Abstract
Aim
This study aimed to investigate the clinical benefits of stereotactic radiosurgery (SRS) in patients with > 10 brain metastases (BM) compared to patients with 2–10 BM.
Methods
The study included multiple BM patients who underwent SRS between 2014 and 2022, excluding patients who underwent whole brain radiotherapy, had a Karnofsky Performance Status score < 60, suspected leptomeningeal disease, or a single BM lesion. Patients were divided into two groups (2–10 and > 10 BM groups) and matched 2:1 based on propensity scores. The primary endpoint was overall survival (OS) in the matched dataset, with intracranial progression-free survival (PFS) as the secondary endpoint. Non-inferiority was established if the upper limit of the 95% confidence interval (CI) of the adjusted hazard ratio was below 1.3.
Results
Of the 1042 patients identified, 434 met eligibility criteria. After propensity score matching, 240 patients were analyzed (160 in the BM 2–10 group and 80 in the > 10 BM group). The median OS was 18.2 months in the 2–10 BM group and 19.4 months in the > 10 BM group (P = 0.60). The adjusted hazard ratio was 0.86 (95% CI: 0.59–1.24), indicating non-inferiority. PFS was not significantly different between the groups (4.8 months vs. 4.8 months, P = 0.94). The number of BM did not significantly impact OS or PFS.
Conclusions
SRS for selected patients with > 10 BM was non-inferior in terms of OS compared to those with 2–10 BM in a propensity score-matched dataset.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BM) are the most common intracranial tumors in adults, with an incidence of 8–10% among patients with cancer [1]. Stereotactic radiosurgery (SRS) is an effective local therapy, along with whole-brain radiotherapy (WBRT) and surgery [2,3,4,5]. SRS has been established as the standard of care (SOC) for patients with 1–4 BM in several randomized controlled trials [6,7,8,9,10]. Although WBRT remains the SOC for patients with extensive BM, the role of SRS is expanding as an alternative treatment for such patients. A multicenter prospective observational study, JLGK0901, demonstrated that SRS for up to 5–10 BM was non-inferior to SRS for 2–4 BM in terms of overall survival (OS) [11, 12]. The National Comprehensive Cancer Network (NCCN) guidelines define patients with limited BM as those "for whom SRS represents an effective alternative to WBRT, but with more cognitive protection" rather than a specific number of BM [2]. Despite insufficient evidence, SRS has frequently been performed alone in patients with an extended number of BM in clinical practice [13]. A recent randomized controlled trial (NCT01592968) comparing SRS to WBRT for non-melanoma patients with 4–15 BM revealed that SRS is associated with a decreased risk of neurocognitive deterioration compared to WBRT without negatively affecting overall survival [14]. However, this trial was prematurely terminated owing to slow enrolment; therefore, further research is required to support the expanding benefit of SRS for more extensive BM.
Recent advances in targeted therapies and immunotherapies have demonstrated the effectiveness of some regimens in the central nervous system (CNS). Patients with non-small cell lung cancer (NSCLC), breast cancer, or melanoma are recommended for specific targeted therapy or immune checkpoint inhibitors (ICI) treatment for BM [3, 15]. In addition, several studies have reported promising results from combining local and systemic therapies [16,17,18,19,20,21,22,23,24,25,26]. This study aimed to evaluate the efficacy of SRS in patients with > 10 BM compared to patients with 2–10 BM after adjusting for variables that may affect prognosis.
Methods
Study design, patient selection, and treatment
This single-center retrospective study involved patients diagnosed with BM between 2014 and 2022 from an institutional disease database. The Institutional Review Board approved this study, and all participants provided informed consent with an opt-out form that stated that participants were included unless they explicitly decided to exclude themselves [27]. All analyses were conducted according to the relevant guidelines and regulations.
The eligibility criteria were: (1) diagnosis of multiple BM using computed tomography or magnetic resonance imaging, and (2) SRS performed for BM between 2014 and 2022. The exclusion criteria were: (1) treatment with WBRT, (2) Karnofsky Performance Status score (KPS) < 60 at the time of BM diagnosis, (3) suspected leptomeningeal disease (LMD), and (4) single BM lesion. Patients with a single BM were excluded because previous studies have shown that such patients have a better prognosis than those with multiple BM [11,12,13].
Patients with newly diagnosed multiple BM were determined for local and systemic therapy (ST) based on their KPS, neurologic symptoms, expected oncologic prognosis, extracranial lesions (ECM), and the presence of reasonable systemic regimen options. Patients with KPS ≥ 60 were considered candidates for SRS if their systemic disease was stable or if reasonable ST options still existed, if the multiple BM were not amenable to surgical resection, and if SRS was technically feasible for all lesions. Thin-slice contrast-enhanced MRI images (T1-weighted images after contrast) were performed on SRS candidates for a detailed diagnosis of BM. At treatment planning, all visible lesions contrasted with 1 mm slice axial, sagittal, and coronal images were delineated as target lesions. According to the guidelines [2, 3], SRS was performed with LINAC or Gamma Knife Surgery (GKS), generally a single fraction for lesions < 2 cm and multi-fraction for larger lesions (also called fractionated SRS or stereotactic radiotherapy). GKS was considered favorable for the asymptomatic patients, each with small and multiple BM lesions (> 10–15). SRS was performed using the following platforms in this study: the Helical Tomotherapy (Hi-Art system, Accuray Inc., Sunnyvale, CA) until 2017, the Varian TrueBeam system (Varian Medical Systems, Palo Alto, CA) since 2018, and the Leksell Gamma Knife (Electa AB, Kungstensgatan, Sweden) in the whole study period. The Helical Tomotherapy and the TrueBeam systems were both available to choose the single-isocenter multitarget therapy (intensity-modulated radiotherapy or volumetric modulated arc therapy) [28, 29]. Single-shot SRS was performed for BM < 2 cm at a maximum dose of 16–22 Gy. Fractionated SRS (e.g., 27–30 Gy in 3 fractions or 30–35 Gy in 5 fractions) was considered for larger lesions. WBRT was performed in patients unsuitable for SRS; these patients were not included in this study, but to confirm the patient selection results, we performed survival estimates in the supplementary. Indications for WBRT were comprehensively determined by neurosurgeons, radiation oncologists, and primary disease physicians for the following reasons: short estimated prognosis, no effective systemic regimen remaining, oncologic characteristics of primary cancer, technical challenges of SRS, number, and location of BMs, and diagnostic status. However, they were determined on an individual case basis and were not standardized during the study period.
The following data were collected: number of BM, age, sex, cancer type, neurologic symptoms, KPS at BM diagnosis, ECM, graded prognostic assessment score (GPA) at BM diagnosis, the maximum diameter of the largest tumor, total tumor volume, and type of systemic therapy. Information on epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) rearrangement, and programmed death-ligand 1 expression was also obtained from NSCLC patients. The timing of SRS for BM was classified into two types: synchronous BM was diagnosis of BM within 6 months of the primary cancer diagnosis, and metachronous BM was the diagnosis of BM later. OS was defined as the time from the date of SRS to death. Intracranial progression-free survival (PFS) was defined as the time from the date of SRS to death or CNS progression. Patients were divided into two groups: 2–10 BM and > 10 BM, and propensity score matching was performed to balance the potential prognostic factors between the two groups. The primary endpoint was OS in the matched dataset with PFS as the secondary endpoint. Patient follow-up data of this study were censored on August 31, 2022.
Sample size calculation
Sample size calculations for comparing survival curves were performed according to Jung et al. [30]. The raw dataset was divided into two groups according to the number of BM (2–10 BM vs. > 10 BM); the 1-year survival rate of patients with BM was assumed to be 55% in both groups according to recent studies [26, 31, 32]. Given the small population of patients with > 10 BM who underwent SRS and the different baseline characteristics of the two groups, we performed propensity score matching to create the selected dataset assigned in a 2:1 ratio, in which the features were balanced between the two groups. The non-inferiority comparison between the two groups required a minimum of 237 patients to achieve > 70% power in the matched data set. Based on the JLGK0901 study [11, 12], the non-inferiority margin was considered the adjusted hazard ratio (HR) of 1.3. Non-inferiority was established if the upper limit of the one-sided 95% confidence interval (CI) for the two-group difference in mortality was less than the margin at an α level of 0.10.
Statistical analysis
The patient characteristics were compared between the groups using the chi-square test and analysis of variance. The propensity score for balancing the potential prognostic factors was calculated using multivariable logistic regression based on the following characteristics: number of BM (2–10 vs. > 10), GPA (0–1.0 vs. 1.5–2.0 vs. 2.5–3.0), KPS (60 vs. 70 vs. 80 vs. 90 vs. 100), the diameter of the largest BM (< 10 vs. 10–19 vs. 20–29 vs. ≥ 30 mm), age (< 63 years vs. ≥ 63 years), ECM (positive vs. negative), cancer type (NSCLC-adenocarcinoma vs. NSCLC-non-adenocarcinoma vs. small cell lung cancer vs. breast cancer vs. other types), EGFR/ALK mutation (positive vs. negative), PD-L1 (50–100% vs. 1–49% vs. negative/unknown), systemic regimens (ICI vs. targeted therapy vs. chemotherapy vs. RT alone), the timing of SRS (synchronous BM vs. metachronous BM). The estimated propensity score was matched 2:1 (2–10 BM, n = 160; > 10 BM, n = 80) without replacement. The calipers of width are equal to 0.2 of the standard deviation of the propensity score logit.
Survival analysis was performed on the raw and matched datasets using the log-rank test, and survival curves were estimated using the Kaplan–Meier method. The proportional hazards assumption between the groups (2–10 vs. > 10 BM) was checked using Schoenfeld residuals, with no significant violation (P = 0.87). The multivariate Cox proportional hazards models were used to identify the factors associated with an increased risk of mortality and progression. The primary analysis was performed in the matched dataset using Cox regression to calculate hazard ratios (HR) with 1-sided 95% CI, adjusted for significant prognostic factors. Statistical analyses were performed using R statistical software version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at P < 0.05, and all tests were 1-sided.
Results
Patient
We identified 1042 patients diagnosed with BM between 2014 and 2022, and 434 eligible patients were analyzed: 324 and 110 were in the 2–10 BM and > 10 BM groups, respectively (Fig. 1). A total of 608 patients were excluded (KPS < 60 in 78, LMD in 104, WBRT in 186, and single BM in 240). The following reasons were observed why WBRT was selected; Shortly expected prognosis or no effective regimen remained (n = 118), Primary cancer was SCLC, and WBRT was the preferred choice at the time (n = 35), SRS was deemed unsuitable at the time (a large number of BM: n = 14, metastases of the brainstem: n = 3, unknown: n = 8) Neurologic symptoms were poorly controlled (n = 5), and not performed contrast-enhanced MRI (n = 3).
Table 1 presents the patient characteristics of this study. In the raw dataset, the BM > 10 group tended to have some biased features, such as a worse GPA score (P = 0.031), more NSCLC-Adenocarcinoma (P = 0.007) with EGFR/ALK or PD-L1 positive, younger patients (P = 0.013), more ECM (P = 0.015), and more combined treatment with ST (P = 0.013).
Based on the calculated propensity score, 240 patients were 2:1 matched between the 2–10 BM group (n = 160) and > 10 BM group (n = 80). Each character in the matched dataset was well-balanced, as shown in Table 1, and the average number of tumors per patient was 10 in the entire dataset. The 2–10 BM group had a median of four lesions, while the > 10 BM group had a median of 16 lesions, with 11–20 lesions accounting for 65% of cases. No patients with a GPA of 3.5–4.0 were included in the matched dataset. NSCLC accounted for 80% of the cancer types, and 60% of patients with NSCLC-adenocarcinoma had EGFR/ALK mutations. ST was combined with SRS in 80% of the patients; particularly ICI/targeted therapy was used in 50%.
Survival analysis and adverse events
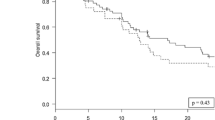
After the final follow-up, 144 deaths and 212 intracranial progressions were recorded in the matched dataset. The median follow-up duration for overall survival (OS) in the matched dataset was 338 days. The median survival time (MST) was 18.2 months (95% CI: 13.9–24.3) in the 2–10 BM group and 19.4 months (95% CI: 12.5–26.2) in the > 10 BM group (P = 0.60; Fig. 2C). After adjusting for significant variables in a multivariable model, including KPS, ECM, sex, cancer type, PD-L1 expression, and systemic regimens, the number of BM did not show a statistically significant difference in OS (Table 2). The adjusted HR was 0.86 (95% CI: 0.59–1.24). The upper limit of the 95% CI was below the non-inferiority margin of 1.3, indicating non-inferiority.
In the raw dataset, the PFS curve of the > 10 BM group was slightly lower than that of the 2–10 BM group (P = 0.061). However, in the matched dataset, PFS was not significantly different between the two groups: 4.8 months (95% CI: 3.8–5.7) and 4.8 months (95% CI: 3.6–6.0) in the 2–10 and > 10 BM group, respectively (P = 0.94; Fig. 2D). The adjusted HR was 1.02 (95% CI: 0.76–1.36). The number of BM did not show a significant difference in PFS (Table 3). Patients with intracranial recurrence were classified by site of recurrence described in table S1: in the 2–10 BM group, of a total of 140 intracranial recurrences, 10 were local, 100 were distant brain recurrences, and 30 were both. In the > 10 BM group, of 72 patients with intracranial recurrence, 8 had local recurrence, 30 had distant brain recurrence, and 34 had both. Salvage radiotherapy was performed in 70 patients (43.7%) and 42 patients (52.6%) in the BM 2–10 and BM > 10 groups, with no significant difference between the two groups (P = 0.64).
Treatment-related adverse events occurred in 30 patients (12.5%): 16 patients in Grade 1 (6.7%), 13 patients in Grade 2 (5.4%), and 1 patient in Grade 3 (0.4%). The proportion of patients who experienced any grade of adverse events did not differ between the two groups (table S2). No patients experienced grade 4–5 adverse events. Tumor bleeding occurred in 5 patients (2.1%) in the BM 2–10 group, and radiographic radiation necrosis developed in 9 patients (3.8%): 7 and 2 patients in the BM 2–10 and BM > 10 groups, respectively.
Discussion
This study investigated the non-inferiority of SRS in patients with > 10 BM versus 2–10 BM in terms of OS. After adjusting for significant prognostic factors, MST was 18.2 months in the 2–10 BM group versus 19.4 months in the > 10 BM group. An adjusted HR of 0.86 (95% CI: 0.59–1.24) indicated non-inferiority. The efficacy of SRS for a large number of BM has not been established [6, 9]. We believe that the results of this study are valuable because the efficacy of SRS for selected patients with > 10 BM was evaluated, including detailed patient characteristics such as systemic regimens and target gene mutations that may affect survival. The data showed a low incidence of treatment-related adverse events in both groups and similar intracranial recurrence rates or the achievement rate of salvage treatment between the two groups.
Despite the lack of sufficient evidence, SRS has been performed alone for patients with a higher number of BM in clinical practice; Hughes et al. reported that of 2083 cases that underwent SRS for 1–15 BM between 1991 and 2013 at multiple centers in the United States and Canada, 212 cases had 5–15 BM [13]. In our study, 171 patients had 5–15 BM, and 69 patients had > 15 BM. Recent technological and clinical advances in radiotherapy may support this gap between evidence and clinical practice. For example, LINAC-based single-isocenter SRS was achieved to shorten the treatment time without increasing the risk of local recurrence compared to conventional multi-isocenter SRS [33, 34].
The results of this study showed that the SRS for BM > 10 was not inferior to that of 2–10 BM in terms of OS. Although the difference between the two groups in the matched data set was not significant, shared characteristics among patients with multiple BM could have influenced the results (Table 1). Approximately 80% of the patients had NSCLC, and 60% of them had EGFR mutations or ALK rearrangements. A prior Japanese multicenter observational study (JLGK0901), which also included patients with lung cancer (80% of the study population), demonstrated the non-inferiority of SRS for 5–10 BM compared to 2–4 BM in terms of OS [11, 12]. Recent systemic regimens, especially targeted therapies for NSCLC, have high efficacy in the CNS. The FLAURA study reported that the EGFR inhibitor osimertinib improved PFS and OS in patients with BM compared to conventional EGFR inhibitors [35]. Zhang et al. also conducted a meta-analysis of ALK inhibitors in patients with NSCLC and BM and reported that second-generation alectinib significantly improved intracranial response efficiency when compared to first-generation crizotinib [36]. The present study showed that targeted agents were an independent prognostic factor, and the high rate (40%) of its combination in both groups might have influenced the finding of no significant difference in OS between the two groups.
In previous studies, total tumor volume has been reported as an important prognostic factor for local control [37,38,39]. However, in contrast to JLGK0901 [11, 12], the present study showed no difference in the total tumor volume between the two groups and did not affect OS or intracranial progression. Table S3 summarizes the major reports of SRS for patients with > 10 BM [13, 40,41,42,43,44], including JLGK0901 [11, 12]. Although the listed studies are similar in terms of the number of BM and cumulative tumor volume, the present study is notable in that patients with multiple BM have expected more prolonged survival than before. Compared to similar studies, the present study analyzed more recent cases from 2014 to 2022, and recent advances in cancer treatment may affect survival outcomes. In addition, previous studies did not specify the details of combined therapy; however, we collected details on genetic mutations and ST combinations, especially ICI/targeted therapies, to examine the impact of such treatments. Among the significant factors affecting survival in the multivariate analysis of this study, better KPS scores, female sex, and no ECM have been reported in previous studies [11, 41, 44]. In addition, the present study found that PD-L1 > 1% and targeted therapy were favorable prognostic factors. We conducted a retrospective analysis using the same database to compare the efficacy of SRS + ST with ST alone or SRS alone as the BM treatment [26]. This study included 928 patients diagnosed with BM between 2016 and 2021, without limiting the number of lesions treated. In a propensity score-adjusted dataset for treatment selection, we found that the combination of SRS and ST had significantly better OS and PFS than monotherapy (SRS alone or ST alone). Specifically, MST was 23.1 months in the SRS + ST group and 17.2 months in the monotherapy group (P = 0.036), while the median PFS was 7.4 months and 5.0 months in the SRS + ST and monotherapy group, respectively (P < 0.001) [26].
SRS for 1–3 BM is associated with less cognitive impairment than WBRT; however, the neurocognitive advantage of SRS over WBRT when treating ≥ 4 BM is still unknown. In a Japanese prospective observational study (JLGK0901), SRS for 5–10 BM was shown to have similar cognitive dysfunction as SRS for 1–4 BM. The NCT01592968 trial compared patients with 4–15 BM receiving WBRT versus SRS, with neurocognitive function and intracranial control as the primary endpoints. Unfortunately, this trial was prematurely terminated due to slow accrual, but the investigators concluded that SRS reduces the risk of neurocognitive deterioration compared with WBRT without compromising OS [14]. Another ongoing phase III trial (NCT03550391) compared SRS and hippocampal-avoidant WBRT plus memantine for 5–15 BM. Although the results have not yet been published, SRS is an increasingly focused treatment for extended BM.
This study had several significant limitations. First, it was a single-center retrospective study. While the patient backgrounds were well-balanced in the matched dataset, treatment selection was not random. Furthermore, SRS for patients with multiple BM, especially > 10 BM, has not yet been established as SOC, and only carefully selected patients were considered candidates. Figure S1 shows the difference in survival curves between excluded WBRT patients (n = 186) and the matched dataset. The indications for WBRT were detected, such as the short estimated prognosis and the lack of remaining effective systemic regimens. However, they were determined in each case and not standardized during the study period. Second, although there were no restrictions on tumor type in the study enrollment, most patients had NSCLC. Third, this study did not consider the specifics of ST regimens, adverse events including cognitive dysfunction, or quality of life in patients with BM.
In conclusion, SRS for patients with more than 10 BM was non-inferior compared to patients with 2–10 BM in terms of OS in a propensity-matched dataset. The results of this study suggest that SRS may be an effective local treatment option for appropriately selected patients, even if they have more than 10 BM.
Data availability
Research data are stored in an institutional repository and anonymized numerical data will be shared upon request to the corresponding author. Research image data are not available at this time.
References
Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94:2698–2705
NCCN guidelines available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (Accessed on January 10, 2023). https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed 10 Jan 2023
Gondi V, Bauman G, Bradfield L et al (2022) Radiation therapy for brain metastases: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol 12:265–282
Vogelbaum MA, Brown PD, Messersmith H et al (2022) Treatment for brain metastases: ASCO-SNO-ASTRO Guideline. J Clin Oncol 40:492–516
Soon YY, Tham IWK, Lim KH et al (2014) Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009454.pub2
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases. JAMA 316:401
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29:134–141
Andrews DW, Scott CB, Sperduto PW et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491
Chang EL, Wefel JS, Hess KR et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387–395
Yamamoto M, Serizawa T, Higuchi Y et al (2017) A multi-institutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901 Study Update): irradiation-related complications and long-term maintenance of mini-mental state examination scores. Int J Radiat Oncol Biol Phys 99:31–40
Hughes RT, Masters AH, McTyre ER et al (2019) Initial SRS for patients with 5 to 15 brain metastases: results of a multi-institutional experience. Int J Radiat Oncol Biol Phys 104:1091–1098
Li J, Ludmir EB, Wang Y et al (2020) Stereotactic radiosurgery versus whole-brain radiation therapy for patients with 4–15 brain metastases: a phase III randomized controlled trial. Int J Radiat Oncol Biol Phys 108:S21–S22
Ramakrishna N, Anders CK, Lin NU et al (2022) Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO Guideline Update. J Clin Oncol 40:2636–2655
Chen L, Douglass J, Kleinberg L et al (2018) Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell carcinoma. Int J Radiat Oncol Biol Phys 100:916–925
Geraud A, Xu HP, Beuzeboc P, Kirova YM (2017) Preliminary experience of the concurrent use of radiosurgery and T-DM1 for brain metastases in HER2-positive metastatic breast cancer. J Neurooncol 131:69–72
Skrepnik T, Sundararajan S, Cui H, Stea B (2017) Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology 6:e1283461
Pomeranz Krummel DA, Nasti TH, Izar B et al (2020) Impact of sequencing radiation therapy and immune checkpoint inhibitors in the treatment of melanoma brain metastases. Int J Radiat Oncol Biol Phys 108:157–163
Yang Y, Deng L, Yang Y et al (2022) Efficacy and safety of combined brain radiotherapy and immunotherapy in non-small-cell lung cancer with brain metastases: a systematic review and meta-analysis. Clin Lung Cancer 23:95–107
Kotecha R, Kim JM, Miller JA et al (2019) The impact of sequencing PD-1/PD-L1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro Oncol 21:1060–1068
Ramakrishna R, Formenti S (2019) Radiosurgery and immunotherapy in the treatment of brain metastases. World Neurosurg 130:615–622
Borius P-Y, Régis J, Carpentier A et al (2021) Safety of radiosurgery concurrent with systemic therapy (chemotherapy, targeted therapy, and/or immunotherapy) in brain metastases: a systematic review. Cancer Metastasis Rev 40:341–354
Schapira E, Hubbeling H, Yeap BY et al (2018) Improved overall survival and locoregional disease control with concurrent PD-1 pathway inhibitors and stereotactic radiosurgery for lung cancer patients with brain metastases. Int J Radiat Oncol Biol Phys 101:624–629
Tonse R, Tom MC, Mehta MP et al (2021) Integration of systemic therapy and stereotactic radiosurgery for brain metastases. Cancers. https://doi.org/10.3390/cancers13153682
Koide Y, Nagai N, Miyauchi R et al (2022) Radiotherapy or systemic therapy versus combined therapy in patients with brain metastases: a propensity-score matched study. J Neurooncol 160:191–200
Koide Y, Tomita N, Adachi S et al (2019) Retrospective analysis of hypofractionated stereotactic radiotherapy for tumors larger than 2 cm. Nagoya J Med Sci 81:397–406
Koide Y, Nagai N, Miyauchi R et al (2023) Recent trends of characteristics and treatments in adults with newly diagnosed brain metastases. Jpn J Clin Oncol. https://doi.org/10.1093/jjco/hyad026
Eba J, Nakamura K (2022) Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol 52:539–544
Jung S-H, Chow S-C (2012) On sample size calculation for comparing survival curves under general hypothesis testing. J Biopharm Stat 22:485–495
Sperduto PW, Mesko S, Li J et al (2020) Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol 38:3773–3784
Sperduto PW, De B, Li J et al (2022) Graded prognostic assessment (GPA) for patients with lung cancer and brain metastases: initial report of the small cell lung cancer GPA and update of the non-small cell lung cancer gpa including the effect of programmed death ligand 1 and other prognostic factors. Int J Radiat Oncol Biol Phys 114:60–74
Kraft J, van Timmeren JE, Mayinger M et al (2021) Distance to isocenter is not associated with an increased risk for local failure in LINAC-based single-isocenter SRS or SRT for multiple brain metastases. Radiother Oncol 159:168–175
Ruggieri R, Naccarato S, Mazzola R et al (2018) Linac-based VMAT radiosurgery for multiple brain lesions: comparison between a conventional multi-isocenter approach and a new dedicated mono-isocenter technique. Radiat Oncol 13:38
Reungwetwattana T, Nakagawa K, Cho BC, et al (2018) CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol JCO2018783118
Zhang Z, Guo H, Lu Y et al (2019) Anaplastic lymphoma kinase inhibitors in non-small cell lung cancer patients with brain metastases: a meta-analysis. J Thorac Dis 11:1397–1409
Garsa AA, Badiyan SN, DeWees T et al (2014) Predictors of individual tumor local control after stereotactic radiosurgery for non-small cell lung cancer brain metastases. Int J Radiat Oncol Biol Phys 90:407–413
Ko PH, Kim HJ, Lee JS, Kim WC (2020) Tumor volume and sphericity as predictors of local control after stereotactic radiosurgery for limited number (1–4) brain metastases from nonsmall cell lung cancer. Asia Pac J Clin Oncol 16:165–171
Suzuki S, Inoue T, Ishido K (2016) Factors influencing local tumor control after Gamma Knife radiosurgery for intracranial metastases from breast cancer. J Clin Neurosci 33:154–158
Chang WS, Kim HY, Chang JW et al (2010) Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: is stereotactic radiosurgery effective for multiple brain metastases? J Neurosurg 113(Suppl):73–78
Grandhi R, Kondziolka D, Panczykowski D et al (2012) Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg 117:237–245
Rava P, Leonard K, Sioshansi S et al (2013) Survival among patients with 10 or more brain metastases treated with stereotactic radiosurgery: Clinical article. J Neurosurg 119:457–462
Salvetti DJ, Nagaraja TG, McNeill IT et al (2013) Gamma Knife surgery for the treatment of 5 to 15 metastases to the brain: clinical article. J Neurosurg 118:1250–1257
Yamamoto M, Kawabe T, Sato Y et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases: a case-matched study comparing treatment results for patients with 2–9 versus 10 or more tumors. J Neurosurg 121(Suppl):16–25
Acknowledgements
The authors thank all the patients, investigators, and institutions involved in this study. The authors thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS, Grant Number 23K14669, 20K16402), and Aichi Cancer Research Foundation.
Author information
Authors and Affiliations
Contributions
N.N and Y.K. wrote the main manuscript text and Y.K. prepared figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
YK has a speaker bureau from Hitachi Co., and has received research funding from Japan Society for the Promotion of Science (JSPS, Grant Number 23K14669, 20K16402), and Aichi Cancer Research Foundation. TK has speaker bureaus from Hitachi Co., Bristle Myers Squibb., Accuray Co., Elekta Co., Ono Pharmaceutical Co., AstraZeneca Co., Taiho Pharmaceutical Co., Canon Co., and Janssen Pharmaceutical Co.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagai, N., Koide, Y., Shindo, Y. et al. Retrospective non-inferiority study of stereotactic radiosurgery for more than ten brain metastases. J Neurooncol 163, 385–395 (2023). https://doi.org/10.1007/s11060-023-04358-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04358-8