Abstract
The diagnosis of leptomeningeal metastases (LM) of solid tumors is complicated due to low sensitivities of both magnetic resonance imaging (MRI) and cytology. MRI has a sensitivity of 76% for the diagnosis of LM and cerebrospinal fluid (CSF) cytology has a sensitivity of 44–67% at first lumbar puncture which increases to 84–91% upon second CSF sampling. Epithelial cell adhesion molecule (EpCAM) is expressed by solid tumors of epithelial origin like non-small-cell lung cancer, breast cancer or ovarium cancer. Recently, a CELLSEARCH® assay and flow cytometry laboratory techniques have been developed to detect circulating tumor cells (CTCs) of epithelial origin in CSF. These laboratory techniques are based on capture antibodies labelled with different fluorescent tags against EpCAM. In this review, we provide an overview of the available laboratory techniques and diagnostic accuracy for tumor cell detection in CSF. The reported sensitivities of the EpCAM-based CTC assays for the diagnosis of LM across the different studies are highly promising and vary between 76 and 100%. An overview of the different EpCAM-based techniques for the enumeration of CTCs in the CSF is given and a comparison is made with CSF cytology for the diagnoses of LM from epithelial tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two to eight percent of patients with solid tumors develop LM. Diagnosis of LM is currently based on clinical symptoms and typical contrast enhancement of the leptomeninges on MRI of brain and/or spine. However, MRI has a low sensitivity (76%) and specificity (77%) for the diagnosis of LM [1]. When MRI results are inconclusive, a LP is performed to obtain CSF. Sensitivity of CSF cytology, however, is also low: 44–67% at first LP, increasing to 84–91% upon second sampling [2,3,4,5,6,7,8,9,10]. EpCAM is a cell–cell adhesion molecule and a mitogenic signal transducer after regulated intramembrane proteolysis [11, 12]. Solid tumors of epithelial origin like non-small-cell lung cancer, breast cancer or ovarium cancer express transmembrane glycoprotein EpCAM (also known as CD326) [13]. In blood donors with nonmalignant diseases the background of EpCAM + cells is extremely low with only 0.3% having ≥ 2 CTC per 7.5 mL [14]. EpCAM + CTCs in blood have been detected in patients with metastasized epithelial tumors, like ovarian cancer, breast cancer and colorectal cancer and prostate and have prognostic value when CTC numbers are higher than 0.3–5 CTC/mL [15,16,17,18]. Therefore, multiple research groups started to investigate assays to detect and count EpCAM + CTCs in CSF in patients with already diagnosed LM or clinically suspected LM. To improve CSF diagnostics, the enumeration of CTCs by flow cytometry and Veridex CELLSEARCH® has been introduced [4,5,6, 19, 20]. The CELLSEARCH® assay is an FDA-approved assay to detect and count CTC from solid tumors in blood [21, 22]. Currently, two major EpCAM-based techniques have been studied: the CELLSEARCH® technology to detect CTCs in blood which has been adapted to detect CTCs in CSF and flow cytometry assays. In this review, an overview is given of the different assays and their performance in CSF for the enumeration of EpCAM + CTCs. The EpCAM-based techniques are compared with CSF cytology for the diagnosis of LM from epithelial tumors.
Methods
In June 2017, PubMed was searched for studies with the following terms “Cerebrospinal Fluid” [Mesh] and “Neoplastic Cells, Circulating“ [Mesh], CELLSEARCH and cerebrospinal fluid or EpCAM and cerebrospinal fluid. The references of the selected articles were also reviewed for inclusion in this review. Articles in which non-EpCAM based assays where used for other tumor types such as melanoma or lymphoma were excluded. Reported CTC numbers in the various articles were standardized to cells/mL, if possible.
Results
The initial article search resulted in 21, 6, and 25 hits, respectively. Eight articles were included for data extraction after reviewing of the abstracts. One additional article was included after reviewing the references of the selected articles.
CELLSEARCH technique
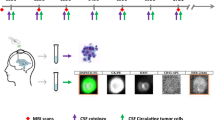
The CELLSEARCH® assay is an FDA-approved assay to detect CTC in blood [21, 22]. The CELLSEARCH® system consists of the CellTracks Autoprep, CellTracks Magnest and the CellTracks Analyzer II [23]. First, blood is drawn in the CellSave collection tube which preserves the sample up to 96 h. Then, the blood is gently mixed with a dedicated dilution buffer provided in the CELLSEARCH® kit and centrifuged at 800×g at room temperature for 10 min [24]. Subsequently, the sample is transferred to the CellTracks Autoprep part of the CELLSEARCH® System. In the CellTracks Autoprep, the EpCAM + CTCs are immunomagnetically enriched and the fluorescently labeled antibodies are added. Anti-EpCAM ferrofluid is added to the aspirated plasma/dilution buffer layer to select for cells of epithelial origin by immunomagnetically enrichment [25]. Captured cells are fixed and permeabilized with the CELLSEARCH® proprietary permeabilization reagents and subsequently stained with 4′6-diamidino-2-phenylindole, dihydrochloride (DAPI) for nuclear staining. Anti-CD45-allophycocyan (CD45-APC) was added to label leukocytes and distinguish them from tumor cells. Anti-cytokeratin (CK) 8, 18-Phycoerythrin (PE), and anti-cytokeratin 19 Phycoerythrin (CK-PE) were added to stain the epithelial tumor cells. Next, cells are deposited in the cartridge that is positioned in the CellTracks Magnest. Thereafter, the CellTracks Analyzer II generates images of the cells using filters for DAPI, PE, and APC. Cells that are stained with both DAPI and PE are automatically identified as CTCs and placed in an image gallery. (see for overview of the CellTracks Analyzer II; Fig. 1a). Finally, a reviewer observes the images and makes the final decision on the identification of CTCs, which are defined as nucleated DAPI + cells, lacking CD45 and expressing CK-PE. An example of gallery images of tumor cells detected by CELLSEARCH® in CSF (B1) and peripheral blood (B2) is given in Fig. 1b.
a Schematic representation of the CellTracks Analyzer II, used in the CellSearch® system [36]. Cells that have been enriched immunomagnetically and fluorescently labelled in the CellTracks Autoprep machine are magnetically (N, S = magnet North and South) aligned to nickel (Ni) lines at the inner surface of the Magnest chamber. The light from a laser diode is focused onto these cells via a normal CD-player objective. The fluorescent light is collected via the same objective and separated through a combination of filters onto the photodiode detectors (PD). Fluorescent images of the events of interest can be acquired by inserting a removable mirror and band pass filter. The fluorescent light captured by the CD objective is then focused onto the camera (CCD). The magnets and chamber (Magnest cartridge) are positioned on a computer-controlled stage and the cells cross the laser focus one after another when the stage is moved in the Y-direction. While scanning, a feedback system uses the Ni-lines to keep the laser focused on the aligned cells. b Gallery of images of tumor cells in CSF and peripheral blood using CELLSEARCH® technology [37]. Gallery of images of tumor cells in CSF (B1) and peripheral blood (B2) detected by CELLSEARCH® technology [4]. CTC are defined as ≥ 4 µm in diameter, nucleated DAPI+ (purple), CD45-, and CK-PE+ (green). In the CSF sample, CTCs were either found as isolated cells or in clusters. Their morphology was similar to the CTCs found in the peripheral blood but without any apoptotic features, which were present in some of the CTCs in blood samples (arrow, shrunken cell containing CK inclusion). Scale bar is 10 μm. CSF cerebrospinal fluid, CTC circulating tumor cell, CK cytokeratin
In the CELLSEARCH® assay plasma is aspirated based on the optical differences between plasma, buffy coat and erythrocytes. To use the CELLSEARCH® assay in CSF instead of blood, it is necessary to make some modifications to the original method. An overview of the CELLSEARCH® studies using CSF is given in Table 1. To calibrate the CELLSEARCH® system, the control mode is normally used [5]. In the control mode, a clear suspension of prestained fixed breast cancer cells is used and no separation line to aspirate the right fluid fraction is needed. Therefore, this mode can be used to aspirate the clear CSF automatically. Lee et al. used the control mode and Patel et al. spiked the CSF in blood for calibration of the CELLSEARCH® system [5, 20]. Le Rhun et al. and Tu et al. darkened the outside of the tube with a black felt-tip up to the fluid level to mimic the level of sedimented erythrocytes to allow for the selection of the clear CSF [4, 19]. The reported sensitivity and specificity for the diagnosis of LM of both types of modified CELLSEARCH® assays for CSF are shown in Table 2.
Flow cytometry
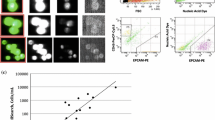
In fluorescence activated cell sorting systems (FACS) for CTCs enumeration of epithelial origin, different fluorescently labelled EpCAM antibodies are used to stain and count the cells. An overview of the FACS technology is depicted in Fig. 2a Milojkovic Kerklaan et al. and Lee et al. used immunomagnetic enrichment with anti-EpCAM MicroBeads prior to FACS analysis [5, 8]. To distinguish between CTCs and leukocytes, anti-CD45-fluorescein isothiocyanate (FITC) for leucocyte labeling was added. FACS plots of CSF obtained by this method are shown in Fig. 2b. In addition to these markers, Acosta et al. used anti-CD33 to improve differentiation between monocyte/macrophages/granulocytes (CD45− CD33+ CD326+) and epithelial cells (CD45− CD33− CD326−) [26]. Milojkovic Kerklaan et al. and Subirá et al. used Hoechst33258 and DRAQ5, respectively, for nuclear DNA-staining whereas Lee et al. did not use a DNA-dye [9, 10]. An overview of flow cytometry studies is given in Table 1. The reported sensitivity and specificity of these assays for the diagnosis of LM are shown in Table 2.
a Schematic representation of fluorescence activated cell sorting (FACS). Cells in the sample are focused into a stream of single cells by hydrodynamic focusing with sheath fluid. A laser is focused on the middle of this stream. Forward scatter is measured in a straight line opposite the laser beam and is used to distinguish cells on the basis of size. Sideward scatter and fluorescence is measured perpendicular to the laser beam and provide, respectively, information about internal complexity and amount of cell-bound fluorescently labelled antibody or dye. The signals from the photodiode detectors (PD) are processed by a computer using flow cytometry software. b Representative examples of epithelial cell adhesion molecule (EpCAM)-based flow cytometry plots of cerebrospinal fluid (CSF) from three individual patients. Circulating tumor cells (CTC) are defined as EpCAM+ and CD45− and will therefore be sorted to the CTC gate. b1: Non-small cell lung cancer patient with LM with EpCAM-positive CTCs (162 CTCs/mL); CSF cytology was positive (not shown). b2: Breast cancer patient with LM with EpCAM-positive CTCs (3 CTCs/mL). CSF cytology in this patient was negative (not shown). b3: Breast cancer patient without LM. No EpCAM-positive CTCs in CSF. CSF cytology in this patient was also negative (not shown). FACS fluorescence activated cell sorting systems, CTC circulating tumor cell, EpCAM epithelial cell adhesion molecule, LC leucocytes
Discussion
The diagnosis of LM is hampered by the low sensitivities of its diagnostic tools: MRI of brain and/or spine and CSF cytology. Although CSF cytology still is the gold standard for LM with a reported sensitivity of 44–67% at the first CSF examination, LM can also be diagnosed by the combination of neurological symptoms compatible with LM and leptomeningeal contrast enhancement in patients with known (metastasized) tumors [2]. The low sensitivity of cytology could be attributed partially to the spill of tumor cells at cytospin preparation. Furthermore, limited sample volume, delayed sample processing and sample collection at a suboptimal site (LP when there are mainly intracranial LM) [27]. Leptomeningeal contrast enhancement on MRI has a sensitivity of 76% for LM [1]. Currently, multiple techniques are used to detect and count EpCAM + CTCs in CSF to improve the CSF diagnostics for LM in patients with epithelial tumors. The reported results of the EpCAM-based techniques in CSF are highly promising with a detection limit of 0.4 CTC/mL. However, these techniques are not yet fully ready for clinical implementation due to lack of assay standardization and proper multicenter validation studies with adequate control groups. These studies are required for each individual CTC assay before clinical implementation. Furthermore, patients groups that have been investigated so far were rather small ranging from 6 to 144 patients. Sensitivity of the EpCAM based techniques may be lower in larger patients studies suspected for LM as it is known that tumor cells of epithelial origin can lose EpCAM expression due to epithelial to mesenchymal cell transition [28]. This may explain that patients with LM can have positive CSF cytology but no detectable CTCs [19].
The FDA-approved CELLSEARCH®-assay was initially validated in blood in a prospective, double-blind, multicenter clinical trial involving 177 metastatic breast cancer patients at 20 clinical centers [29]. The reported sensitivities of the EpCAM-based CTC assays for the diagnosis of LM across the different studies are highly promising and vary between 76 and 100%. However, none of the studied EpCAM assays for the enumeration of CTCs in CSF have yet been shown to be statistically significant better than CSF cytology [4,5,6,7,8,9,10, 19, 20, 26].
This can be attributed to the insufficient number of patients ultimately diagnosed with LM in the study cohorts. Furthermore, in order to establish the real value of the new techniques in CSF, standardization of the patient selection process is critical to ensure selection of patients with true diagnostic uncertainty of LM. A patient population with a true diagnostic uncertainty with clinical suspicion of LM was investigated in only two studies [6, 8]. All other studies that reported sensitivity for tumor cell detection in CSF also included patients with already proven LM based on MRI and/or CSF cytology [4, 5, 9, 10]. Future validation studies should be performed in properly defined study populations with a clinical suspicion on LM in prospective, multicenter triple blind (clinician, lab technician and patient) studies. A possible risk in CSF analysis is the detection of CTCs in the CSF due to contamination with blood in a traumatic LP. When high numbers of CTCs per mL blood are present, contamination of 5 mL CSF with just a few µL of blood may raise CTC levels above the detection limit, which can possibly effect the specificity of the assay [14]. Therefore, it is recommended to determine CTC-numbers in blood simultaneously with CSF, which up till now only has been done in one study [8].
The question which technique, CELLSEARCH® or flow cytometry, is optimal to detect epithelial tumor cells in CSF is unresolved as comparable sensitivity and specificity rates can be gained with both methods (Table 2). No direct comparison with adequate power between both methods in patients with a clinical suspicion on LM has been done hitherto [5]. The CELLSEARCH® method requires specific reviewer training to minimalize inter-reviewer discordant results [30]. Besides, a major limitation of the CELLSEARCH® analysis is the requirement of CELLSEARCH® reagents, CELLSEARCH® laboratory equipment and central laboratories equipped with CellTracks Autoprep, the CellTracks Analyzer II and trained operators. These prerequisites may limit wide-spread application’ [23]. Flow cytometry assays for CTCs utilize standard flow cytometry equipment, which makes these assays more widely applicable and can potentially shorten the time to LM diagnosis compared to the CELLSEARCH® analysis. Another important merit of flow cytometry is their reliance on a predefined tumor cell gate, which allows fully automatic identification and enumeration of CTCs in CSF. From an analytical perspective it makes sense to perform a pre-enrichment step using magnetic cell sorting with ferrolabelled antibodies against EpCAM to lower the amount of cellular background events. This has been applied in the CELLSEARCH® assay and in some flow cytometry assays [5, 8]. An overview of the benefits and drawbacks of flow cytometry and CELLSEARCH assays is given in Table 3.
A critical review of the randomized trials in LM using intra-CSF therapy, of which five of them enrolled patients with solid tumors, revealed that all these studies have methodological limitations with a lack of standardization for the evaluation of treatment response and long time-periods needed for accrual [31]. Also phase one clinical trials in patients with LM with targeted agents failed due to slow patient accrual [32, 33]. To improve the accrual rate of (early) LM patients and the reliability of response evaluation in clinical trials, CTC assays in CSF are promising tools as tumor cells can be quantified at very low levels. As LM often has a devastating course with median reported survival between 2 and 5 months [34], it is important to include patients with a low CSF tumor burden. A validated and sensitive CTC assay in CSF that can diagnose patients at an early LM stage when CSF cytology is still negative, is crucial. This was demonstrated by Milojkovic-Kerklaan et al., who reported that the EpCAM-based flow cytometry assay in CSF brings higher sensitivity than CSF cytology for the diagnosis of LM, especially when CTC numbers in the CSF drop below 50 cells/mL [8]. The specificity of the different EpCAM assays varies between 84 and 100%. Future large scale study cohorts need to reveal the true sensitivity and specificity of CTC assays in CSF. It is of particular interest to determine the optimal cut-off value for the number of CTCs per mL with an optimal sensitivity and specificity by using Receiver Operating Curves.
CSF cytology is a non-quantitative method with a low sensitivity, which renders the technique insufficient for monitoring of treatment response. A sensitive quantitative technique enables patient treatment response monitoring. A decrease in the CTC number would be indicative for a response to treatment. In several articles described in our review, sequential CSF samples from patients have been obtained for treatment monitoring using CTC enumeration [4, 5, 19, 20]. Lee et al. showed that in three of seven patients who had been treated for LM, no CTCs were detectable after treatment. CSF clearance of CTCs was associated with the longest survival with an average of 2 years [5]. Although the number of studies performed so far are limited, CTC enumeration in CSF has the potential to be a sensitive, specific, and quantitative biomarker for evaluating treatment response in LM. The new CTC assays do not only have the potential to be more sensitive, specific and quantitative in the diagnosis and treatment of LM, they also provide the possibility of expanding our knowledge on the pathophysiology of LM. Single cell analysis and the use of other molecular markers in the identification of the cells in the CSF may help to understand why this highly malignant cells metastasize to the CSF. Recently, Cordone et al. showed the presence of syndecan-1 and MUC-1 overexpression and the putative stem cell markers CD15, CD24, CD44 and CD133 on CTCs in the CSF of breast cancer patients with LM [35].
In conclusion, we have shown in our review that the EpCAM-based assays are promising new techniques for epithelial tumor cell detection in CSF, although assay standardization and proper multicenter validation studies are needed before clinical implementation. Furthermore, the possibility of detecting (and isolating) low numbers of tumor cells in the CSF using flow cytometry assays opens new ways to further understand why these malignant cells metastasize to the central nervous system.
References
Straathof CS, de Bruin HG, Dippel DW, Vecht CJ (1999) The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol 246(9):810–814
Wasserstrom WR, Glass JP, Posner JB (1982) Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 49(4):759–772
van Oostenbrugge RJ, Twijnstra A (1999) Presenting features and value of diagnostic procedures in leptomeningeal metastases. Neurology 53(2):382–385
Tu Q, Wu X, Le Rhun E, Blonski M, Wittwer B, Taillandier L, De Carvalho Bittencourt M, Faure GC (2015) CellSearch technology applied to the detection and quantification of tumor cells in CSF of patients with lung cancer leptomeningeal metastasis. Lung Cancer 90(2):352–357. https://doi.org/10.1016/j.lungcan.2015.09.008
Lee JS, Melisko ME, Magbanua MJ, Kablanian AT, Scott JH, Rugo HS, Park JW (2015) Detection of cerebrospinal fluid tumor cells and its clinical relevance in leptomeningeal metastasis of breast cancer. Breast Cancer Res Treat 154(2):339–349. https://doi.org/10.1007/s10549-015-3610-1
Nayak L, Fleisher M, Gonzalez-Espinoza R, Lin O, Panageas K, Reiner A, Liu CM, Deangelis LM, Omuro A (2013) Rare cell capture technology for the diagnosis of leptomeningeal metastasis in solid tumors. Neurology 80(17):1598–1605. https://doi.org/10.1212/WNL.0b013e31828f183f
Jiang BY, Li YS, Guo WB, Zhang XC, Chen Z, Su J, Zhong W, Yang XN, Yang J, Shao YW, Huang B, Liu YH, Zhou Q, Tu HY, Chen HJ, Wang Z, Xu C, Wang BC, Wu SY, Gao CY, Zhang X, Wu YL (2017) Detection of driver and resistance mutations in leptomeningeal metastases of NSCLC by next-generation sequencing of cerebrospinal fluid circulating tumor cells. Clin Cancer Res. https://doi.org/10.1158/1078-0432.ccr-17-0047
Milojkovic Kerklaan B, Pluim D, Bol M, Hofland I, Westerga J, van Tinteren H, Beijnen JH, Boogerd W, Schellens JH, Brandsma D (2016) EpCAM-based flow cytometry in cerebrospinal fluid greatly improves diagnostic accuracy of leptomeningeal metastases from epithelial tumors. Neuro Oncol 18(6):855–862. https://doi.org/10.1093/neuonc/nov273
Subira D, Serrano C, Castanon S, Gonzalo R, Illan J, Pardo J, Martinez-Garcia M, Millastre E, Aparisi F, Navarro M, Domine M, Gil-Bazo I, Perez Segura P, Gil M, Bruna J (2012) Role of flow cytometry immunophenotyping in the diagnosis of leptomeningeal carcinomatosis. Neuro Oncol 14(1):43–52. https://doi.org/10.1093/neuonc/nor172
Subira D, Simo M, Illan J, Serrano C, Castanon S, Gonzalo R, Granizo JJ, Martinez-Garcia M, Navarro M, Pardo J, Bruna J (2015) Diagnostic and prognostic significance of flow cytometry immunophenotyping in patients with leptomeningeal carcinomatosis. Clin Exp Metastasis 32(4):383–391. https://doi.org/10.1007/s10585-015-9716-3
Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, Gires O (2009) Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol 11(2):162–171. https://doi.org/10.1038/ncb1824
Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO (1994) Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol 125(2):437–446
Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S (2004) Frequent EpCam protein expression in human carcinomas. Hum Pathol 35(1):122–128
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10(20):6897–6904. https://doi.org/10.1158/1078-0432.CCR-04-0378
Zhou Y, Bian B, Yuan X, Xie G, Ma Y, Shen L (2015) Prognostic value of circulating tumor cells in ovarian cancer: a meta-analysis. PLoS ONE 10(6):e0130873. https://doi.org/10.1371/journal.pone.0130873
Lv Q, Gong L, Zhang T, Ye J, Chai L, Ni C, Mao Y (2016) Prognostic value of circulating tumor cells in metastatic breast cancer: a systemic review and meta-analysis. Clin Transl Oncol 18(3):322–330. https://doi.org/10.1007/s12094-015-1372-1
Huang X, Gao P, Song Y, Sun J, Chen X, Zhao J, Xu H, Wang Z (2015) Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch system in colorectal cancer. BMC Cancer 15:202. https://doi.org/10.1186/s12885-015-1218-9
Ma X, Xiao Z, Li X, Wang F, Zhang J, Zhou R, Wang J, Liu L (2014) Prognostic role of circulating tumor cells and disseminated tumor cells in patients with prostate cancer: a systematic review and meta-analysis. Tumour Biol 35(6):5551–5560. https://doi.org/10.1007/s13277-014-1731-5
Le Rhun E, Massin F, Tu Q, Bonneterre J, Bittencourt Mde C, Faure GC (2012) Development of a new method for identification and quantification in cerebrospinal fluid of malignant cells from breast carcinoma leptomeningeal metastasis. BMC Clin Pathol 12:21. https://doi.org/10.1186/1472-6890-12-21
Patel AS, Allen JE, Dicker DT, Peters KL, Sheehan JM, Glantz MJ, El-Deiry WS (2011) Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget 2(10):752–760. https://doi.org/10.18632/oncotarget.336
Andree KC, van Dalum G, Terstappen LW (2016) Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol 10(3):395–407. https://doi.org/10.1016/j.molonc.2015.12.002
CellSearch™ (2017) Circulating Tumor Cell Kit premarket notification—expanded indications for use-colorectal. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf7/k071729.pdf Accessed 21 Mar 2017
de Wit S, van Dalum G, Terstappen LW (2014) Detection of circulating tumor cells. Scientifica 2014:819362. https://doi.org/10.1155/2014/819362
Cellsearch® (2017) Circulating Tumor Cell Kit (Epithelial). Available at http://documents.cellsearchctc.com/pdf/e631600004/e631600004_EN.pdf Accessed 22 Mar 2017
Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Janicke F, Jackson S, Gornet T, Cristofanilli M, Pantel K (2007) Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 13(3):920–928. https://doi.org/10.1158/1078-0432.CCR-06-1695
Acosta M, Pereira J, Arroz M (2016) Screening of carcinoma metastasis by flow cytometry: a study of 238 cases. Cytometry B Clin Cytom 90(3):289–294. https://doi.org/10.1002/cyto.b.21258
Glantz MJ, Cole BF, Glantz LK, Cobb J, Mills P, Lekos A, Walters BC, Recht LD (1998) Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer 82(4):733–739
Hyun KA, Koo GB, Han H, Sohn J, Choi W, Kim SI, Jung HI, Kim YS (2016) Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 7(17):24677–24687. https://doi.org/10.18632/oncotarget.8250
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12(14 Pt 1):4218–4224. https://doi.org/10.1158/1078-0432.CCR-05-2821
Kraan J, Sleijfer S, Strijbos MH, Ignatiadis M, Peeters D, Pierga JY, Farace F, Riethdorf S, Fehm T, Zorzino L, Tibbe AG, Maestro M, Gisbert-Criado R, Denton G, de Bono JS, Dive C, Foekens JA, Gratama JW (2011) External quality assurance of circulating tumor cell enumeration using the CellSearch® system: a feasibility study. Cytometry B Clin Cytom 80(2):112–118. https://doi.org/10.1002/cyto.b.20573
Chamberlain M, Soffietti R, Raizer J, Ruda R, Brandsma D, Boogerd W, Taillibert S, Groves MD, Le Rhun E, Junck L, van den Bent M, Wen PY, Jaeckle KA (2014) Leptomeningeal metastasis: a response assessment in neuro-oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol 16(9):1176–1185. https://doi.org/10.1093/neuonc/nou089
Jackman DM, Cioffredi LA, Jacobs L, Sharmeen F, Morse LK, Lucca J, Plotkin SR, Marcoux PJ, Rabin MS, Lynch TJ, Johnson BE, Kesari S (2015) A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget 6(6):4527–4536. https://doi.org/10.18632/oncotarget.2886
Wu PF, Lin CH, Kuo CH, Chen WW, Yeh DC, Liao HW, Huang SM, Cheng AL, Lu YS (2015) A pilot study of bevacizumab combined with etoposide and cisplatin in breast cancer patients with leptomeningeal carcinomatosis. BMC Cancer 15:299. https://doi.org/10.1186/s12885-015-1290-1
Brower JV, Saha S, Rosenberg SA, Hullett CR, Ian Robins H (2016) Management of leptomeningeal metastases: prognostic factors and associated outcomes. J Clin Neurosci 27:130–137. https://doi.org/10.1016/j.jocn.2015.11.012
Cordone I, Masi S, Summa V, Carosi M, Vidiri A, Fabi A, Pasquale A, Conti L, Rosito I, Carapella CM, Villani V, Pace A (2017) Overexpression of syndecan-1, MUC-1, and putative stem cell markers in breast cancer leptomeningeal metastasis: a cerebrospinal fluid flow cytometry study. Breast Cancer Res 19(1):46. https://doi.org/10.1186/s13058-017-0827-4
Tibbe AG, de Grooth BG, Greve J, Dolan GJ, Terstappen LW (2002) Imaging technique implemented in CellTracks system. Cytometry 47(4):248–255
Tu Q, Wu X, Le Rhun E, Blonski M, Wittwer B, Taillandier L, De Carvalho Bittencourt M, Faure GC.(2015) CellSearch technology applied to the detection and quantification of tumor cells in CSF of patients with lung cancer leptomeningeal metastasis, Pages No. 352–357 Reprinted from Lung Cancer: vol 90 number 2 Copyright 2015, with permission from Elsevier
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
van Bussel, M.T.J., Pluim, D., Bol, M. et al. EpCAM-based assays for epithelial tumor cell detection in cerebrospinal fluid. J Neurooncol 137, 1–10 (2018). https://doi.org/10.1007/s11060-017-2691-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2691-6