Abstract
Purpose
The purpose of this study was to retrospectively evaluate the new treatment paradigm of staged stereotactic radiosurgery (SRS) for the treatment of large brain metastases (BM) compared to the standard of surgical resection followed by SRS.
Methods
We evaluated 78 patients with large BM treated 2012–2017 with surgical resection and postoperative SRS (surgery + SRS) or staged SRS separated by 1 month. Overall survival (OS) was estimated using the Kaplan Meier method and compared across groups using the log-rank test. Cumulative incidence of neurologic death and local and distant brain failure (LF, DBF) were estimated using competing risk methodology.
Results
Forty patients were treated with surgery + SRS and 38 patients were treated with staged SRS. Median follow-up was 23.2 months (95% CI 20.5–39.3). Median OS was 13.2 months for staged SRS compared to surgery + SRS 9.7 months (p = 0.53). Cumulative incidence of neurologic death at 1 year was 23% after surgery + SRS, 27% after staged SRS (p = 0.69); cumulative incidence of LF at 1 year was 6% and 8% (p = 0.65) and 1-year DBF was 59% and 21% (p ≤ 0.01). Overall rates of leptomeningeal failure and radiation necrosis were similar between the groups (p = 0.63 and p = 1.0).
Conclusions
Though surgery and postoperative SRS is the standard, staged SRS represents an attractive treatment paradigm for treating large BM without sacrificing LC or survival, and potentially decreases DBF. Prospective studies are needed to validate these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stereotactic radiosurgery (SRS) has been shown to be an effective treatment for BM [1]. It has been widely adopted in clinical practice because it is generally considered to be a minimally invasive procedure that allows for limited delay in the initiation of systemic chemotherapy [2] or immunotherapy and a reduction in cognitive toxicity compared to whole brain radiotherapy (WBRT) [3]. However, the use of SRS is limited in large BM as larger volumes have decreased LC rates [4] and an increased risk for toxicity such as radiation necrosis [5]. A commonly practiced standard treatment for brain tumors greater than 3 cm in maximum diameter is surgical resection if tumors are in a resectable location and if the burden of disease, either in the brain or outside of brain, warrants aggressive treatment [6]. Traditionally, these large tumors have been treated with surgery and WBRT, possibly followed by a SRS boost [7, 8].
Staged radiosurgery is a novel technique for treating larger BM which utilizes the tendency of BM to decrease in size soon after radiotherapy. The BM is treated with SRS, time is allowed for tumor response, and then the lesion is consolidated with a second-stage of SRS. In the time between stages, inflammation and edema from first stage of treatment may be allowed to decrease. Recently, both hypofractionated radiotherapy [9] and staged radiosurgery [10, 11] have emerged as additional safe and effective treatment options for large BM. However, the advantage of staged radiosurgery over hypofractionated radiotherapy is an increased biologically effective dose (BED) [11]. It is unclear whether these options improve LC or have greater toxicity than surgical resection as the primary intervention.
Current data supports BM resection followed by radiosurgery to the resection cavity as an acceptable standard of care. In 1998 a randomized trial by Patchell and colleagues demonstrated that resection alone for BM had poor LC with failure rates of 46% for resection alone versus 10% for surgery and WBRT (p < 0.001) [12]. Further, the EORTC 22952-26001 trial confirmed the inadequacy of surgery alone for BM, finding recurrence in the surgical cavity of 59% [13]. Concerns about toxicity of WBRT led a randomized phase 3 trial that showed potential for improved quality of life and cognition for patients undergoing postoperative SRS compared to WBRT [14]. This has led many to accept post-operative SRS for BM as a reasonable standard of care.
Alternatively, it has been suggested that surgical resection of BM may increase the risk of intraoperative seeding, and subsequent leptomeningeal failure [15, 16]. WBRT may potentially mitigate this risk, while SRS may not; however, disagreement exists [17].
To our knowledge there are no studies comparing staged radiosurgery to the current standard of resection followed by SRS to the resection cavity. As we previously have reported our series of staged SRS with good outcomes, we have decided to compare clinical outcomes of staged SRS with a contemporaneous cohort of patients treated with surgery followed by SRS, which can be seen as an acceptable standard of care. We have chosen LC, DBF, and neurologic death as critical outcomes to compare between cohorts because surgery has demonstrated improved LC and fewer neurological deaths for larger BM over traditional whole brain radiation [18], bringing into question whether it will maintain that advantage over an optimized SRS approach. Moreover, it is unclear if postponement of systemic therapy for wound healing in the surgical cohort may affect DBF.
Materials and methods
Data acquisition
This study was approved by the Institutional Review Board at Wake Forest School of Medicine (IRB00026908). We retrospectively reviewed our institutional database of patients with BM treated with Gamma Knife® radiosurgery between 2012 and 2017. Eligibility for inclusion in the surgical cohort were as follows: the patients must have had craniotomy for resection of a brain metastasis that was ate least 6 cc in volume followed by cavity-directed SRS within 30 days. Eligibility for inclusion in the staged SRS cohort was as follows: patients must have received SRS directed towards the same lesion separated by 1 month that was at least 6 cm3 in volume. Eligible patients were identified through the institutional Gamma Knife database. 32 of 38 patients in the staged SRS arm were previously included our original publication documenting our outcomes of staged SRS [11]. A single patient from the prior series was excluded from the present study for not meeting the volume criteria. Patients in both cohorts were consecutively treated eligible patients.
Electronic medical records were reviewed to determine patient characteristics such as age, sex, Karnofsky Performance Status, primary histology and number of brain lesions. Outcomes such as survival, LC, and radiation necrosis were determined by review of the electronic medical records.
Radiosurgery technique
Patients were apprised of the risks and benefits of surgery, hypofractionated radiotherapy, and staged SRS. While hypofractionated radiotherapy is available at our institution, our institutional bias has been toward staged radiosurgery because of the higher BED.
Per institutional practice, a patient was considered for staged SRS if at least one lesion was approximately 6 cm3 in a single fraction and were poor candidates for surgical resection. Staged SRS technique was previously described [11], but in short, the dose chosen for initial SRS treatment was based on guidelines published by Shaw et al. [5]. The prescription doses for the second stage of SRS was at the discretion of the treating physician, but generally prescribed such as the sum of the total prescribed to the margin was equal to 30 Gy. The first and second stages were separated by approximately 1 month.
For patients receiving SRS to the resection cavity, the technique was performed and previously described by Jensen et al. [19]. The radiosurgical dose was prescribed to the rim of enhancement around the resection cavity. A margin around this rim of enhancement was not routinely applied. Dose selection for cavities was consistent with NCCTG N107C/CEC·3 guidelines [14].
Response assessment
Post-treatment MRI was performed 6–8 weeks following initial SRS and then every 3 months thereafter. Volumetric data were acquired from the GammaPlan treatment planning system. Each metastasis was manually contoured slice-by-slice on the T1-axial contrast-enhanced MRI within the GammaPlan system. The volume of each metastasis was calculated based on these contours. Contoured volumes of the lesions and cavities treated were acquired at each stage for staged SRS and volume of the cavity at SRS. Local failure was defined as a histologically-proven recurrence or a 25% increase in the size of the enhancing lesion on T1 contrast axial MRI slice, with a corresponding increase in perfusion on perfusion-weighted imaging. Distant brain failure was defined as a new metastasis at a site that was outside of the radiosurgical treatment volume. Determination of leptomeningeal failure was done in the manner as previously described by Wang et al. [19, 20]. Clear MRI evidence of new dural based nodular enhancement that was confirmed by neuroradiology report, diffuse leptomeningeal enhancement or positive CSF cytology after treatment with SRS were all considered leptomeningeal failure.
Neurologic death was defined as previously reported by McTyre et al. [21]. In brief, patients with progressive neurologic decline at time of death, irrespective of status of their extracranial disease status, were considered to have died of neurologic death. In addition, patients with severe neurologic dysfunction who died of intercurrent disease were also considered to have had neurologic death. Post-treatment toxicity was defined as per the RTOG CNS toxicity grading criteria within 90 days of treatment.
An increasing lesion size on follow-up imaging was evaluated with radiological and/or pathological information to differentiate progression from radiation necrosis. Specifically, stabilization or decrease in size of a previously enlarged lesion and/or decreased cerebral blood volume on perfusion MRI was considered to be due to RN. Whereas, continued lesion enlargement and/or increased cerebral blood volume was considered to be tumor progression. If lesion was indeterminate, it was reviewed by a multispecialty brain tumor board for adjudication.
Statistical analysis
Categorical data were described using count (frequency) and continuous data were summarized using mean (range) for normally and median (range) for non-normally distributed variables. Patients were stratified by treatment received (staged SRS or surgery followed by cavity-directed SRS), as above. Categorical variables were compared across strata using the Chi square test and Fisher’s exact test, as appropriate, and continuous variables were compared using the t-test and the Wilcoxon-Mann-Whitney test for normally and non-normally-distributed variables, respectively. Time-to-event data were calculated from the date of first staged procedure (either SRS or surgery). Follow-up was determined using the reverse Kaplan–Meier method. Kaplan–Meier estimates of overall survival were generated and compared using the log-rank test. Cumulative incidence rates of local failure, DBF, and neurologic death were estimated using competing risk methodology [22]. Competing risk regression modeling was utilized to estimate the effects of patient/treatment factors on the hazard of DBF. Findings were determined to be statistically significant if they reached a p value of < 0.05. All statistical analyses were performed using R version 3.5 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Patient characteristics are summarized in Table 1. A total of 40 patients with 40 lesions were treated with surgery + SRS and 38 patients with 45 lesions were treated with staged SRS. Median follow-up was 23.2 months (95% CI 20.5–39.3). Median tumor volume for those treated with surgery + SRS and staged SRS were 14.9 cm3 and 13.5 cm3, respectively (p = 0.50). All patients treated with surgery + SRS had one lesion. Staged SRS was performed on 1 lesion in 33 (87%) patients, 2 lesions in 3 (8%) of patients, and 3 lesions in 2 (5%) of patients. Median dose to the tumor cavity after surgical resection was 16.5 Gy (range 9–20). Median minimum dose for patients treated with staged SRS was 15 Gy (range 9–22) for the first treatment and 13.5 Gy (range 9–18) for the second. Median reduction in treatment volume was 54% for surgery + SRS and 36% for staged SRS (p = 0.60). Tumor characteristics are summarized in Table 2.
Disease control and survival
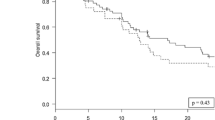
Cumulative incidence of local failure at 1, 2 and 3 years was 8%, 13%, and 13% after staged SRS and 6%, 14%, and 19% after surgery + SRS (p = 0.65, Fig. 1a). Cumulative incidence of DBF at 1 year was 21% and 59% for staged SRS and surgery + SRS, respectively (p < 0.01, Fig. 1b). On univariate analysis, the number of staged metastases treated was associated with DBF (sHR 1.91, 95% CI 1.21–3.00, p = 0.01) while age, gender, primary histology, prior WBRT and post-treatment chemotherapy were not. In the 14 patients receiving chemotherapy, time to chemotherapy (in days) was not associated with DBF (p = 0.67). Cumulative incidence of leptomeningeal failure at 1 year was 6% for surgery + SRS and 8% for staged SRS (p = 0.63). Clinical outcomes are summarized in Table 3. At the time of this study, 65% of patients had died. Median overall survival for staged SRS was 13.2 months compared to 9.7 months for surgery followed by SRS (p = 0.53, Fig. 2). Cumulative incidence of neurologic death at 1 year was 27% for staged SRS and 23% for surgery followed by SRS (p = 0.69).
Radiation necrosis and adverse events
For surgery followed by SRS, 15% of patients experienced RN compared to 13% for staged SRS patients (p = 1.0). 3 of 38 patients in the staged SRS group developed Radiation Therapy Oncology Group grade > 2 toxicity (2 patients had grade 4 toxicities and 1 had grade 3). One patient with grade 4 toxicity experienced intratumoral hemorrhage in the thalamus 4 weeks after this lesion was treated with the second stage of radiosurgery. The patient presented with foot drop and expressive aphasia and was hospitalized for 3 days; however, after a course of steroids, the patient showed significant improvement in speech and ambulation. The second patient with grade 3 toxicity had imaging changes suggestive of expanding necrosis and underwent Laser Interstitial Thermal Therapy (LITT). The third patient with grade 4 toxicity had expanding radiation necrosis to the staged treatment area that required hospitalization and treatment with LITT.
Seven of 40 patients with surgery followed by SRS experienced Radiation Therapy Oncology group grade > 2 toxicity (2 patients had grade 4 and 5 patients had grade 3 toxicity). All five patients who had grade 3 toxicity were found to have areas of expanding vasogenic edema suggestive of radiation necrosis and treated with LITT therapy. Two patients had grade 4 toxicity. One patient had right hemispheric focal seizures and was hospitalized for mental status changes. He was treated with a course of steroids but soon thereafter put on hospice. The second patient had memory impairment and seizures that resulted in hospitalization for a subdural hemorrhage. Subsequently she developed severe gait abnormalities because of a pseudomeningocele and required shunt placement.
Discussion
Management of patients with large BM can be complicated by multiple issues including progressive extracranial disease, BM in high risk surgical locations, or poor patient performance status. Surgery, when feasible, remains a standard treatment option due to the ability to immediately decompress symptoms and decrease edema. A minimally invasive alternative to surgery such as staged radiosurgery may provide advantages which include limiting the time to initiation of systemic therapy, the ability to treat lesions in eloquent regions, and the provision of a treatment option to patients who are poor surgical candidates.
Our institutional bias has been to provide staged radiosurgery for these patients, however, a major competing technique would be hypofractionated SRS. We feel a primary advantage of staged radiosurgery is an increased BED as compared to the hypofractionated approach (75 Gy for staged radiosurgery versus 48 Gy for hypofractionated radiotherapy using an α/β = 10). While the utilization of a increased BED will potentially lead to a greater local control, the risk of radiation necrosis is also likely increased. Several studies have demonstrated the feasibility of hypofractionated radiotherapy for the treatment of BM. A phase 2 trial by Ernst-Stecken et al. treating 51 patients with 72 BM (median diameter 2.27 cm) with hypofractionated SRS showed local control of 76% at 12 months [23]. Another study by Lockney and colleagues found that in 195 patients with 231 BM treated with hypofractionated SRS had LC of 83% at 12 months [24]. Prospective studies are needed to further compare staged SRS and hypofractionated SRS.
While staged SRS has been reported as feasible, it had not yet been directly compared to surgical outcomes. In the present series, patients from the same institution that were managed with either staged SRS or surgery with postoperative SRS were compared with respect to clinical outcomes. The cohort reviewed included patients with BM that were clinically determined to be too large to be sufficiently treated with single fraction SRS. We aimed to look at five meaningful endpoints: LC, leptomeningeal failure, DBF, neurologic death, and treatment-related toxicity.
Local control rates for SRS alone in tumors greater than 3 cm have been reported to be as low as 37% [25]. As such, surgery has been incorporated as a standard treatment for larger BM. Multiple randomized trials have demonstrated a LC benefit to surgery + WBRT versus WBRT [18, 26]. However, because surgical resection of a brain metastasis does not intend for clear surgical margins, the local recurrence rate without adjuvant therapy is high [12]. Adjuvant SRS to the resection cavity has been demonstrated in 2 randomized trials to be an effective and non-toxic adjuvant therapy for a resected brain metastasis [14, 27]. Even with combined surgery and SRS, resection cavities greater than 2.5 cm can have local recurrence rates as high as 40% at 1 year [28]. In the present study, there was no statistical difference in the LC rates between the non-surgical or surgical populations. Non-surgical LC is similar to that of surgical management, suggesting that it can be considered a viable alternative in cases where surgery is not feasible or may place the patient at higher risk.
Leptomeningeal disease is a known and often devastating sequela of surgical resection of a brain metastasis. It is unclear if SRS to the resection cavity (instead of larger field radiation such as WBRT) may increase the likelihood of leptomeningeal failure though there are at least reports that suggest as much [29]. It is also unknown if primary radiosurgical management could decrease leptomeningeal dissemination over a surgical approach by decreasing surgical contamination of the CSF. In the present series, there was no difference in leptomeningeal failure between the surgical and non-surgical patients. These data suggest that leptomeningeal failure may be driven more by the biology of the patient’s cancer than by the potential surgical seeding of the cerebral spinal fluid. These results agree with a series by Huang et al. which suggested that surgical resection was not a risk factor for leptomeningeal failure after radiosurgery [15].
In the present series, patients with staged radiosurgery experienced a lower likelihood of DBF than patients in the surgical cohort. One possible explanation for this is that surgery can potentially interrupt or delay the delivery of systemic treatment to allow for wound healing postoperatively. Multiple series have demonstrated that SRS can safely be delivered concurrently with systemic therapy [2, 30]. With control of systemic disease having been closely linked to later development of new BM [31], the lack of interruption of systemic therapy may have led to greater distant brain control. A series from Shen et al. from John Hopkins suggested that patients receiving early systemic therapy (without delay of SRS) had better clinical outcomes compared to those delaying their systemic therapy [2].
Patients with larger BM are at increased risk of dying from neurologic causes [32]. In modern brain metastases series, the likelihood of dying of neurologic death is approximately 20% [13]. The risk of neurologic death from BM is generally mediated by a combination of the degree to which BM affect performance status and cognitive function [13, 33], and certainly rises in the setting of inability to control intracranial disease [34]. In the present series, there was no difference in neurologic death rate between the surgical and non-surgical populations. This is an important finding because neurologic death is a potential benchmark by which one can measure success of brain metastasis-directed therapies. Prior series comparing surgical and non-surgical management of BM have traditionally shown that surgery can decrease neurologic death compared to SRS alone for larger BM [18].
There are several limitations to the present study. As a retrospective review, it is subject to patient selection biases including patient preferences, severity of clinical condition, and tumor characteristics and location. These biases could have bearing on the outcomes, particularly survival, of this study. Patients with severe symptoms or significant edema were likely to have been triaged towards surgery given the ability of surgery to alleviate symptoms of mass effect. There was also a difference in the number of BM treated in each group; however, Baschnagel et al. showed that overall BM tumor volume may be a better predictor of clinical outcomes than the number of BM [35] and our study had no significant difference in volume between groups (p = 0.5). In addition, it is possible that given the small number of local failure events and the limited patient numbers that the present study did not have adequate power to detect differences in LC. However, the fact that there were not large differences in either neurologic death or LC in this series is significant as it re-affirms the possibility that staged SRS is a viable alternative to surgical management in properly selected patients with larger BM. The present study, unlike previous studies of staged SRS where there was no comparator cohort, does compare outcomes to a cohort at the same institution where patients and the multi-disciplinary management team had a surgical option [10, 11]. Prospective studies would necessary to validate the findings in the present study.
References
Devoid H-M, McTyre ER, Page BR et al (2016) Recent advances in radiosurgical management of brain metastases. Front Biosci 8:203–214
Shen CJ, Kummerlowe MN, Redmond KJ et al (2016) Stereotactic radiosurgery: treatment of brain metastasis without interruption of systemic therapy. Int J Radiat Oncol Biol Phys 95:735–742
Greene-Schloesser D, Robbins ME, Peiffer AM et al (2012) Radiation-induced brain injury: a review. Front Oncol 2:73
Black PJ, Page BR, Lucas JT Jr et al (2015) Factors that determine local control with gamma knife radiosurgery: the role of primary histology. J Radiosurg SBRT 3:281–286
Shaw E, Scott C, Souhami L et al (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 9005. Int J Radiat Oncol Biol Phys 47:291–298
Ellis TL, Neal MT, Chan MD (2012) The role of surgery, radiosurgery and whole brain radiation therapy in the management of patients with metastatic brain tumors. Int J Surg Oncol 2012:952345
Andrews DW, Scott CB, Sperduto PW et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Arvold ND, Lee EQ, Mehta MP et al (2016) Updates in the management of brain metastases. Neuro Oncol 18:1043–1065
Eaton BR, Gebhardt B, Prabhu R et al (2013) Hypofractionated radiosurgery for intact or resected brain metastases: defining the optimal dose and fractionation. Radiat Oncol 8:135
Angelov L, Mohammadi AM, Bennett EE et al (2017) Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases ≥ 2 cm. J Neurosurg 129:366–382
Dohm A, McTyre ER, Okoukoni C et al (2017) Staged stereotactic radiosurgery for large brain metastases: local control and clinical outcomes of a one-two punch technique. Neurosurgery https://doi.org/10.1093/neuros/nyx355
Patchell RA, Tibbs PA, Regine WF et al (1998) Postoperative radiotherapy in the treatment of single metastases to the brain. JAMA https://doi.org/10.1001/jama.280.17.1485
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29:134–141
Brown PD, Ballman KV, Cerhan JH et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1049–1060
Huang AJ, Huang KE, Page BR et al (2014) Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. J Neurooncol 120:163–169
Johnson MD, Avkshtol V, Baschnagel AM et al (2016) Surgical resection of brain metastases and the risk of leptomeningeal recurrence in patients treated With stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 94:537–543
Ojerholm E, Lee JYK, Thawani JP et al (2014) Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg 121 (Suppl):75–83
Patchell RA, Tibbs PA, Walsh JW et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500
Jensen CA, Chan MD, McCoy TP et al (2011) Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg 114:1585–1591
Wang EC, Huang AJ, Huang KE et al (2017) Leptomeningeal failure in patients with breast cancer receiving stereotactic radiosurgery for brain metastases. J Clin Neurosci 43:6–10
McTyre ER, Johnson AG, Ruiz J et al (2017) Predictors of neurologic and nonneurologic death in patients with brain metastasis initially treated with upfront stereotactic radiosurgery without whole-brain radiation therapy. Neuro Oncol 19:558–566
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Ernst-Stecken A, Ganslandt O, Lambrecht U et al (2006) Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol 81:18–24
Lockney NA, Wang DG, Gutin PH et al (2017) Clinical outcomes of patients with limited brain metastases treated with hypofractionated (5 × 6 Gy) conformal radiotherapy. Radiother Oncol 123:203–208
Shiau CY, Sneed PK, Shu HK et al (1997) Radiosurgery for brain metastases: relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Phys 37:375–383
Vecht CJ, Haaxma-Reiche H, Noordijk EM et al (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery. Ann Neurol 33:583–590
Mahajan A et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048
Patel KR, Burri SH, Asher AL et al (2016) Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery 79:279–285
Johnson AG, Ruiz J, Hughes R et al (2015) Impact of systemic targeted agents on the clinical outcomes of patients with brain metastases. Oncotarget 6:18945–18955
Ayala-Peacock DN, Attia A, Braunstein SE et al (2017) Prediction of new brain metastases after radiosurgery: validation and analysis of performance of a multi-institutional nomogram. J Neurooncol 135:403–411
Lucas JT Jr, Colmer HG 4th, White L et al (2015) Competing risk analysis of neurologic versus nonneurologic death in patients undergoing radiosurgical salvage after whole-brain radiation therapy failure: who actually dies of their brain metastases? Int J Radiat Oncol Biol Phys 92:1008–1015
Farris M, McTyre ER, Cramer CK et al (2017) Brain metastasis velocity: a novel prognostic metric predictive of overall survival and freedom from whole-brain radiation therapy after distant brain failure following upfront radiosurgery alone. Int J Radiat Oncol Biol Phys 98:131–141
Wroński M, Maor MH, Davis BJ et al (1997) External radiation of brain metastases from renal carcinoma: a retrospective study of 119 patients from the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys 37:753–759
Baschnagel AM, Meyer KD, Chen PY et al (2013) Tumor volume as a predictor of survival and local control in patients with brain metastases treated with gamma knife surgery. J Neurosurg 119:1139–1144
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to disclose and informed consent was obtained from all human participants in compliance with our IRB standards.
Rights and permissions
About this article
Cite this article
Dohm, A.E., Hughes, R., Wheless, W. et al. Surgical resection and postoperative radiosurgery versus staged radiosurgery for large brain metastases. J Neurooncol 140, 749–756 (2018). https://doi.org/10.1007/s11060-018-03008-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03008-8