Abstract
Purpose
The optimal interfraction intervals for fractionated radiosurgery has yet to be established. We investigated the outcome of fractionated gamma knife radiosurgery (FGKRS) for large brain metastases (BMs) according to different interfraction intervals.
Methods
Between September 2016 and May 2018, a total of 45 patients who underwent FGKRS for BMs were enrolled in this study. They were divided into two groups (standard fractionation over 3 consecutive days with a 24-h interfraction interval versus prolonged fractionation over 4 or 5 days with an interfraction interval of at least 48-h). BMs with ≥ 2 cm in maximum diameter or ≥ 5 cm3 in volume were included in analysis.
Results
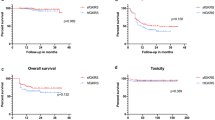
Among 52 BMs treated with 3-fraction GKRS, 25 (48.1%) were treated with standard fractionation scheme, and 27 (51.9%) with prolonged fractionation scheme. The median follow-up period was 10.5 months (range 5–25). Local tumor control rates of the standard group were 88.9% at 6 months and 77.8% at 12 months, whereas those of the prolonged group were 100% at 6 and 12 months (p = 0.023, log-rank test). In multivariate analysis, fractionation scheme (hazard ratio [HR] 0.294, 95% CI 0.099–0.873; p = 0.027) and tumor volume (HR 0.200, 95% CI 0.051–0.781; p = 0.021) were revealed as the only significant factors affecting the local tumor control after 3-fraction GKRS.
Conclusions
Our preliminary tumor control results suggest a promising role of 3-fraction GKRS with an interfraction interval of at least 48-h. This fractionation regimen could be an effective and safe treatment option in the management of large BMs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BMs) are the most common intracranial malignant tumors, accounting for an estimated 20% to 40% of patients with cancer [1]. The treatment options available for BMs include surgical resection, whole brain radiation therapy (WBRT), stereotactic radiosurgery, such as Gamma Knife radiosurgery (GKRS), systemic chemotherapy, or some combination of these modalities [2, 3]. Of them, GKRS has increasingly become the essential treatment modality for BMs, thereby affording excellent local tumor control and prolonging survival time [2, 4,5,6]. It can also reduce neurocognitive toxicities associated with WBRT [2, 6,7,8]. However, local recurrence after GKRS is not uncommon, especially for large BMs and occasionally warrants additional GKRS, increasing the risk of radiation injury to the normal tissue. Although surgical resection has been the standard treatment for large BMs, it is sometimes limited due to location in eloquent structures, poor performance status, and the extent of the systemic disease. In this respect, fractionated GKRS (FGKRS) has been emerging as a safe and effective regimen for large BMs as well as benign tumors abutting the critical neurovascular structures [2, 6, 8,9,10,11]. The rationale for using multifraction SRS is a potential radiobiological advantage that can result in a decreased incidence of radionecrosis [12, 13].
The optimal fractionation scheme for BMs is not well defined and remains controversial. In this preliminary study, we retrospectively assessed and analyzed the impact of fractioanted radiosurgery using gamma knife with different fractionation schedules on local tumor control in patients harboring large BMs. We present a single institution retrospective series documenting the impact of FGKRS with respect to different fractionation schedules.
Materials and methods
Patient population
After institutional review board approval (SMC 2019-01-135) was obtained, we performed a retrospective analysis of 1690 brain metastasis patients who underwent Gamma Knife radiosurgery at Samsung Medical Center between September 2016 to May 2018 and identified 218 patients treated with FGKRS for BMs. FGKRS has been usually recommended for large BMs in order to improve the treatment efficacy and reduce radiation-induced toxicity. The number of fractions (ranging from 2 to 5) or dose per fraction (ranging from 5 to 10 Gy) were chosen based on the patient’s clinical condition, characteristics of brain lesions (i.e., tumor volume, number of metastatic lesions, pathology, etc.), and preference of the patients and treating neurosurgeons. Because the purpose of this study was to evaluate the impact of interfraction interval on the outcome, it was necessary to minimize the influence of other confounding variables, such as different number of fractions or wide range of dose. Therefore, the analysis was limited to 3-fraction GKRS with 8–10 Gy per fraction, and 65 patients were found. Among them, 20 patients were excluded from the analysis and could be divided into two groups. One group of the patients with poor performance status at the time of GKRS was lost to follow-up, because they expired early due to systemic causes and therefore, follow-up imaging could not be performed. The other excluded group included those who underwent GKRS following either surgical resection or Ommaya reservoir placement for large cystic lesions. As a result, a total of 45 patients (25 females and 20 males) were enrolled in the final study cohort. The median age at the time of diagnosis of BMs was 59 years (range 22–80 years). The eligibility criteria included (1) adults aged ≥ 18 year with 1 to 5 brain metastatic lesions; (2) no previous history of surgical resection; (3) a maximal diameter ≥ 2 cm or the largest tumor volume ≥ 5cm3; (4) 3-fraction GKRS with a total dose of 24–30 Gy; (5) no previous history of WBRT; and (6) no radiological evidence of leptomeningeal seeding at the time of FGKRS. The patients were divided into two groups according to fractionation scheme: standard fractionation over 3 consecutive days with a 24-h interfraction interval and prolonged fractionation over 4 or 5 days with an interfraction interval of at least 48-h (Fig. 1).
Evaluation of tumor response using tumor volumetry
For each patient, clinical follow-up data were obtained during office evaluations of the treated patients after radiosurgery. Gadolinium-enhanced MRI of the brain was performed using a 3T MR scanner (Intera Achieva, Philips Medical Systems, Best, Netherlands) at the time of FGKRS and at 3-month intervals thereafter, and the images were exported to Leksell GammaPlan software (version 11.0.3, Elekta Instrument AB, Stockholm, Sweden) for assessment of the changes in tumor volume. Based on follow-up MR images, volumetric statistics included the volume reduction (%∆V) from baseline to last follow-up and the volume rate of change represented as percent rate of change (PRC), in percent/month at 6 months and 12 months [14]. Tumor responses after GKRS were classified into 2 different categories: tumor control (a decrease of > 15% volume or follow-up volume ± 15% of the initial volume) or tumor progression (an increase of > 15% volume) [14,15,16,17]. Follow-up MR scans included a contrast-enhanced cerebral blood volume (rCBV) map in order to differentiate radiation necrosis from tumor progression. Increased rCBV suggests tumor progression, whereas localized decreased rCBV suggests radiation necrosis. The radiation necrosis was not counted as an event in the response analysis. All radiographic data were reviewed by a dedicated neuroradiologist.
Evaluation of radiation-induced complications
Radiation-induced complications were defined as newly developed or aggravated neurological deficits after GKRS unless tumor progression occurred. The Radiation Therapy Oncology Group (RTOG) CNS toxicity criteria was used to assess radiation-induced complications after FGKRS of difference schemes: Grade 1, mild neurologic symptoms (no medication required); Grade 2, moderate neurologic symptoms (outpatient medication required); Grade 3, severe neurologic symptoms; Grade 4, life-threatening neurologic symptoms (e.g., uncontrolled seizures, paralysis, or coma); includes clinically or radiographically suspected radionecrosis and histologically proven radionecrosis at the time of an operation [18]. Severe complications were considered to have an RTOG CNS toxicity Grade ≥ 3.
Radiosurgical technique
The GKRS procedure was performed using a Leksell Gamma Knife® Icon™ (Elekta Instrument AB, Stockholm, Sweden), and the Leksell GammaPlan was used for treatment planning. Forty patients (88.9%) underwent frameless GKRS using an individually customized mask, while 5 patients (11.1%) underwent GKRS using the Leksell stereotactic frame. In order to prevent acute brain swelling, all patients received 5 mg dexamethasone intravenously immediately before the procedure and thereafter completed a 7-day course of dexamethasone following the procedure. Most of the patients were immobilized using a thermoplastic mask system, which consisted of customized 3-point Efficast® thermoplastic masks (Orfit Industries, Wijnegem, Belgium) and cradles (Moldcare®; Alcare Co, Tokyo, Japan). A high-resolution MRI scan was performed the day before the first fraction of GKRS; postcontrast T1-weighted axial images with a slice thickness of 1.0 mm and T2-weighted FLAIR images with a slice thickness of 2.0 mm were obtained for treatment planning. A planning cone beam computed tomography (CBCT) was subsequently performed and co-registered with MR images for target localization and verification. Gamma Knife® Icon™ tracks patient movement during treatment using a CBCT, a thermoplastic mask system, and an infrared-based high-definition motion management (HDMM) camera. For those who underwent FGKRS with rigid fixation, on the early morning of the first day of FGKRS, the head frame was placed by the treating neurosurgeon after application of a local anesthetic solution, and it was retained until the last day of the irradiation. Following frame placement, a stereotactic brain MRI scan of the same protocol was performed. Dose planning was performed and approved by the treating neurosurgeons and medical physicist.
Statistical analyses
The primary end point of this study was the local tumor control rate after FGKRS. Before analyses, summary statistics were displayed as means, medians, and ranges for continuous variables and as frequencies and proportions for categorical variables. Categorical variables using the χ2 test or Fisher exact test and continuous variables using the Mann–Whitney U-test were compared to compare proportions and means, respectively. Univariate and multivariate analyses using the Cox proportional hazard model were performed to determine pre-GKRS clinical factors favoring the local tumor control. The local progression-free survival was estimated by the Kaplan–Meier method and log-rank test. p values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS (version 25.0, IBM Co., Armonk, New York, USA).
Results
Patient demographics
The clinical characteristics of the 45 patients are listed in Table 1. At their initial presentation, 23 patients (51.1%) were treated with the 3-fraction GKRS of standard fractionation scheme, and 22 patients (48.9%) received the 3-fraction GKRS of prolonged fractionation scheme. The median duration of follow-up was 10.5 months (range 5–28 months). Lung cancer (n = 28, 62.2%) was the most common primary tumor followed by breast cancer (n = 9, 20.0%). No significant differences were observed between the two treatment groups with respect to Karnofsky Performance Scale score, RTOG recursive partitioning analysis (RPA) class, primary tumor status, number of BMs, and timing of BMs. There was a significant difference between the two groups in terms of presence of extracranial metastases (p = 0.018).
Treatment characteristics
Table 2 summarizes the GKRS treatment characteristics of two different fractionation schemes. Among a total of 68 lesions in 45 patients treated with 3-fraction GKRS, only 52 metastatic lesions with ≥ 2 cm in maximum diameter or ≥ 5 cm3 in volume were included in analysis. The median volume reduction for BMs in the standard scheme group was 55.5% (range − 25.8–100%), whereas it was 81.3% (range 39.1–100%) in the prolonged scheme group (p < 0.01) (Fig. 2b). Tumor volume changes for each metastatic lesion in both groups are shown in Fig. 2c.
Box plot showing the target tumor volumes in the standard and prolonged fractionation schedule groups (a). Box plot showing the significant difference in volume reduction (from baseline to last follow-up) between the two treatment groups (b). Tumor response to 3-fraction GKRS as assessed by relative change in volume between pretreatment and last follow-up MRI (c)
Local tumor control after 3-fraction GKRS with different interfraction intervals
The local tumor control rates of the standard scheme group were 88.9% at 6 months and 77.8% at 12 months, whereas those of the prolonged scheme group at 6 and 12 months were 100% (p = 0.023, log-rank test) (Fig. 3). In multivariate analysis, fractionation scheme (hazard ratio [HR] 0.294, 95% CI 0.099–0.873; p = 0.027) and tumor volume (HR 0.200, 95% CI 0.051–0.781; p = 0.021) were revealed as the only significant factors affecting the local tumor control after 3-fraction GKRS (Table 3).
CNS toxicity
Overall, radiation-induced complications according to RTOG CNS toxicity criteria occurred in in 8 patients (17.8%) after 3-fraction GKRS. The median tumor volume of the 8 treated lesions with radiation necrosis was 18.1 cm3 (range 13.3–37.9 cm3). No significant difference was found between the two treatment groups (p = 0.136) (Table 2). Among 6 patients in the standard scheme group, 2 patients (treated with 9 Gy × 3 and 10 Gy × 3, respectively) and 2 patients (treated with 9 Gy × 3 and 10 Gy × 3, respectively) developed RTOG grade 3 and 4 toxicities after treatment, respectively, eventually undergoing surgical resection for symptom relief. In the same group, 1 patient (treated with 9 Gy × 3) with RTOG grade 1 toxicity was managed with observation alone, whereas the other patient with grade 2 toxicity was tolerable with oral pain medication. In the prolonged scheme group, 1 patient (treated with 10 Gy × 3) with RTOG grade 2 toxicity improved with steroid administration alone, whereas the other patient (treated with 8 Gy × 3) with grade 4 toxicity underwent surgical resection. No RTOG CNS grade 5 toxicity after FGKRS was observed in both groups.
Discussion
The emerging role of FGKRS in the treatment for large BMs
Large BMs located in eloquent areas and patients with poor performance status are deemed appropriate for FGKRS, if surgical resection is not indicated [9]. Similar rates of local tumor control and lower risk of radiation-induced neurotoxicity after fractionated SRS have been previously reported in several studies [19,20,21,22,23]. It has been proposed that fractionated SRS with 2 to 5 fractions could be considered as an alternative approach for large-volume targets to improve the therapeutic ratio by allowing greater total doses to be delivered safely with minimal normal tissue toxicity [24]. A recent report concluded that multifraction SRS at a dose of 27 Gy in 3 consecutive fractions was an effective treatment modality for BMs larger than 2 cm in diameter and was associated with improved local control and reduced risk of radiation necrosis as compared with single-fraction SRS [12].
Radiobiology of fractionated SRS
The optimum radiotherapeutic strategy is defined as the treatment scheme that maximizes tumor biologically effective dose (BED) while keeping normal tissue BED constant [25]. However, the linear–quadratic (LQ) model and BED have been suggested to be incorrect when used for hypofractionation [26]. The LQ model overestimates the effect of high fractional doses of radiation, and BED is particularly incorrect when used for tumor responses in vivo, since it does not take reoxygenation into account [26,27,28]. Given that the biological effects of SRS are attributable to irreversible cellular damage and vascular occlusion, it has been suggested that SRS kills tumor cells not only through directly damaging DNA but also by causing vascular damage and increasing tumor hypoxia, thereby inducing indirect/secondary tumor cell death [29, 30]. Nevertheless, SRS has the potential to damage normal tissues nearby target volumes. In contrast, fractionated SRS does not damage surrounding tissues to the same extent as it better enables cellular reoxygenation and target volume redistribution, thus better preserving normal tissues than single-fraction SRS. Fractionated administration of radiation dose potentially minimizes toxicity to late-responding healthy tissues, with a low α/β ratio compared to a single acute dose of radiation for a given level of tumor damage, according to the LQ model of cellular survival [31]. Given that during fractionation surviving hypoxic tumor cells reoxygenate and become more sensitive to subsequent irradiation, reoxygenation between dose fractions is a pivotal phenomenon that should be fully utilized in fractionated SRS [26]. In in vivo tumor cells, rapid reoxygenation of hypoxic tumor cells may counterbalance the sublethal damage repair during fractionated radiation therapy [26]. In previous laboratory studies using three different murine tumors, reoxygenation was not complete within 24 h after irradiation, and with significantly lower hypoxic fractions at 24 h after irradiation, reoxygenation seemed to proceed further until 72 h after irradiation [26, 32]. In other words, longer interfraction intervals other than a 24-h interval may allow more reoxygenation to occur. In line with this finding regarding the importance of reoxygenation during fractionation, our results showed that FGKRS with interfraction intervals of at least 48 h was associated with a more favorable outcome.
GKRS with different fractionation schedules for BMs
The optimal FGKRS schedule for BMs has not yet been established. A few studies reported on the outcomes of FGKRS for BMs using several fractionated schemes with interfraction intervals of 1 to 4-weeks. Higuchi et al. reported 6- and 12-month local control rates of 89.8% and 75.9%, respectively, with 30 Gy in 3 fractions with a 2-week interfraction interval [33]. In line with the results of their pilot study, Yomo et al. showed an 85% and 64% local control rate at 6 and 12 months, respectively, after the delivery of 20–30 Gy in 2 fractions with an interfraction interval of 3–4 weeks [34, 35]. However, given that from a biologic point of view, the additional advantage of dose fractionation over a short treatment time has been suggested for malignant tumors, Aoyama et al. reported a local PFS rate of 81% at 1 year in the treatment of BMs with 35 Gy 4 fractions over a 4–6 days [7, 36]. Similarly, Kim et al. performed FGKRS with three consecutive days for large BMs and reported a 1-year local control rate of 90% [19]. In the present study, a 1-year local PFS of FGKRS with a 24-h interfraction interval for large BMs was 80.8%, whereas that of FGKRS with prolonged schedule including at least a 48-h interval was 100%. The latter fractionation schedule seemed to be tolerable and effective for large BMs. This may be attributable to 48- to 72-h interfraction intervals that permit more efficient reoxygenation of hypoxic tumor cells and redistribution of the cell cycle to a more sensitive phase. In addition, considering that 2 or 3 procedures with interfraction intervals of 2- or 3-weeks may be burdensome from the patient’s perspective, shorter interfraction intervals over the shorter treatment period, as described in this study, may be suitable for FGKRS in the treatment of large BMs, with similar tumor control rates and comparable toxicity rates. In addition, given that the pin-based, rigid head frame system is devoid of reproducibility once it was removed, Gamma Knife® Icon™ was developed to facilitate fractionated radiosurgery as well as frameless fixation, enabling on-board verification of patient position and correction using a quality CBCT system [37].
Radiation-related complications after FGKRS
The most common late-delayed radiation effect of SRS is the development of radiation-induced necrosis at a rate of 2–15% [7, 23, 38]. The risk of radiation-induced necrosis generally increases with higher doses, prior history of radiation therapy, and larger target volumes. Minniti et al. published a retrospective study that compared single-fraction SRS to a median dose of 18 Gy with multifraction SRS to a total dose of 27 Gy in 3 fractions in the patients with large BMs; the incidence of radionecrosis after multifraction SRS was 14.4% compared with 27.7% after single-session SRS [12]. In this study, 6 (26.1%) and 2 (9.1%) in the standard and prolonged groups manifested neurological symptoms, respectively, although no significant difference was observed between two different fractionation schemes (Table 4).
Study limitations
There are several limitations to this preliminary study. First, the current study was retrospective in design, based on a single institutional experience. Unknown bias may be inherent in patient selection for FGKRS in the treatment of large BMs. Second, our sample size was relatively small and approximately one-third of 65 patients who underwent 3-fraction GKRS were excluded due to a variety of reasons. Such a small cohort and relatively large number of cases excluded in analysis may limit the generalizability and statistical power. Third, FGKRS was applied using 3 different dose regimens with a total dose of 24, 27, and 30 Gy. The distribution of dose was not statistically different in the standard and prolonged interval groups, and it is not likely to be a major factor of different outcomes in this study. However, the combined effect of dose per fraction and interfraction interval might affect the efficacy and toxicity of treatment. Therefore, optimal fractionation schedule should be always considered with dose per fraction or total dose, and optimal dose needs to be defined according to each specific fractionation schedule in future studies. Fourth, a multiparametric study is encouraged to improve diagnostic accuracy for assessment of tumor response, because rCBV analysis for posttreatment evaluation may encompass treatment-related inflammation, leading to underestimation of viable tumor angiogenesis [39]. Lastly, the relatively short follow-up period could have underestimated the risk of delayed radiation-induced neurotoxicity and tumor progression. A longer follow-up study of a larger cohort is necessary to consolidate the efficacy and safety of FGKRS with an interfraction interval of at least 48-h. Furthermore, prospective confirmation of the preliminary results from the study is warranted.
Conclusion
In summary, this study represents a single institution’s preliminary experience with 3-fraction GKRS of standard and prolonged interfraction intervals, although it is limited by relatively short follow-up and by the absence of prospective assessment of patient outcomes. Our preliminary tumor control results suggest a promising role of 3-fraction GKRS with an interfraction interval of at least 48-h. This fractionation regimen could be an effective and safe treatment option in the management of large BMs, while decreasing the risk of radiation-related complications. A randomized controlled study is necessary to determine the efficacy and safety of the fractionation regimen in this study.
References
Lin X, DeAngelis LM (2015) Treatment of brain metastases. J Clin Oncol 33:3475–3484. https://doi.org/10.1200/JCO.2015.60.9503
Hasegawa T, Kato T, Yamamoto T, Iizuka H, Nishikawa T, Ito H, Kato N (2017) Multisession gamma knife surgery for large brain metastases. J Neurooncol 131:517–524. https://doi.org/10.1007/s11060-016-2317-4
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG 2nd, Deming R, Burri SH, Menard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401–409. https://doi.org/10.1001/jama.2016.9839
Higuchi Y, Yamamoto M, Serizawa T, Aiyama H, Sato Y, Barfod BE (2018) Modern management for brain metastasis patients using stereotactic radiosurgery: literature review and the authors' gamma knife treatment experiences. Cancer Manag Res 10:1889–1899. https://doi.org/10.2147/CMAR.S116718
Serizawa T, Higuchi Y, Yamamoto M, Matsunaga S, Nagano O, Sato Y, Aoyagi K, Yomo S, Koiso T, Hasegawa T, Nakazaki K, Moriki A, Kondoh T, Nagatomo Y, Okamoto H, Kohda Y, Kawai H, Shidoh S, Shibazaki T, Onoue S, Kenai H, Inoue A, Mori H (2018) Comparison of treatment results between 3- and 2-stage gamma knife radiosurgery for large brain metastases: a retrospective multi-institutional study. J Neurosurg. https://doi.org/10.3171/2018.4.JNS172596
Yamamoto M, Serizawa T, Higuchi Y, Sato Y, Kawagishi J, Yamanaka K, Shuto T, Akabane A, Jokura H, Yomo S, Nagano O, Aoyama H (2017) A multi-institutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901 study update): irradiation-related complications and long-term maintenance of mini-mental state examination scores. Int J Radiat Oncol Biol Phys 99:31–40. https://doi.org/10.1016/j.ijrobp.2017.04.037
Aoyama H, Shirato H, Onimaru R, Kagei K, Ikeda J, Ishii N, Sawamura Y, Miyasaka K (2003) Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys 56:793–800
Serizawa T, Higuchi Y, Nagano O, Sato Y, Yamamoto M, Ono J, Saeki N, Miyakawa A, Hirai T (2012) Analysis of 2000 cases treated with gamma knife surgery: validating eligibility criteria for a prospective multi-institutional study of stereotactic radiosurgery alone for treatment of patients with 1–10 brain metastases (JLGK0901) in Japan. J Radiosurg SBRT 2:19–27
McTyre E, Helis CA, Farris M, Wilkins L, Sloan D, Hinson WH, Bourland JD, Dezarn WA, Munley MT, Watabe K, Xing F, Laxton AW, Tatter SB, Chan MD (2017) Emerging indications for fractionated gamma knife radiosurgery. Neurosurgery 80:210–216. https://doi.org/10.1227/NEU.0000000000001227
Yamamoto M, Kawabe T, Sato Y, Higuchi Y, Nariai T, Barfod BE, Kasuya H, Urakawa Y (2013) A case-matched study of stereotactic radiosurgery for patients with multiple brain metastases: comparing treatment results for 1–4 vs %3e/= 5 tumors: clinical article. J Neurosurg 118:1258–1268. https://doi.org/10.3171/2013.3.JNS121900
Yamamoto M, Kawabe T, Sato Y, Higuchi Y, Nariai T, Watanabe S, Kasuya H (2014) Stereotactic radiosurgery for patients with multiple brain metastases: a case-matched study comparing treatment results for patients with 2–9 versus 10 or more tumors. J Neurosurg 121(Suppl):16–25. https://doi.org/10.3171/2014.8.GKS141421
Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, Osti M, Enrici RM, Esposito V (2016) Single-Fraction Versus Multifraction (3 x 9 Gy) Stereotactic radiosurgery for large (%3e2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148. https://doi.org/10.1016/j.ijrobp.2016.03.013
Minniti G, Esposito V, Clarke E, Scaringi C, Lanzetta G, Salvati M, Raco A, Bozzao A, Maurizi Enrici R (2013) Multidose stereotactic radiosurgery (9 Gy x 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys 86:623–629. https://doi.org/10.1016/j.ijrobp.2013.03.037
Harrison G, Kano H, Lunsford LD, Flickinger JC, Kondziolka D (2016) Quantitative tumor volumetric responses after Gamma Knife radiosurgery for meningiomas. J Neurosurg 124:146–154. https://doi.org/10.3171/2014.12.JNS141341
Da Silva AN, Nagayama K, Schlesinger D, Sheehan JP (2009) Early brain tumor metastasis reduction following gamma knife surgery. J Neurosurg 110:547–552. https://doi.org/10.3171/2008.4.17537
Huang CW, Tu HT, Chuang CY, Chang CS, Chou HH, Lee MT, Huang CF (2018) Gamma Knife radiosurgery for large vestibular schwannomas greater than 3 cm in diameter. J Neurosurg 128:1380–1387. https://doi.org/10.3171/2016.12.JNS161530
Snell JW, Sheehan J, Stroila M, Steiner L (2006) Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. Tech Note J Neurosurg 104:157–162. https://doi.org/10.3171/jns.2006.104.1.157
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47:291–298
Kim JW, Park HR, Lee JM, Kim JW, Chung HT, Kim DG, Jung HW, Paek SH (2016) Fractionated stereotactic gamma knife radiosurgery for large brain metastases: a retrospective, single center study. PLoS ONE 11:e0163304. https://doi.org/10.1371/journal.pone.0163304
Jeong WJ, Park JH, Lee EJ, Kim JH, Kim CJ, Cho YH (2015) Efficacy and safety of fractionated stereotactic radiosurgery for large brain metastases. J Korean Neurosurg Soc 58:217–224. https://doi.org/10.3340/jkns.2015.58.3.217
Minniti G, D'Angelillo RM, Scaringi C, Trodella LE, Clarke E, Matteucci P, Osti MF, Ramella S, Enrici RM, Trodella L (2014) Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol 117:295–301. https://doi.org/10.1007/s11060-014-1388-3
Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, Romano A, Enrici RM (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48. https://doi.org/10.1186/1748-717X-6-48
Fahrig A, Ganslandt O, Lambrecht U, Grabenbauer G, Kleinert G, Sauer R, Hamm K (2007) Hypofractionated stereotactic radiotherapy for brain metastases: results from three different dose concepts. Strahlenther Onkol 183:625–630. https://doi.org/10.1007/s00066-007-1714-1
Brown JM, Carlson DJ, Brenner DJ (2014) The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys 88:254–262. https://doi.org/10.1016/j.ijrobp.2013.07.022
Yang Y, Xing L (2005) Optimization of radiotherapy dose-time fractionation with consideration of tumor specific biology. Med Phys 32:3666–3677. https://doi.org/10.1118/1.2126167
Shibamoto Y, Miyakawa A, Otsuka S, Iwata H (2016) Radiobiology of hypofractionated stereotactic radiotherapy: what are the optimal fractionation schedules? J Radiat Res 57(Suppl 1):i76–i82. https://doi.org/10.1093/jrr/rrw015
Shibamoto Y, Otsuka S, Iwata H, Sugie C, Ogino H, Tomita N (2012) Radiobiological evaluation of the radiation dose as used in high-precision radiotherapy: effect of prolonged delivery time and applicability of the linear-quadratic model. J Radiat Res 53:1–9
Iwata H, Matsufuji N, Toshito T, Akagi T, Otsuka S, Shibamoto Y (2013) Compatibility of the repairable-conditionally repairable, multi-target and linear-quadratic models in converting hypofractionated radiation doses to single doses. J Radiat Res 54:367–373. https://doi.org/10.1093/jrr/rrs089
Song CW, Glatstein E, Marks LB, Emami B, Grimm J, Sperduto PW, Kim MS, Hui S, Dusenbery KE, Cho LC (2019) Biological principles of stereotactic body radiation therapy (SBRT) and stereotactic radiation surgery (SRS): indirect cell death. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2019.02.047
Coffey RJ (1993) Boost Gamma Knife radiosurgery in the treatment of primary glial tumors. Stereotact Funct Neurosurg 61(Suppl 1):59–64. https://doi.org/10.1159/000100661
Giubilei C, Ingrosso G, D'Andrea M, Benassi M, Santoni R (2009) Hypofractionated stereotactic radiotherapy in combination with whole brain radiotherapy for brain metastases. J Neurooncol 91:207–212. https://doi.org/10.1007/s11060-008-9700-8
Shibamoto Y, Hashizume C, Baba F, Ayakawa S, Manabe Y, Nagai A, Miyakawa A, Murai T, Iwata H, Mori Y, Mimura M, Ishikura S (2012) Stereotactic body radiotherapy using a radiobiology-based regimen for stage I nonsmall cell lung cancer: a multicenter study. Cancer 118:2078–2084. https://doi.org/10.1002/cncr.26470
Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M, Iwadate Y, Saeki N (2009) Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 74:1543–1548. https://doi.org/10.1016/j.ijrobp.2008.10.035
Yomo S, Hayashi M, Nicholson C (2012) A prospective pilot study of two-session gamma knife surgery for large metastatic brain tumors. J Neurooncol 109:159–165. https://doi.org/10.1007/s11060-012-0882-8
Yomo S, Hayashi M (2014) A minimally invasive treatment option for large metastatic brain tumors: long-term results of two-session Gamma Knife stereotactic radiosurgery. Radiat Oncol 9:132. https://doi.org/10.1186/1748-717X-9-132
Brenner DJ, Martel MK, Hall EJ (1991) Fractionated regimens for stereotactic radiotherapy of recurrent tumors in the brain. Int J Radiat Oncol Biol Phys 21:819–824
Park HR, Park KW, Lee JM, Kim JH, Jeong SS, Kim JW, Chung HT, Kim DG, Paek SH (2019) Frameless fractionated Gamma Knife radiosurgery with ICON for large metastatic brain tumors. J Korean Med Sci 34:e57. https://doi.org/10.3346/jkms.2019.34.e57
Kim YJ, Cho KH, Kim JY, Lim YK, Min HS, Lee SH, Kim HJ, Gwak HS, Yoo H, Lee SH (2011) Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys 81:483–489. https://doi.org/10.1016/j.ijrobp.2010.05.033
Cha J, Kim ST, Kim HJ, Kim BJ, Kim YK, Lee JY, Jeon P, Kim KH, Kong DS, Nam DH (2014) Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am J Neuroradiol 35:1309–1317. https://doi.org/10.3174/ajnr.A3876
Dohm A, McTyre ER, Okoukoni C, Henson A, Cramer CK, LeCompte MC, Ruiz J, Munley MT, Qasem S, Lo H-W, Xing F, Watabe K, Laxton AW, Tatter SB, Chan MD (2018) Staged stereotactic radiosurgery for large brain metastases: local control and clinical outcomes of a one-two punch technique. Neurosurgery 83(1):114–121
Angelov L, Mohammadi AM, Bennett EE, Abbassy M, Elson P, Chao ST, Montgomery JS, Habboub G, Vogelbaum MA, Suh JH, Murphy ES, Ahluwalia MS, Nagel SJ, Barnett GH (2018) Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases >/= 2 cm. J Neurosurg 129:366–382. https://doi.org/10.3171/2017.3.JNS162532
Yamamoto M, Higuchi Y, Serizawa T, Kawabe T, Nagano O, Sato Y, Koiso T, Watanabe S, Aiyama H, Kasuya H (2018) Three-stage Gamma Knife treatment for metastatic brain tumors larger than 10 cm3: a 2-institute study including re-analyses of earlier results using competing risk analysis. J Neurosurg 129:77–85. https://doi.org/10.3171/2018.7.GKS181392
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
This study was approved by our institutional review board as minimal risk, thus negating need for written consent from each patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeon, C., Cho, K.R., Choi, J.W. et al. Outcome of three-fraction gamma knife radiosurgery for brain metastases according to fractionation scheme: preliminary results. J Neurooncol 145, 65–74 (2019). https://doi.org/10.1007/s11060-019-03267-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03267-z