Abstract
Background
Atypical meningiomas (WHO grade II) have high recurrence rate. However, data on the effect of radiotherapy (RT) is still conflicting. The aim of this study was to evaluate the influence of postoperative RT on the recurrence of primary atypical intracranial meningiomas.
Methods
The medical records of all patients who underwent surgery (2007–2017 in 4 neurosurgical departments) for a histologically diagnosed primary atypical meningioma were reviewed to assess progression-free survival (PFS) and prognostic factors.
Results
This analysis included 258 patients with a median age of 60 years (54.7% female). The predominant tumor locations were convexity and falx (60.9%) followed by the skull base (37.2%). Simpson grade I–II resection was achieved in 194 (75.2%) patients, Simpson grade III–IV in 53 patients (20.5%). Tumor progressed in 54 cases (20.9%). Postoperative RT was performed in 46 cases (17.8%). RT was more often applied after incomplete resection (37.7% vs. 13.4% Simpson III–IV vs. I–II). A multivariate analysis showed a significantly shorter PFS associated with Simpson III–IV [HR 1.19, (95% CI) 1.09–1.29, p < 0.001] and age > 65 years [HR 2.89, (95% CI) 1.56–5.33, p = 0.001]. A subgroup analysis with a minimal follow-up of 36 months revealed that Simpson III–IV [HR 3.01, 95% CI 1.31–6.931.03–1.24, p = 0.009] and age > 65 years [HR 2.48, 95% CI 1.20–5.13, p = 0.014] reduced PFS. The impact of postoperative RT on PFS remained statistically insignificant, even in a propensity-score matched survival analysis [n = 46; p = 0.438; OR 0.710 (0.299–1.687)].
Conclusions
In the present study, postoperative RT did not improve PFS. The most important prognostic factors remain the extent of resection and age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are mostly benign, extra-axial tumors, arising from non-neuroepithelial progenitor cells, the arachnoidal cap cells, and comprise up to 30% of all intracranial tumors [1, 2]. These tumors are classified by the WHO according to their prognosis and their recurrence rate into benign (grade I), which comprise many histological subtypes, atypical (grade II) and anaplastic (grade III) [3, 4]. Grade I tumors make up the majority of these tumors, whereas the grade III meningiomas are very rare. The revisions of the WHO classification in 2007 produced a marked increase in atypical meningiomas, from previously 5% [5] to 20–35% [6, 7].
Atypical meningiomas have a greater recurrence rate than grade I tumors. According to an inverse relationship, every recurrence leads to shorter disease-free survival [8]. Therefore, the postoperative treatment of grade II meningiomas poses significant challenges. Up to 30–60% of atypical meningiomas recur within 5 years after surgical resection [9,10,11,12,13]. Many potential prognostic factors for tumor recurrence have been studied and most were not consistently accepted [11]. The value of postoperative RT of these tumors is still under debate because findings of retrospective studies of mostly small cohorts are conflicting and results of a prospective study are still pending.
Therefore, in this study, we aimed to evaluate the influence of adjuvant RT on progression free survival after surgery for primary atypical intracranial meningiomas in the largest cohort analyzed, so far.
Patients and methods
Patient sample, study design and outcome measures
A retrospective multi-center database of atypical meningiomas included surgically treated consecutive patients from 4 neurosurgical departments over a time period of 10 years (2007–2017). The inclusion criteria were: newly diagnosed primary atypical meningioma, at least one postoperative follow-up at ≥ 3 months after surgery, and no history of neurofibromatosis or previous brain RT. Demographic and clinical data such as sex, age at time of operation, tumor location, extent of tumor resection, postoperative treatment, follow-up period, and recurrence rate were assessed.
Tumor location was categorized as convexity and falcine, skull base and intraventricular.
Postoperative follow-up was performed by clinical investigation and evaluation of neuroimages either from magnetic resonance imaging (MRI) or, when not available/contraindicated, from computed tomography (CT) scans. Recurrence was recorded when a re-growth was observed in follow-up imaging. Time from date of operation until the date of neuro-imaging data indicating a tumor recurrence was defined as progression-free survival (PFS).
Pathological diagnosis was made based on the latest WHO criteria for classification of meningiomas from 2007 [3]. Simpson grade was used to classify the extent of tumor resection [14].
Statistical analysis
Categorical data were described by absolute and relative frequency and continuous data were described by mean, standard error and range.
PFS was defined as the time from the operation to the detection of the tumor regrowth based on imaging.
Univariate analysis for PFS was performed using a Cox regression model test and described using Kaplan–Meier plots. A multivariate Cox regression model was used to evaluate possible prognostic factors for meningioma recurrence. A Chi-Squared test was used to evaluate the equality of distribution among groups. The factors studied were age, sex, treating institution, postoperative RT, tumor location and complete vs. partial tumor resection. A propensity matching for RT was performed with matching factors age and Simpson resection grade and tumor location to further evaluate the influence of RT. A p-value less than 0.05 was considered as statistically significant. Adjustment for multiple testing was not performed.
Results
Demographic and treatment data
The analysis included 258 patients. The patient characteristics are summarized in Table 1. Mean age at the time of diagnosis was 60 years (range 12–84 years) and 141 were female. The tumor locations were convexity and falcine (60.9%), followed by skull base (37.2%) and intraventricular (1.9%) (Table 1).
Gross total resection (Simpson grade I–II) was achieved in 194 (75.2%) patients and subtotal resection (Simpson grade III–IV) in 53 (20.5%) patients. In 11 cases the extent of resection was not reported (4.3%). No patients who underwent biopsy only (Simpson grade V) were found in the database. Subtotal resection was performed mostly for skull base tumors (32.3% of skull base tumors) (Table 2).
Adjuvant RT was performed in 46 (17.8%) patients. The decision for adjuvant RT was mainly based on an incomplete resection. When compared with non-irradiated patients, RT was more often applied after incomplete resection (13.4% vs. 37.7% RT for Simpson grades I–II vs. III–IV resections, respectively; p < 0.001). In 43 patients (93.5%) RT was performed during the initial postoperative phase (mean 128.3 days ± 177.4 SD). Another 3 patients (6.5%) underwent RT in case of tumor recurrence. The proportion of patients selected for RT differed among the 4 centers (center 1: 15.0%; center 2: 14.3%; center 3: 9.5%; and center 4: 31%; p = 0.007). Most cases received a conventional radiotherapy [41 patients, 89.1%; either intensity modulated radiotherapy (IMRT) or 3D conformal radiotherapy (3DCRT)], while 2 patients underwent a stereotactic radiosurgery (4.3%). The treatment modality is unknown in 3 cases (6.5%). Mean radiation doses in the conventionally irradiated patients were 56.6 ± 6.7 Gy and 23 ± 0.1 Gy in the 2 patients treated by stereotactic radiosurgery. Tumor recurrence was observed in 11 of the 46 irradiated patients (23.9%) (Table 3).
Follow-up and recurrence rate
Median follow-up was 31 months (range 3–309). Tumor recurrence was observed in 54 (20.9%) patients, among these 11 had previous adjuvant RT (20.1%). The median progression-free survival of the relapsed patients was 91 months (95% CI 78.8–103.2). In 14.4% of patients recurrence was observed after Simpson grades I–II and in 30.2% of patients after subtotal resection according to Simpson grades III–IV.
Progression-free survival
Univariate analysis for progression-free survival was performed by categorizing patients according to the tumor location: skull base or convexity/falcine meningiomas. No significant link was found between RT and location (p = 0.468) as well as location and tumor relapse (p = 0.491).
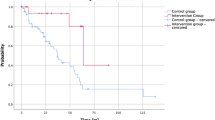
As univariate analysis revealed, RT itself was also not associated with prolonged PFS (p = 0.269, HR 0.35–1.34) (Fig. 1a). The 5-years PFS of irradiated patients was 70.7% compared to 68.8% of those without RT. No influence of radiation treatment on PFS was evident after categorization of patients according to complete (Simpson grade I–II) and incomplete resection (Simpson grade III–IV) (p = 0.654, p = 0.846) (Fig. 2).
a Progression-free survival (PFS) of patients with and without RT showing no significant difference; b PFS of patients with age 65 and less vs. > 65 years, showing a significant difference in PFS; c PFS of patients with Simpson grade I-IV, showing that grades I and II have a better PFS; d PFS of patients with Simpson grades I + II vs. III + IV underlining the results of c
Age significantly influenced tumor recurrence. The group of 89 patients older than 65 years had a significantly shorter PFS on univariate analysis [p = 0.004 HR 2.29 (1.31–4.00)] than did those who were < 65 years of age (Fig. 1b).
The factors treating center (p = 0.567, HR 0.74–1.18), sex (p = 0.078, HR 0.35–1.06) and tumor location (p = 0.589, HR 0.69–1.92) did not influence PFS.
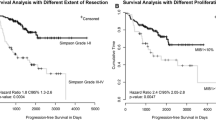
The extent of resection significantly influenced tumor recurrence. Patients with Simpson grade III–IV resection had significantly shorter PFS in comparison to patients with grade I–II resection [p < 0.001 HR 3.47 (1.84–6.52)].
A multivariate regression analysis of the independent variables RT, age and extent of resection demonstrated that a significantly shorter PFS was associated with Simpson grade III–IV in comparison to grade I–II (HR 1.19, 95% CI 1.09–1.29, p < 0.001; Fig. 1c, d) and age > 65 years (HR 2.89, 95% CI 1.56–5.33, p = 0.001). The 5-years PFS was 78.9% for the Simpson grade I + II and 34.1% for the Simpson grade III + IV group (p < 0.001).
A multivariate analysis of a subgroup with a minimal follow-up of 36 months (112 patients including 18 RT cases) also showed that the following factors significantly reduced PFS: Simpson grades III–IV (HR 3.01, 95% CI 1.31–6.93, p = 0.009) and age > 65 years (HR 2.48, 95% CI 1.20–5.13, p = 0.014).
To further evaluate the influence of RT, a propensity-matched survival analysis was performed. The propensity scores were calculated using age and Simpson resection grade. After propensity matching, no statistical difference in PFS was found [n = 46 (exact 2, fuzzy 44); p = 0.438; HR 0.710 (0.299–1.687)].
Propensity scores were calculated again using age, location and Simpson resection grade. Again, no statistical difference in PFS was found [n = 46 (exact 5, fuzzy 41); p = 0.934; HR 0.965 (0.417–2.237)].
Discussion
Atypical meningiomas are a heterogeneous group of tumors; altogether they have a high, 30–60%, 5-year recurrence rate [9,10,11,12,13, 15, 16]. Tumor recurrence is associated with markedly reduced overall survival rates after resection [15]. Talacchi et al. reported a 5-year second tumor recurrence of > 70% of patients after total resection and up to 90% after subtotal resection [8]. Therefore, postoperative treatment, especially the effectiveness of adjuvant RT, has been intensely evaluated. All current findings are derived from retrospective studies and are inconsistent. Study results about adjuvant RT that are certain enough to support a treatment guideline are not available. However, cohorts of available studies are small which hampering relevant conclusions.
Therefore, we aimed to assess the influence of RT by analyzing the clinical course of a large cohort of primary intracranial atypical meningioma patients from four neurosurgical sites treated within the last ten years. Despite having a retrospective character, this study is the largest multi-centric case series to analyze PFS and prognostic factors of atypical meningiomas. The database consisted of 258 patients, 46 were subjected to adjuvant RT.
The median age of 60 years is consistent with other reports of atypical meningiomas [12, 17,18,19]. The tumors occurred slightly more often in females. Traditionally, atypical tumors are non-gender-specific or occur more frequently in males and higher prevalence in females is more typical of benign meningiomas [1, 12, 18, 20, 21].
The extent of resection was usually extensive; approximately 80% of patients had a Simpson grade I or II independent of the treating center. The decision for adjuvant RT correlated with the Simpson resection grade. Patients who underwent a less extensive resection, grades III and IV, were significantly more often subjected to adjuvant therapy than were grade I and II resected patients. A study conducted in German neurosurgical centers reported that 74.1% of patients were offered adjuvant RT after incomplete and 17.9% after complete resection [22]. In a systematic meta-analysis of gross total resected atypical meningiomas, pooling 757 patients with atypical meningiomas, Hasan et al. reported of no influence of adjuvant RT on the 5-year PFS. This supports the selection of subtotally resected patients for adjuvant RT [23].
Unlike the extent of resection, the decision for adjuvant RT in this study differed significantly among the four participating centers. Marcus et al. surveyed British and Irish neurosurgeons regarding their opinion and practice of adjuvant RT for WHO grade I and II meningiomas. Although 59% of the neurosurgeons would advise adjuvant RT after incomplete resection, only 20% did so [24]. These observations underline the need for clear treatment guidelines for these tumors.
Progression-free survival
We observed a significant difference between PFS after gross total and subtotal tumor resection. After a Simpson grade I or II resection, patients had a significantly longer PFS than after a Simpson grade III or IV resection. Most studies agree that gross total resection results in a much lower tumor recurrence rate than after subtotal resection [13, 16, 17, 20, 25, 26]. This supports the recommendation of primary surgical treatment with the aim of gross total resection.
Some series showed older age to be associated with earlier tumor recurrence and shorter overall survival [9, 15, 17, 20, 27]. In our series, we also found that older patients (> 65 years) had a significantly shorter PFS. In accordance with our findings a multicentric French study concluded that the only factors increasing PFS in atypical meningiomas were age less than 60 years and Simpson Grade I removal in atypical meningiomas [17].
In agreement with the results of the entire study cohort, a multivariate analysis of a subgroup with a follow-up period of at least 36 months (112 patients including 18 RT cases) revealed the Simpson grade III–IV and age > 65 years as negative predictive factors for PFS.
In propensity-matched pairs, no significant difference in PFS was found between RT and non-RT group.
Tumor location has also been found to be associated with PFS. Masalha et al. reported a longer PFS of patients harboring an atypical meningioma in the anterior or posterior fossa [26]. Furthermore, the location parasagittal-falcine and non-skull base was found to be associated with an earlier recurrence rate [28, 29]. However, many series reported of no association of tumor location and PFS [15, 18, 21, 27]. In our study location had no impact on PFS.
Radiotherapy
In the present study, adjuvant RT did not influence the PFS. Moreover, a subgroup analysis of patients with a minimal follow-up of > 36 months of follow-up revealed adjuvant RT to predict shorter PFS. However, published findings on the use of adjuvant RT are controversially.
Nowak et al. recently reported a clear trend towards fewer recurrences after adjuvant RT; however, no statistical significance could be established [11]. The same results were observed by Aghi et al., who reported no tumor recurrence in irradiated patients after gross total resection, but the difference did not reach statistical significance [15]. Komotar et al. demonstrated a trend of fewer tumor recurrences after adjuvant RT [19]. In the latter two studies, no subtotally resected patients were included. In our study a decision towards an adjuvant RT was mainly based on remnant tumor in 20 cases (43.5% Simpson grade III + IV resection grades). A systematic review addressing this question was published in 2014 [30]. It concluded that adjuvant RT significantly improves local control of atypical meningiomas after subtotal resection; however, this was not supported by findings after gross total resection. The authors hypothesized that due to decreasing recurrence rate after high dose RT, adjuvant RT should be effective in this patient subpopulation as well [30].
Many studies do not find adjuvant RT significantly advantageous after gross total resection. Published finding on PFS after subtotal resection is more consistent. In such cases, adjuvant RT is usually recommended. For example, the large series reported by Mair et al. included 114 patients and demonstrated a significant benefit of RT only after subtotal resection; no significant benefit was reported for patients after gross total resection (Simpson grade I–II) [31]. In line with these data there was no increase in PFS achieved by adjuvant RT after gross total resection in our series (23 patients; 56.5%) (Fig. 2). A Korean study showed no tumor recurrence after Simpson grade I resection, with and without RT; therefore, in such cases only clinical and radiographic observation was recommended [18]. Following less extensive resection, adjuvant RT was suggested, because it increased PFS [18]. Hardesty et al. analyzed patients after gross total removal and showed that RT (stereotactic or intensity modulated) did not influence PFS; they suggested close observation as a reasonable strategy in such patients [25]. Cao et al. reported that gross total resection was associated with better PFS and overall survival, but they established no relation with RT and survival [10]. Moreover, a recent study by Jenkinson et al. showed that gross total resection increased PFS (but not overall survival) [21]. Adjuvant RT did not influence PFS nor overall survival regardless of the extent of resection. Consistent with these results, in their single center series of atypical meningiomas, Masalha et al. showed no impact of RT on PFS [26]. Another recent meta-analysis addressing the impact of adjuvant RT on atypical meningiomas selected 17 papers including 1,761 patients, and concluded that there is no evidence that RT decreases recurrence rate of atypical meningiomas [32].
Several other studies reported detrimental effects of RT as well. In a French multicenter study, adjuvant RT was associated with shorter PFS [9]. Another study reported that RT led to no survival advantage and could even be a detrimental factor [33].
Late toxicity is one of the most feared side effects of adjuvant therapy, even though its prevalence after meningioma resection is rare. A recent study showed, that up to 13% of irradiated patients present with signs of late toxicity; approximately a half of them show signs of cognitive deficits [9]. In the present study the majority of irradiated patients underwent conventional RT (89.1%) with a mean dose of 56.6 (± 6.7) Gy. No side effects clearly related to RT were reported. Conventional postoperative RT is usually well tolerated [15]; grade 2 and 3 side effects occurring in up to 10% of patients [9]. A RT dose lower than 53 Gy has been shown to be inadequate to improve postoperative outcome [2]. On the other hand, a study by Hug et al., using an earlier classification of meningiomas, revealed that a RT dose greater than 60 Gy significantly improved local control [34]. In addition, Boskos et al. reported similar findings [35]. However it has also been reported that very aggressive RT with more than 60 Gy does not improve outcome and only increases side effects [36]. Therefore, possible side effects should always be acknowledged, but not exaggerated, when considering adjuvant RT.
Other influencing factors
In our study, the recurrence of atypical meningiomas was more frequent in the first 29 months independent from RT. Katz et al. and Nowak et al. observed that most of the tumor recurrences occur within 4 years, after that they rarely develop recurrent disease [11, 36]. This lead to the hypothesis that other factors influenced early recurrence of atypical meningiomas, which could help stratify atypical meningiomas into subgroups according to the risk of recurrence. Despite the fact that among the irradiated patients 56.5% were graded as Simpson grade I–II, the decision for adjuvant RT was individually made due to unknown reasons. For the non-recurring meningiomas, the adjuvant RT might be unnecessary. Regarding this, there is a strong need for defined risk stratification factors. For example, a Norwegian group suggested a reconsideration of histopathological criteria to achieve stronger prognostic values (i.e. two out of three: absence of psammoma bodies, presence of necrosis, and/or ≥ 4 mitoses per 10 high power fields) [37]. Another study has shown sheeting/loss of lobular architecture and prominent nucleoli to be predictive markers for tumor recurrence [15]. Recent advances in genetic and molecular diagnostics is slowly leading to a better understanding of the biological behavior of meningiomas [38]. Sahm et al. performed a genome-wide methylation pattern analysis and found significant correlations of distinct methylation classes with tumor growth and recurrence patterns. They suggested a new classification of meningiomas by methylation classes to improve decision making for adjuvant treatment strategies [38]. In addition, epigenetic changes, such as the loss of histone H3K27me3, have been described to correlate with a poor outcome and a shorter PFS [39]. Some studies identified telomerase reverse transcriptase (TERT) promotor mutations in approximately 6% of all meningioma patients that were associated with shorter PFS [40,41,42]. These molecular markers need to be further evaluated for their potential for risk assessment; they could support more targeted decisions about adjuvant treatment after surgery.
Conclusion
Our findings demonstrated no significant difference in PFS with and without postoperative RT. Therefore, in accordance to these findings and the literature review, we suggest that in patients who undergo gross total resection, a close clinical and radiographic follow-up is a reasonable postoperative treatment strategy. After an incomplete resection, postoperative RT might be considered as an option for tumor control which is, however, not supported by the present data.
Limitations of this study
Due to its retrospective design, this study has significant drawbacks. Maybe the worse performance of irradiated meningiomas in our study is due to surgical related decisions—individual decisions on the application of RT cannot be excluded. More definitive answers are expected from the results of ongoing prospective studies.
References
Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, Vecht C (2008) Meningioma. Crit Rev Oncol Hematol 67:153–171. https://doi.org/10.1016/j.critrevonc.2008.01.010
Modha A, Gutin PH (2005) Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery 57:538–550 (discussion 538–550)
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Jääskeläinen J, Haltia M, Servo A (1986) Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol 25:233–242
Willis J, Smith C, Ironside JW, Erridge S, Whittle IR, Everington D (2005) The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol 31:141–149. https://doi.org/10.1111/j.1365-2990.2004.00621.x
Rogers L, Gilbert M, Vogelbaum MA (2010) Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol 99:393–405. https://doi.org/10.1007/s11060-010-0343-1
Talacchi A, Muggiolu F, De Carlo A, Nicolato A, Locatelli F, Meglio M (2016) Recurrent atypical meningiomas: combining surgery and radiosurgery in one effective multimodal treatment. World Neurosurg 87:565–572. https://doi.org/10.1016/j.wneu.2015.10.013
Pasquier D, Bijmolt S, Veninga T, Rezvoy N, Villa S, Krengli M, Weber DC, Baumert BG, Canyilmaz E, Yalman D, Szutowicz E, Tzuk-Shina T, Mirimanoff RO, Network RC (2008) Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys 71:1388–1393. https://doi.org/10.1016/j.ijrobp.2007.12.020
Cao X, Hao S, Wu Z, Wang L, Jia G, Zhang L, Zhang J (2015) Treatment Response and prognosis after recurrence of atypical meningiomas. World Neurosurg 84:1014–1019. https://doi.org/10.1016/j.wneu.2015.05.032
Nowak A, Dziedzic T, Krych P, Czernicki T, Kunert P, Marchel A (2015) Benign versus atypical meningiomas: risk factors predicting recurrence. Neurol Neurochir Pol 49:1–10. https://doi.org/10.1016/j.pjnns.2014.11.003
Pisćević I, Villa A, Milićević M, Ilić R, Nikitović M, Cavallo LM, Grujičić D (2015) The influence of adjuvant radiotherapy in atypical and anaplastic meningiomas: a series of 88 patients in a single institution. World Neurosurg 83:987–995. https://doi.org/10.1016/j.wneu.2015.02.021
Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH (2000) Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys 46:57–61
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20:22–39
Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, Curry WT, Barker FG (2009) Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64:56–60. https://doi.org/10.1227/01.NEU.0000330399.55586.63 (discussion 60)
Klinger DR, Flores BC, Lewis JJ, Hatanpaa K, Choe K, Mickey B, Barnett S (2015) Atypical meningiomas: recurrence, reoperation, and radiotherapy. World Neurosurg 84:839–845. https://doi.org/10.1016/j.wneu.2015.04.033
Durand A, Labrousse F, Jouvet A, Bauchet L, Kalamaridès M, Menei P, Deruty R, Moreau JJ, Fèvre-Montange M, Guyotat J (2009) WHO grade II and III meningiomas: a study of prognostic factors. J Neurooncol 95:367–375. https://doi.org/10.1007/s11060-009-9934-0
Jo K, Park H-J, Nam D-H, Lee J-I, Kong D-S, Park K, Kim JH (2010) Treatment of atypical meningioma. J Clin Neurosci 17:1362–1366. https://doi.org/10.1016/j.jocn.2010.03.036
Komotar RJ, Iorgulescu JB, Raper DMS, Holland EC, Beal K, Bilsky MH, Brennan CW, Tabar V, Sherman JH, Yamada Y, Gutin PH (2012) The role of radiotherapy following gross-total resection of atypical meningiomas. J Neurosurg 117:679–686. https://doi.org/10.3171/2012.7.JNS112113
Zaher A, Abdelbari Mattar M, Zayed DH, Ellatif RA, Ashamallah SA (2013) Atypical meningioma: a study of prognostic factors. World Neurosurg 80:549–553. https://doi.org/10.1016/j.wneu.2013.07.001
Jenkinson MD, Waqar M, Farah JO, Farrell M, Barbagallo GMV, McManus R, Looby S, Hussey D, Fitzpatrick D, Certo F, Javadpour M (2016) Early adjuvant radiotherapy in the treatment of atypical meningioma. J Clin Neurosci 28:87–92. https://doi.org/10.1016/j.jocn.2015.09.021
Simon M, Boström J, Koch P, Schramm J (2006) Interinstitutional variance of postoperative radiotherapy and follow up for meningiomas in Germany: impact of changes of the WHO classification. J Neurol Neurosurg Psychiatry 77:767–773. https://doi.org/10.1136/jnnp.2005.077974
Hasan S, Young M, Albert T, Shah AH, Okoye C, Bregy A, Lo SS, Ishkanian F, Komotar RJ (2015) The role of adjuvant radiotherapy after gross total resection of atypical meningiomas. World Neurosurg 83:808–815. https://doi.org/10.1016/j.wneu.2014.12.037
Heufelder MJ, Sterker I, Trantakis C, Schneider J-P, Meixensberger J, Hemprich A, Frerich B (2009) Reconstructive and ophthalmologic outcomes following resection of spheno-orbital meningiomas. Ophthal Plast Reconstr Surg 25:223–236. https://doi.org/10.1097/IOP.0b013e3181a1f345
Hardesty DA, Wolf AB, Brachman DG, McBride HL, Youssef E, Nakaji P, Porter RW, Smith KA, Spetzler RF, Sanai N (2013) The impact of adjuvant stereotactic radiosurgery on atypical meningioma recurrence following aggressive microsurgical resection. J Neurosurg 119:475–481. https://doi.org/10.3171/2012.12.JNS12414
Masalha W, Heiland DH, Franco P, Delev D, Haaker JG, Schnell O, Scheiwe C, Grauvogel J (2018) Atypical meningioma: progression-free survival in 161 cases treated at our institution with surgery versus surgery and radiotherapy. J Neurooncol 136:147–154. https://doi.org/10.1007/s11060-017-2634-2
Champeaux C, Wilson E, Shieff C, Khan AA, Thorne L (2016) WHO grade II meningioma: a retrospective study for outcome and prognostic factor assessment. J Neurooncol 129:337–345. https://doi.org/10.1007/s11060-016-2181-2
Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, McDermott MW, Berger MS, Parsa AT (2011) Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer 117:1272–1278. https://doi.org/10.1002/cncr.25591
Vranic A, Popovic M, Cör A, Prestor B, Pizem J (2010) Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: a study of 86 patients. Neurosurgery 67:1124–1132. https://doi.org/10.1227/NEU.0b013e3181eb95b7
Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, Sun MZ, Barani IJ, James CD, Parsa AT (2014) Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol 16:628–636. https://doi.org/10.1093/neuonc/nou025
Mair R, Morris K, Scott I, Carroll TA (2011) Radiotherapy for atypical meningiomas. J Neurosurg 115:811–819. https://doi.org/10.3171/2011.5.JNS11112
Pereira BJA, de Almeida AN, Paiva WS, Teixeira MJ, Marie SKN (2018) Impact of radiotherapy in atypical meningioma recurrence: literature review. Neurosurg Rev. https://doi.org/10.1007/s10143-018-0959-8
Stessin AM, Schwartz A, Judanin G, Pannullo SC, Boockvar JA, Schwartz TH, Stieg PE, Wernicke AG (2012) Does adjuvant external-beam radiotherapy improve outcomes for nonbenign meningiomas? A Surveillance, Epidemiology, and End Results (SEER)-based analysis. J Neurosurg 117:669–675. https://doi.org/10.3171/2012.7.JNS111439
Hug EB, Devries A, Thornton AF, Munzenride JE, Pardo FS, Hedley-Whyte ET, Bussiere MR, Ojemann R (2000) Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol 48:151–160
Boskos C, Feuvret L, Noel G, Habrand J-L, Pommier P, Alapetite C, Mammar H, Ferrand R, Boisserie G, Mazeron J-J (2009) Combined proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int J Radiat Oncol Biol Phys 75:399–406. https://doi.org/10.1016/j.ijrobp.2008.10.053
Katz TS, Amdur RJ, Yachnis AT, Mendenhall WM, Morris CG (2005) Pushing the limits of radiotherapy for atypical and malignant meningioma. Am J Clin Oncol 28:70–74
Backer-Grøndahl T, Moen BH, Arnli MB, Torseth K, Torp SH (2014) Immunohistochemical characterization of brain-invasive meningiomas. Int J Clin Exp Pathol 7:7206–7219
Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, Okonechnikov K, Koelsche C, Reuss DE, Capper D, Sturm D, Wirsching H-G, Berghoff AS, Baumgarten P, Kratz A, Huang K, Wefers AK, Hovestadt V, Sill M, Ellis HP, Kurian KM, Okuducu AF, Jungk C, Drueschler K, Schick M, Bewerunge-Hudler M, Mawrin C, Seiz-Rosenhagen M, Ketter R, Simon M, Westphal M, Lamszus K, Becker A, Koch A, Schittenhelm J, Rushing EJ, Collins VP, Brehmer S, Chavez L, Platten M, Hänggi D, Unterberg A, Paulus W, Wick W, Pfister SM, Mittelbronn M, Preusser M, Herold-Mende C, Weller M, von Deimling A (2017) DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 18:682–694. https://doi.org/10.1016/S1470-2045(17)30155-9
Katz LM, Hielscher T, Liechty B, Silverman J, Zagzag D, Sen R, Wu P, Golfinos JG, Reuss D, Neidert MC, Wirsching H-G, Baumgarten P, Herold-Mende C, Wick W, Harter PN, Weller M, von Deimling A, Snuderl M, Sen C, Sahm F (2018) Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathol 135:955–963. https://doi.org/10.1007/s00401-018-1844-9
Abedalthagafi MS, Bi WL, Merrill PH, Gibson WJ, Rose MF, Du Z, Francis JM, Du R, Dunn IF, Ligon AH, Beroukhim R, Santagata S (2015) ARID1A and TERT promoter mutations in dedifferentiated meningioma. Cancer Genet 208:345–350. https://doi.org/10.1016/j.cancergen.2015.03.005
Sahm F, Schrimpf D, Olar A, Koelsche C, Reuss D, Bissel J, Kratz A, Capper D, Schefzyk S, Hielscher T, Wang Q, Sulman EP, Adeberg S, Koch A, Okuducu AF, Brehmer S, Schittenhelm J, Becker A, Brokinkel B, Schmidt M, Ull T, Gousias K, Kessler AF, Lamszus K, Debus J, Mawrin C, Kim Y-J, Simon M, Ketter R, Paulus W, Aldape KD, Herold-Mende C, von Deimling A (2016) TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv377
Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M (2014) High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol 24:184–189. https://doi.org/10.1111/bpa.12110
Acknowledgements
This work has been presented as a plenary talk at the meeting of the European Association of Neurosurgical Societies (EANS) in Brussels/ Belgium on 23rd October 2018.
Funding
This research was not supported by any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The ethics committee of Rhineland-Palatinate, Germany reviewed and approved this study [837.476.17 (11310)].
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keric, N., Kalasauskas, D., Freyschlag, C.F. et al. Impact of postoperative radiotherapy on recurrence of primary intracranial atypical meningiomas. J Neurooncol 146, 347–355 (2020). https://doi.org/10.1007/s11060-019-03382-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03382-x