact Funct Neurosurg 98:371–377. https://doi.org/10.1159/000508737
Yu J, Li Y, Quan T, Li X, Peng C, Zeng J, Liang S, Huang M, He Y, Deng Y (2020) Initial gamma knife radiosurgery for nonfunctioning pituitary adenomas: results from a 26-year experience. Endocrine 68:399–410. https://doi.org/10.1007/s12020-020-02260-1
Lee CC, Sheehan JP, Kano H, Akpinar B, Martinez-Alvarez R, Martinez-Moreno N, Guo WY, Lunsford LD, Liu KD (2017) Gamma knife radiosurgery for hemangioma of the cavernous sinus. J Neurosurg 126:1498–1505. https://doi.org/10.3171/2016.4.JNS152097
Wang Y, Li P, Zhang XJ, Xu YY, Wang W (2016) Gamma knife surgery for cavernous sinus hemanginoma: A report of 32 cases. World Neurosurg 94:18–25. https://doi.org/10.1016/j.wneu.2016.06.094
Lee CC, Trifiletti DM, Sahgal A, DeSalles A, Fariselli L, Hayashi M, Levivier M, Ma L, Álvarez RM, Paddick I, Regis J, Ryu S, Slotman B, Sheehan J (2018) Stereotactic radiosurgery for benign (World Health Organization Grade I) cavernous sinus meningiomas-international stereotactic radiosurgery society (ISRS) practice guideline: a systematic review. Neurosurgery 83:1128–1142. https://doi.org/10.1093/neuros/nyy009
Nicolato A, Foroni R, Alessandrini F, Bricolo A, Gerosa M (2002) Radiosurgical treatment of cavernous sinus meningiomas: experience with 122 treated patients. Neurosurgery 51:1153–1159; discussion 1159–1161. https://doi.org/10.1097/00006123-200211000-00009
Maruyama K, Shin M, Kurita H, Kawahara N, Morita A, Kirino T (2004) Proposed treatment strategy for cavernous sinus meningiomas: a prospective study. Neurosurgery 55:1068–1075. https://doi.org/10.1227/01.neu.0000140839.47922.5a
Williams BJ, Yen CP, Starke RM, Basina B, Nguyen J, Rainey J, Sherman JH, Schlesinger D, Sheehan JP (2011) Gamma Knife surgery for parasellar meningiomas: long-term results including complications, predictive factors, and progression-free survival. J Neurosurg 114:1571–1577. https://doi.org/10.3171/2011.1.JNS091939
Zeiler FA, McDonald PJ, Kaufmann AM, Fewer D, Butler J, Schroeder G, West M (2012) Gamma Knife radiosurgery of cavernous sinus meningiomas: an institutional review. Can J Neurol Sci 39:757–762. https://doi.org/10.1017/s0317167100015572
Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL (2013) Single-fraction radiosurgery of benign cavernous sinus meningiomas. J Neurosurg 119:675–682. https://doi.org/10.3171/2013.5.JNS13206
Fariselli L, Biroli A, Signorelli A, Broggi M, Marchetti M, Biroli F (2016) The cavernous sinus meningiomas’ dilemma: surgery or stereotactic radiosurgery? Rep Pract Oncol Radiother 21:379–385. https://doi.org/10.1016/j.rpor.2015.05.002
Leroy HA, Tuleasca C, Reyns N, Levivier M (2018) Radiosurgery and fractionated radiotherapy for cavernous sinus meningioma: a systematic review and meta-analysis. Acta Neurochir 160:2367–2378. https://doi.org/10.1007/s00701-018-3711-9
Park KJ, Kano H, Iyer A, Liu X, Tonetti DA, Lehocky C, Faramand A, Niranjan A, Flickinger JC, Kondziolka D, Lunsford LD (2018) Gamma Knife stereotactic radiosurgery for cavernous sinus meningioma: long-term follow-up in 200 patients. J Neurosurg 130:1799–1808. https://doi.org/10.3171/2018.2.JNS172361
Hung YC, Lee CC, Guo WY, Shiau CY, Chang YC, Pan DH, Sheehan JP, Chung WY (2019) Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas: post-treatment long-term clinical outcomes, complications, and volume changes. J Neurooncol 143:261–270. https://doi.org/10.1007/s11060-019-03090-6
Hafez RF, Morgan MS, Fahmy OM (2015) Stereotactic gamma knife surgery safety and efficacy in the management of symptomatic benign confined cavernous sinus meningioma. Acta Neurochir 157:1559–1564. https://doi.org/10.1007/s00701-015-2509-2
Kano H, Park KJ, Kondziolka D, Iyer A, Liu X, Tonetti D, Flickinger JC, Lunsford LD (2013) Does prior microsurgery improve or worsen the outcomes of stereotactic radiosurgery for cavernous sinus meningiomas? Neurosurgery 73:401–410. https://doi.org/10.1227/01.neu.0000431471.64289.3d
Sun S, Liu A, Zhang Y (2019) Long-term follow-up studies of gamma knife radiosurgery for postsurgical nonfunctioning pituitary adenomas. World Neurosurg 24:e715–e723. https://doi.org/10.1016/j.wneu.2019.01.009
Wang X, Zhu H, Knisely J, Mei G, Liu X, Dai J, Mao Y, Pan L, Qin Z, Wang E (2018) Hypofractionated stereotactic radiosurgery: a new treatment strategy for giant cavernous sinus hemangiomas. J Neurosurg 128:60–67. https://doi.org/10.3171/2016.10.JNS16693
Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, Parsa AT (2010) Factors affecting outcome following treatment of patients with cavernous sinus meningiomas. J Neurosurg 113:1087–1092. https://doi.org/10.3171/2010.3.JNS091807
Graffeo CS, Link MJ, Stafford SL, Parney IF, Foote RL, Pollock BE (2020) Risk of internal carotid artery stenosis or occlusion after single-fraction radiosurgery for benign parasellar tumors. J Neurosurg 133:1388–1395. https://doi.org/10.3171/2019.8.JNS191285
Pikis S, Bunevicius A, Sheehan J (2021) Internal carotid artery stenosis and risk of cerebrovascular ischemia following stereotactic radiosurgery for recurrent or residual pituitary adenomas. Pituitary 24:574–581. https://doi.org/10.1007/s11102-021-01134-7
Acknowledgements
None
Funding
This work was supported by JSPS KAKENHI (Grant Number 19K24042 to Yuki Shinya).
Author information
Authors and Affiliations
Contributions
Conceptualization: MU and YS; Methodology: MU and YS; Formal analysis and investigation: MU and YS; Writing—original draft preparation: MU; Writing—review and editing: YS, HH, MS, MK, AK, and NS; Funding acquisition: YS; Resources: YS, HH, SM, MK, and AK; Supervision: MS and NS.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
The study was approved by the Institutional Review Board of our institution (#2231) and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
All patients provided written informed consent for study participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Umekawa, M., Shinya, Y., Hasegawa, H. et al. Long-term outcomes of stereotactic radiosurgery for skull base tumors involving the cavernous sinus. J Neurooncol 156, 377–386 (2022). https://doi.org/10.1007/s11060-021-03921-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03921-5