Abstract
Background
Stereotactic radiosurgery (SRS) has become a primary option for management for both newly diagnosed vestibular schwannomas (VS), as well as VS that enlarge after initial observation.
Methods
A retrospective review of our prospectively maintained data base found 871 patients who underwent Gamma knife® SRS as their initial (primary) management between 1987 and 2008. Follow-up ranged from 1–25 years (median = 5.2 years) Median tumor volume was 0.9 cc (0.02–36) and median margin dose was 13 Gy (12–25).
Results
Progression free survival (PFS) after SRS was 97% at 3 years, 95% at 5 years, and 94% at 10 years. Freedom from delayed surgical resection was found in 98.7% of patients. Smaller tumor volume was significantly associated with improved PFS. There were 326 patients with serviceable hearing (Gardner–Robertson 1 or 2) at the time of SRS with audiological follow-up of ≥ 1 year. Serviceable hearing preservation rates after SRS were 89.8% at 1 year, 76.9% at 3 years, 68.4% at 5 years, 62.5% at 7 years, and 51.4% at 10 years. Factors associated with improved serviceable hearing preservation included younger age, Gardner-Robertson grade 1 at SRS, and absence of subjective complaints of dysequilibrium or vertigo (vestibulopathy). Fifty-one patients (5.8%) developed trigeminal neuropathy. Fourteen (1.6%) developed a transient House-Brackmann grade 2 or 3 facial neuropathy.
Conclusions
In this report with extended follow-up, primary SRS achieved tumor growth control in 94% of patients. Optimization of long- term cranial nerve outcomes remains an important achievement of this management strategy for VS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vestibular schwannomas (VS) are primary brain tumors that account for 10% of newly diagnosed intracranial tumors and 80% of cerebellopontine angle tumors [1]. Over the last three decades, management options, including observation, microsurgical resection, fractionated radiation therapy, and stereotactic radiosurgery (SRS) have evolved. SRS is now one of the most common strategies for newly diagnosed or progressive VS. Extensive data defining safety, tumor control, and cranial nerve preservation rates have been reported in recent years [2,3,4,5,6]. In this study, we sought to define the long -term outcomes of SRS as a primary management for VS. In order to emphasize long term follow-up, we performed a retrospective review of our prospectively maintained database of patients who underwent primary SRS between 1987 and 2008. This serves as an update and a long-term extension to prior studies from our institution.[3]
Material and methods
During our 32 years experience beginning in 1987, we performed Leksell Gamma Knife® (AB Elekta, Stockholm, Sweden) SRS on 1954 patients with schwannonmas located in the posterior fossa. We reviewed our prospectively maintained Institutional Review Board approved database of 1372 VS patients who underwent SRS between 1987 and 2008. Patients with neurofibromatosis type-2, prior surgical resection, or prior SRS at an outside facility were excluded from this study. Patient clinical and imaging follow up ranged from 1 to 25 years (median = 5.2 years). Fifty patients had > 15 years follow up. Mean age, tumor volume, follow-up, and median margin dose were 57 years (range 18–95), 0.98 cc (0.05–36), 5.2 years (1–25), and 13 Gy (8–20), respectively (Table 1). While dose planning evolved between 1987 and 1992, the typical prescribed minimum tumor margin dose (12–13 Gy) has since remained stable for more than 20 years. During this interval various models of the Leksell Gamma knife were used (U, B, C, 4C, Perfexion).
Preoperative evaluation
All patients underwent high-resolution posterior fossa imaging with magnetic resonance imaging (MRI) or computed tomography (CT) if ineligible for MRI, detailed neurological examination, and audiologic testing that included speech discrimination score and pure-tone average. Hearing was subsequently categorized according to the Gardner-Robertson (GR) hearing classification [7]. Serviceable hearing, GR classes I and II, was defined as a speech discrimination score (SDS) higher than 50% and a pure tone average (PTA) less than 30 dB. The Koos classification was used to assess the relationship between the tumor and the surrounding structures, and served as an indirect indicator of tumor volume [8]. Facial nerve function was evaluated according to the House-Brackmann grading system [9]. Patients were also assessed for trigeminal sensory dysfunction, symptoms of trigeminal neuralgia, tinnitus, and vestibulopathy (dysequilibrium or vertigo symptoms).
Radiosurgical technique
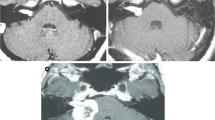
Under conscious sedation and after the injection of a local anesthetic, an imaging-compatible Leksell stereotactic head frame was attached to the head. High-resolution 1.5 or 3 T MR images were obtained with a fiducial system in place, generating 1 to 1.5-mm axial slice thickness that is reformatted into coronal and sagittal images. CT was used if the patient was treated before the availability of MRI (1991) or had a contraindication to MRI. Multiple isocenter conformal dose planning was then performed by an interdisciplinary team consisting of a neurosurgeon, radiation oncologist, and a medical physicist (Fig. 1). The tumor volume, margin dose, and maximum doses were determined jointly. The prescribed tumor margin dose of 12–13 Gy became standard by 1992. Radiation delivery was performed in a single procedure. At the conclusion of the procedure, patients were typically observed for 1–2 h before discharge to home.
(Left) Pre-SRS axial T1-weighted MRI scan with gadolinium enhancement showing an vestibular schwannoma. (Middle) Axial T1-weighed MRI scan with gadolinium enhancement showing tumor regression 4 years after SRS. (Right) Axial T1-weighed MRI scan with gadolinium enhancement showing further tumor regression 8 years after SRS
Follow up
Follow up ranged from 1 to 25 years (median = 5.2 years. Follow up was recommended at 6 month intervals for the first year. If initial tumor control was achieved, then follow up imaging and evaluations were performed at 2, 4, 8, 12, 16, 20 and 24 years (Fig. 2). If a patient reported new or worsening symptoms, then more frequent imaging was obtained. Depending on the quality of imaging, which was often done at an institution closer to home, 10%-20 patients had transient tumor enlargement within the first year after SRS [10]. Sustained tumor progression was defined as + 15% estimated volume in cubic centimeters determined by the measurements of the X, Y, and Z tumor dimensions × 0.5 cm. Continued tumor progression lead to additional intervention (repeat SRS or surgical resection) typically within 3 years.
Formal audiological testing was performed at regular intervals to assess the hearing status in patients with serviceable hearing at the time of SRS. Hearing deterioration was defined as a decline from serviceable to non-serviceable hearing, corresponding to a drop from GR grade I or II to grade III or worse. Trigeminal neuropathy was defined as a subjective or objective decrease in facial sensation and was documented by patient examination. Facial neuropathy was evaluated using the House-Brackmann (HB) grading method.
Statistical analysis
For statistical analysis, Kaplan Meier survival plots were used to determine progression-free survival and hearing preservation rates, based on the date of the SRS procedure and the date of follow-up imaging and audiometry. Univariate analysis was performed on the Kaplan–Meier curves with the use of the log-rank statistic with p < 0.05 set for statistical significance. Multivariate analysis was performed with the Cox proportional hazards model. The suggested cut-off value for variables found to significantly affect tumor response rate and serviceable hearing preservation rate was determined by Youden index based on Receiver operating characteristic (ROC) curve analysis [11]. Statistical analysis was performed with IBM SPSS Statistics 24 (IBM, Armonk, NY).
Results
Tumor growth control
The primary goals of SRS were to arrest further tumor growth, to reduce perioperative risks, and to maintain cranial nerve function whenever possible. We identified 27 (3.1%) patients who despite primary SRS had progressive tumor growth and for whom we recommended additional management. The progression free survival (PFS) after SRS was 97% at 3 years, 95% at 5 years, 95% at 7 years, and 94% at 10 years (Fig. 3). Only an initial smaller tumor volume < 0.56 cc was significantly associated with improved PFS (p = 0.028). Margin dose, age, and Koos class were not associated with PFS (Fig. 2). Patients with a tumor volume ≥ 0.56 cc had a 10-year PFS of 93% compared to a 10 year PFS of 96% in patients whose initial tumor volume was < 0.56 cc (p = 0.028) (Fig. 3). The median time until imaging defined continued progression was 32 months, and was detected between 2 and 3 years after initial radiosurgery in most patients. Only 2 patients had delayed progression more than 10 years after SRS (1% of patients with 10-year follow up). Eleven patients (1.3%) ultimately underwent surgical resection, 15 (1.7%) required CSF diversion, and 6 (0.69%) underwent repeat SRS. All 6 patients who underwent repeat SRS had no further tumor growth after their second SRS procedure. All patients had imaging evidence of continued tumor progression despite initial SRS. One patient also had incomplete surgical resection after initial SRS. All patients were deaf at the time of the second SRS.
Trigeminal nerve outcomes
Fifty-one patients (5.8%) developed mild symptoms of trigeminal neuropathy. Four patients (0.46%) required either repeat SRS or percutaneous retrogasserian glycerol rhizotomy for management of refractory trigeminal neuralgia. A higher margin dose was not associated with trigeminal neuropathy.
Facial nerve outcomes
Fourteen patients (1.6%) developed a HB grade 2 or 3 facial neuropathy. All cases underwent SRS prior to 1997, during an interval in which higher marginal doses were delivered to the tumor. No patient who received a margin dose of ≤ 13 Gy developed any degree of facial weakness.
Hearing and tinnitus
We studied 326 patients with serviceable hearing (GR grade 1 or 2) at the time of SRS, all of whom had audiological follow-up of ≥ 1 year. The last audiological examination demonstrated that 196 (60.1%) retained serviceable hearing (GR grade 1 or 2). Serviceable hearing preservation rates after SRS were 89.8% at 1 year, 76.9% at 3 years, 68.4% at 5 years, 62.5% at 7 years, and 51.4% at 10 years (Fig. 4). In the univariate analysis, factors associated with improved serviceable hearing preservation rates included younger age (p < 0.0001), GR grade I at SRS (p < 0.0001), and absence of vestibular symptoms (p = 0.031) (Table 2). In the multivariate analysis, factors associated with improved serviceable hearing preservation rates included younger age (p < 0.0001, HR 1.04, 95% CI 1.02–1.06), smaller tumor volume (p = 0.038, HR 1.08, 95% CI 1.00–1.16), and pre -procedure GR grade I hearing (p = 0.001, HR 1.98, 95% CI 1.31–2.97) (Table 2).
a Kaplan–Meier curve shows serviceable hearing preservation rate after SRS. b Kaplan–Meier curves comparing serviceable hearing preservation in patients who were < 45 year-old vs. 45–59 year-old vs. ≥ 60 year-old. c Kaplan–Meier curves comparing serviceable hearing preservation in patients who were Gardner-Robertson grade 1 vs. grade 2 at the time of SRS. d Kaplan–Meier curves comparing serviceable hearing preservation in patients with vs. without vertigo at the time of SRS
We found the cut off values of for an age related hearing effect were < 45 years, 45 to 60 years, and ≥ 60 years based on ROC curves. Serviceable hearing preservation rates in patients who were < 45 year-old were 87% at 3 years, 83% at 5 years, and 76% at 10 years (Fig. 2). Serviceable hearing preservation rates in patients who were between 45 and 59 years-old were 78% at 3 years, 69% at 5 years, and 64% at 10 years. Serviceable hearing preservation rates in patients who were ≥ 60 years-old were 66% at 3 years, 54% at 5 years, and 45% at 10 years (Fig. 4). Serviceable hearing preservation rates in patients with initial Gardner-Robertson grade I hearing were 83% at 3 years, 75% at 5 years, and 59% at 10 years. Serviceable hearing preservation rates in patients with Gardner-Robertson grade II at the time of SRS were 56% at 3 years, 49% at 5 years, and 32% at 10 years. Tinnitus was reported by 538 patients (61.5%) prior to SRS. Seventy –nine patients (14.7%) noted reduction in tinnitus, 376 (69.9%) had no change, and 29 (5.4%) reported worsening tinnitus. Fifty-four (10%) had insufficient follow-up information available. Twenty-six patients (7.7%) reported the new onset of tinnitus after SRS.
Early and late SRS risks
Perioperative headache from stereotactic frame placement dissipated within hours and responded to oral acetaminophen. Pin site inflammation occurred in < 0.1% of patients and responded to local care. Rare occipital nerve sensory dysfunction related to the posterior pin site injection of local anesthesia typically resolved within days of treatment. No SRS patients sustained a CSF leak, pulmonary embolus, or treatment related brain hemorrhage. In this long- term study, no cases of delayed brain tumor oncogenesis were reported. Late peritumoral cyst development (usually a trapped CSF cistern posterior to the tumor) was detected in < 0.1% of patients.
Dysequilibrium and vertigo (vestibulopathy)
Three-hundred thirteen patients (35.8%) reported intermittent symptoms of vertigo or disequilibrium at the time of SRS. Ninety-nine (31.6%) noted improvement or resolution of their symptoms during follow-up, 170 (54.3%) had no change, and 28 (8.9%) described worsening vestibulopathy. Sixteen patients (5.1%) had insufficient subsequent follow-up information. Thirty of 562 patients (5.3%) without vestibular symptoms prior to SRS developed such symptoms subsequently. Serviceable hearing preservation rates in patients without associated vestibulopathy were 80% at 3 years, 72% at 5 years, and 55% at 10 years. Serviceable hearing preservation rates in patients with vestibulopathy were 71% at 3 years, 62% at 5 years, and 45% at 10 years. Vestibulopathy was associated with significantly worse serviceable hearing preservation rates (p = 0.031) (Fig. 4 and Table 2).
Long term follow up
Among 191 patients who were followed up of more than 10 years, complete regression was seen in 7 patients (4%), partial regression was seen in 149 (78%), no volume change was seen in 31(16%) and continued tumor progression was seen in 4 (2%). Among 48 patients who had imaging follow-up of more than 15 years, only one patient developed tumor progression and required repeat SRS 14 years after the initial SRS. This tumor was well controlled after repeat SRS. The remaining 41 tumors were smaller in size and 6 were stable in size. Among 31 patients who had hearing follow-up of more than 15 years, 11 of 18 patients who had serviceable hearing maintained serviceable hearing.
Discussion
SRS using various modalties has been utilized as a management for VS for over 50 years and is now the most commonly utilized primary intervention [1]. Stereotactic technology, dosing parameters, and patient selection all have evolved over these years. While prior reports established SRS as a safe and effective option, additional long-term outcome studies remain important to cement the role of radiosurgery. Fractionated stereotactic radiation therapy is a more recent alternative for vestibular schwannoma management and has been offered mostly by centers using linear accelerator based technologies. No randomized trials currently exist to compare outcomes after SRS to fractionated radiation therapy. Most early outcome studies report similar findings of tumor control, and cranial nerve preservation. Longer term outcomes such as the present SRS study will be able to better evaluate late results of tumor control, cranial nerve preservation, delayed oncogenesis, and other late complications of fractionated radiation therapy [12,13,14]. Such studies no doubt will be able to evaluate whether there is any clinical or radiobiological advantage to fractionation, or whether this methodology is simply technology dependent.
The present review has a median follow up of 5.2 years (maximum 25 years) and includes 50 patients with a minimum 15-year follow-up. Our results confirm that both long term tumor control and high rates of functional cranial nerve preservation are possible. With the exception of two late cases, tumor progression occurred within a median of 32 months after the procedure. We found that a pre-treatment tumor volume of ≥ 0.56 cc was associated with a slightly worse progression free survival (PFS) (10 year = 93%), although the Koos grade itself did was not statistically related to tumor response.
While radiosurgical tumor margin doses evolved over the first 10 years of VS experience at our center, they have largely remained constant over the past 21 years [15]. The reduction in prescribed tumor margin dose over time did not decrease tumor control, but did reduce cranial nerve complications such as delayed facial neuropathy, which we did not encounter in the years since the margin dose was reduced to 12 Gy. We suspect that the long term tumor control rates may drop if tumor margin doses are further reduced below 12 Gy [15]. Klijn et al. reported 420 patients who underwent SRS using a prescribe tumor margin dose of 11 Gy. The 5 year tumor control rate was 91.3% [16].
Hearing preservation after SRS
In our previous studies, factors associated with better hearing preservation after SRS included younger patient age, smaller tumor volume, better hearing at the time of SRS, and average cochlear dose < 4.2 Gy [17,18,19,20,21,22]. In addition we found that patients who underwent SRS within two years of diagnosis had better hearing preservation rates [23]. In the present study, younger age, smaller tumor volume, and better hearing at the time of SRS were significantly associated with improved serviceable hearing preservation on multivariate analysis (Table 2). The strongest predictor of serviceable hearing preservation was age at the time of SRS. Observation is considered an initial option for small tumors found in minimally symptomatic patients [13, 24]. The long term hearing preservation rates during observation require further study.[25, 26]. The best timing to initiate SRS is still unknown.
Delayed malignant transformation or oncogenesis after SRS
Delayed oncogenesis is a concern frequently described to patients who are obtaining multispecialty consultation relative to management strategies for their VS. We have reported a single case of a patient with a malignant schwannoma who died from recurrent and progressive disease [27]. In our 32-year institutional experience of over 1950 VS and 16,030 SRS patients, we have not confirmed a case that fulfills the Cahan criteria of a radiation related tumor (different histology in the field of radiation after a period of elapsed time) [28]. Hasegawa et al. reported that the estimated incidence of malignant transformation after vestibular schwannoma radiosurgery ranged from 0.03 to 0.06% [18].
Additional surgical resection after SRS
Delayed microsurgery after radiosurgery has been reported by some to be more difficult. (7, 23–28) We found no consensus positive, negative, or neutral related to the impact of prior SRS in 11 patients who underwent delayed tumor removal because of tumor progression. Wise et al. reported 37 patients who underwent VS surgical resection after SRS. These tumors were matched to non-radiated controls [29]. They found that less than complete resection was utilized more frequently in previously radiated patients. However, they found no difference in major complications such as stroke, hydrocephalus, meningitis, CSF leak, or facial neuropathy.
Conclusion
Our experience in 871 patients who underwent primary VS radiosurgery confirmed that long term tumor control was achieved in 94% of patients at 10 years. Only 1.3% of patients underwent subsequent microsurgery for continued progression. The risk of facial neuropathy approached zero. Serviceable hearing at 10 years was maintained in 76% of patients younger than 45 years of age and in patients whose tumors are smaller than 0.56 cc in volume at the time of the procedure. SRS optimized long term tumor control, eliminated risks associated with alternative surgical management, and facilitated cranial nerve preservation in the present report.
References
Matthies C, Samii M (1997) Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery 40: 1–9. (Discussion 9–10)
Andrews DW, Suarez O, Goldman HW, Downes MB, Bednarz G, Corn BW, Werner-Wasik M, Rosenstock J, Curran WJ Jr (2001) Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys 50:1265–1278
Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D (2005) Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg 102(Suppl):195–199
Mahboubi H, Sahyouni R, Moshtaghi O, Tadokoro K, Ghavami Y, Ziai K, Lin HW, Djalilian HR (2017) CyberKnife for treatment of vestibular schwannoma: a meta-analysis. Otolaryngol Head Neck Surg 157: 7–15. https://doi.org/10.1177/0194599817695805
Meijer OW, Vandertop WP, Baayen JC, Slotman BJ (2003) Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma: a single-institution study. Int J Radiat Oncol Biol Phys 56:1390–1396
Suh JH, Barnett GH, Sohn JW, Kupelian PA, Cohen BH (2000) Results of linear accelerator-based stereotactic radiosurgery for recurrent and newly diagnosed acoustic neuromas. Int J Cancer 90:145–151
Gardner G, Robertson JH (1988) Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol 97:55–66
Koos WT, Day JD, Matula C, Levy DI (1998) Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg 88:506–512. https://doi.org/10.3171/jns.1998.88.3.0506
House JW, Brackmann DE (1985) Facial nerve grading system. Otolaryngol Head Neck Surg 93:146–147
Bowden G, Cavaleri J, Monaco E III, Niranjan A, Flickinger J, Lunsford LD (2017) Cystic vestibular schwannomas respond best to radiosurgery. Neurosurgery 81:490–497. https://doi.org/10.1093/neuros/nyx027
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Bowden GN, Niranjan A, Lunsford LD (2019) Leksell radiosurgery for vestibular schwannomas. Progr Neurol Surg 34:82–90. https://doi.org/10.1159/000493053
Kalogeridi MA, Kougioumtzopoulou A, Zygogianni A, Kouloulias V (2019) Stereotactic radiosurgery and radiotherapy for acoustic neuromas. Neurosurg Rev. https://doi.org/10.1007/s10143-019-01103-6
Tsao MN, Sahgal A, Xu W, De Salles A, Hayashi M, Levivier M, Ma L, Martinez R, Regis J, Ryu S, Slotman BJ, Paddick I (2017) Stereotactic radiosurgery for vestibular schwannoma: International Stereotactic Radiosurgery Society (ISRS) practice guideline. J Radiosurg SBRT 5:5–24
Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD (2001) Results of acoustic neuroma radiosurgery: an analysis of 5 years' experience using current methods. J Neurosurg 94:1–6. https://doi.org/10.3171/jns.2001.94.1.0001
Klijn S, Verheul JB, Beute GN, Leenstra S, Mulder JJ, Kunst HP, Hanssens PE (2016) Gamma Knife radiosurgery for vestibular schwannomas: evaluation of tumor control and its predictors in a large patient cohort in The Netherlands. J Neurosurg 124:1619–1626. https://doi.org/10.3171/2015.4.JNS142415
Carlson ML, Jacob JT, Pollock BE, Neff BA, Tombers NM, Driscoll CL, Link MJ (2013) Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg 118:579–587. https://doi.org/10.3171/2012.9.JNS12919
Hasegawa T, Kato T, Yamamoto T, Naito T, Kato N, Torii J, Ishii K (2018) Long-term hearing outcomes after gamma knife surgery in patients with vestibular schwannoma with hearing preservation: evaluation in 92 patients with serial audiograms. J Neurooncol 138:283–290. https://doi.org/10.1007/s11060-018-2784-x
Kano H, Kondziolka D, Khan A, Flickinger JC, Lunsford LD (2009) Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuroma. J Neurosurg 111:863–873. https://doi.org/10.3171/2008.12.JNS08611
Lobato-Polo J, Kondziolka D, Zorro O, Kano H, Flickinger JC, Lunsford LD (2009) Gamma knife radiosurgery in younger patients with vestibular schwannomas. Neurosurgery 65: 294–300. https://doi.org/10.1227/01.NEU.0000345944.14065.35. (Discussion 300–291)
Roos DE, Potter AE, Zacest AC (2011) Hearing preservation after low dose linac radiosurgery for acoustic neuroma depends on initial hearing and time. Radiother Oncol 101:420–424. https://doi.org/10.1016/j.radonc.2011.06.035
Watanabe S, Yamamoto M, Kawabe T, Koiso T, Yamamoto T, Matsumura A, Kasuya H (2016) Stereotactic radiosurgery for vestibular schwannomas: average 10-year follow-up results focusing on long-term hearing preservation. J Neurosurg 125:64–72. https://doi.org/10.3171/2016.7.GKS161494
Akpinar B, Mousavi SH, McDowell MM, Niranjan A, Faraji AH, Flickinger JC, Lunsford LD (2016) Early radiosurgery improves hearing preservation in vestibular schwannoma patients with normal hearing at the time of diagnosis. Int J Radiat Oncol Biol Phys 95:729–734. https://doi.org/10.1016/j.ijrobp.2016.01.019
Patnaik U, Prasad SC, Tutar H, Giannuzzi AL, Russo A, Sanna M (2015) The long-term outcomes of wait-and-scan and the role of radiotherapy in the management of vestibular schwannomas. Otol Neurotol 36:638–646. https://doi.org/10.1097/MAO.0000000000000657
Kondziolka D, Mousavi SH, Kano H, Flickinger JC, Lunsford LD (2012) The newly diagnosed vestibular schwannoma: radiosurgery, resection, or observation? Neurosurg Focus 33:E8. https://doi.org/10.3171/2012.6.FOCUS12192
Walsh RM, Bath AP, Bance ML, Keller A, Rutka JA (2000) Consequences to hearing during the conservative management of vestibular schwannomas. The Laryngoscope 110:250–255. https://doi.org/10.1097/00005537-200002010-00012
Comey CH, McLaughlin MR, Jho HD, Martinez AJ, Lunsford LD (1998) Death from a malignant cerebellopontine angle triton tumor despite stereotactic radiosurgery. Case report. J Neurosurg 89:653–658. https://doi.org/10.3171/jns.1998.89.4.0653
Cahan WG, Woodard HQ et al (1948) Sarcoma arising in irradiated bone; report of 11 cases. Cancer 1:3–29
Wise SC, Carlson ML, Tveiten OV, Driscoll CL, Myrseth E, Lund-Johansen M, Link MJ (2016) Surgical salvage of recurrent vestibular schwannoma following prior stereotactic radiosurgery. Laryngoscope 126:2580–2586. https://doi.org/10.1002/lary.25943
Acknowledgements
The work described in this report was funded by a research Grant to Dr. Kano from AB Elekta, Stockholm, Sweden.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors other than HK declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Johnson, S., Kano, H., Faramand, A. et al. Long term results of primary radiosurgery for vestibular schwannomas. J Neurooncol 145, 247–255 (2019). https://doi.org/10.1007/s11060-019-03290-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03290-0