Abstract
Circulating tumor cells are commonly observed in the peripheral blood of advanced breast cancer patients. We tested the feasibility of tumor cell detection in the cerebrospinal fluid (CSF) and studied its clinical relevance in leptomeningeal metastasis (LM) of breast cancer. CSF samples were collected from 38 metastatic breast cancer patients known or suspected to have LM. Control CSF samples were collected from 14 individuals without solid tumor malignancy. We used a modified CellSearch™ assay and an alternative EPCAM-based method involving immunomagnetic enrichment followed by flow cytometry (IE/FC) to enumerate CSF tumor cells (CSFTCs). CSFTCs were assayed at time of LM diagnosis and over the course of LM-directed therapy. We analyzed a total of 102 CSF samples with modified CellSearch™. The CSFTC counts were strongly correlated with the corresponding IE/FC results (Pearson’s r = 0.94). Twenty-eight out of 30 samples in which malignant cells were identified by CSF cytology were CSFTC-positive by modified CellSearch™. Baseline CSFTC levels from 21 patients eventually diagnosed with LM were significantly higher than the controls (p = 0.0202), whereas 13 patients deemed not to have LM showed CSFTC results indistinguishable from the controls. In patients with serial samples, it was possible to monitor CSFTC levels as a potential biomarker of treatment response. CSFTC detection using a modified CellSearch™ assay demonstrated high sensitivity in detecting malignant cells in CSF and may be a promising method for diagnosing LM and monitoring LM during treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leptomeningeal metastasis (LM) represents a well-recognized clinical syndrome which can strike advanced cancer patients and is typically associated with extremely high morbidity and mortality [1, 2]. CNS metastasis, including LM, appears to be increasing in incidence; this has been attributed to greater awareness, improved CNS imaging techniques, and longer survival with systemic disease [3–5]. Breast cancer is among the most common solid tumors that give rise to LM, and 10–20 % of breast cancer patients who have parenchymal brain metastases eventually develop LM [6, 7]. Although improved systemic therapies have resulted in better outcomes for many cancer patients, the median survival of patients with LM is 4–6 weeks without treatment and 3–7 months with multimodality therapy [8–11]. This poor prognosis reflects a paucity of management options and therapies for LM. Patients are often diagnosed when their disease is highly advanced and their performance status is compromised, further limiting therapeutic options. In addition to improved therapies, it would be beneficial to have a biomarker-driven approach to address LM, including earlier diagnosis, disease monitoring, and facilitation of clinical trials.
Evaluation by cerebrospinal fluid (CSF) cytology is the gold standard for LM diagnosis. However, CSF cytology has a high false-negative rate, with a sensitivity of only 50–75 % on the first lumbar puncture [12, 13]. Consequently, repeat sampling involving the collection of high volumes of CSF is often required to definitively make the diagnosis [14–16]. Neuroaxial imaging, such as brain or spinal magnetic resonance imaging (MRI), are complementary methods to diagnose LM, but many equivocal MRI findings make these methods less accurate [15, 17].
Circulating tumor cells (CTCs) are rare cells in the peripheral blood considered to be seeding from the primary tumor or other metastatic sites. Detection methods for these rare cells have been developed [18–20], such as the CellSearch™ system [21–24]. Changes in the number of CTCs in response to treatment in metastatic breast cancer, as well as the detection of CTCs in early breast cancer, have both been shown to provide prognostic information [25–28]. In contrast to CTCs, CSF tumor cells (here referred to as CSFTCs) have not been extensively studied beyond standard CSF cytology examination. Here, we used CTC-based techniques to detect, count, and monitor CSFTCs in CSF samples obtained from 38 patients. We investigated the feasibility and accuracy of CSFTC detection in diagnosing LM in breast cancer patients. Finally, we monitored CSFTC numbers and subsequent LM disease progression in a subset of patients.
Methods
Patients and clinical samples
Thirty-eight metastatic breast cancer patients known or suspected to have LM based on clinical symptoms or neuroaxial images were enrolled in this study between November 2005 and January 2014. For controls, an additional 14 patients who had hematologic malignancy with no history of a solid tumor malignancy and no findings consistent with LM were also enrolled. All patients gave informed consent for participation in this study.
CSF samples were collected from the breast cancer patients via lumbar puncture or Ommaya reservoir at initial presentation of neurologic symptoms or abnormal neuroaxial imaging suggestive of LM and/or throughout treatment for diagnosed LM. At each initial CSF collection from the 38 patients, at least 8 mL of CSF were sent for standard cytologic evaluation. CSF cytology was not performed in the follow-up samples if it was not clinically indicated. Based on available volume of the sample, an additional 0.4–4.5 mL of CSF underwent CSFTC counting by CellSearch™ or IE/FC. For the control group, CSF samples (1.0–4.0 mL) were obtained via lumbar puncture. For CellSearch™ analysis, CSF samples were collected in CellSave preservative tubes, which allow for storage at ambient temperature for up to 96 h. CSF samples for IE/FC enumeration were collected into tubes containing ethylenediaminetetraacetic acid (EDTA). In our study, we processed the samples within 24–48 h after CSF collection. The investigator (J.H.S.) who performed the CSFTC analysis was blinded to the patient clinical data.
CSFTC detection
We adapted two different EPCAM-based methods originally designed for CTC enumeration to count CSFTCs.
CellSearch™
CSF samples were analyzed using the CellSearch™ system (Veridex LLC, Warrenn, NJ), a US Food and Drug Administration-cleared method for enumeration of CTCs in 7.5mLs of blood from metastatic breast, prostrate, and colon cancers [18, 24, 25, 29]. The CellSearch™ is a semi-automated system involving an immunomagnetic enrichment step followed by fluorescence microscopy. The enrichment and staining steps are performed within the CellTracks Autoprep machine. Here, the sample is enriched for tumor cells using anti-EPCAM conjugated to magnetic beads. After enrichment, fluorescent dyes are added to the enriched sample. These dyes include 4′,6-diamidino-2-phenylindole (DAPI) to stain the nuclei, anti-cytokeratin conjugated to phycoerythrin (EPCAM-PE) to stain for epithelial cells, and anti-CD45 conjugated to allophycocyanin (CD45–APC) to stain for leukocytes. The enriched sample is then examined via fluorescence microscopy using the CellTracks Analyzer II to enumerate CTCs. The machine automatically scans the enriched sample and acquires fluorescent images. Nucleated, cytokeratin-positive, and CD45-negative events are considered CTCs.
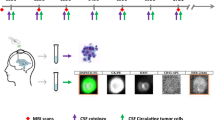
The CellSearch™ CTC kit was used to analyze CSF samples to detect CSFTCs. The dilution buffer (Veridex LLC, Warrenn, NJ) was added when the CSF sample was less than 4.5 mLs. Since the CellSearch™ method was originally designed to enumerate CTCs in the blood, modifications were made to allow for CSF processing. The modifications included the elimination of the centrifugation step prior to enrichment and the use of the “control mode” in the CellTracks Autoprep system. This system relies on the detection of the separation line between the clear phase (plasma) and red phase (containing erythrocytes, white blood cells, and tumor cells, if present) in centrifuged blood. This separation line guides the machine to aspirate the plasma at the correct depth while leaving the red phase portion untouched for the subsequent immunomagnetic enrichment step. As CSF samples are mostly clear with few red blood cells, a separation line cannot be detected. The “control mode,” which is normally used for calibrating the CellSearch™ system, uses a clear suspension of pre-stained fixed cancer cell line [CTC Control Kit (Veridex LLC, Warren, NJ)] and therefore does not require a separation line. After enrichment, the sample was imaged in the Cell Tracks Analyzer II using the “standard mode” (i.e., treated as a blood sample). Similar to CTCs, CSFTCs were also defined as nucleated, cytokeratin-positive, and CD45-negative cells. Cell clusters were counted as a single event. Examples of CSFTC images accessed through the Cell Tracks Analyzer II are shown in Fig. 1a.
Detection of tumor cells in cerebrospinal fluid. a Single and clusters of tumor cells were detected in the cerebrospinal fluid (CSF) using a modified CellSearch™ assay. b Tumor cells were detected using immunomagnetic enrichment followed by flow cytometry (IE/FC). CSFTCs were defined as EPCAM-positive (EPCAM-PE), CD45-negative (CD45–PerCP-Cy5.5), and nucleated. Gates P1 and P2 select for cells that are nucleated. Gates P3 and P4 select for cells that are CD45–PerCP-Cy5.5-negative and EPCAM-PE-positive. P5 gate selects for events that are CD45–PerCP-Cy5.5-positive and EPCAM-PE-negative (e.g., white blood cells/non-tumor control cells). c The correlation of CSFTC counts between two methods is shown. Thirty-two CSF samples were analyzed concurrently for the presence of CSFTCs via IE/FC and modified CellSearch™. The CSFTC counts by modified CellSearch™ were strongly correlated with the corresponding IE/FC results (r = 0.94). SSC side scatter, FSC forward scatter
IE/FC
A second EPCAM-based method involving immunomagnetic enrichment followed by flow cytometry (IE/FC) was used to count CSFTC as previously described with minor modification [30, 31]. Briefly, casein buffer was added to the CSF sample followed by the addition of two different monoclonal antibodies to EPCAM: one conjugated to magnetic beads and the other to phycoerythrin (EPCAM-PE). The sample was then subjected to a magnetic field for 15 min. Unbound cells (supernatant) were then aspirated, leaving a suspension that is enriched for EPCAM-expressing cells. A second round of enrichment was performed by incubating the sample in a magnetic filed for 5 min. Next, a nucleic acid dye and anti-CD45 conjugated to peridinin chlorophyll-Cy5.5 (CD45–PerCP-Cy5.5) were added to the sample. This step was followed by flow cytometric analysis to count CSFTCs defined as nucleated, EPCAM-positive, and CD45-negative (Fig. 1b). A subset of CSF samples was analyzed by this IE/FC method concurrently with a modified CellSearch™ assay to determine the correlation of two different EPCAM-based methods.
Data analysis
We used STATA ver.12 to conduct all statistical analysis. Independent-samples t-test was implemented to compare CSFTC numbers between two groups. The correlation between modified CellSearch™ and IE/FC was calculated using the Pearson correlation coefficient (r). Assay performance characteristics including sensitivity and specificity of conventional CSF cytology and the modified CellSearch™ method were evaluated based on results from initial samples.
Results
Patient characteristics
Metastatic breast cancer patients who were known or suspected to have LM based on clinical symptoms or MRI findings were enrolled in the study (N = 38) (Table 1). The median age of study participants was 47 years old (range 27–76). Thirty-two (84 %) patients had neurologic symptoms including headache, back pain, leg weakness, or altered mental status leading to clinical workup for LM. Six patients did not have neurologic symptoms but had findings suspicious for LM noted on routine follow-up MRI for previously treated parenchymal brain or spine metastases. Among these cases, CSF sampling was recommended by the radiologist based on the imaging findings. Of the 38 total patients, 25 (66 %) patients were estrogen receptor (ER) positive or progesterone receptor (PR) positive, 15 (40 %) patients were HER2-positive, and 8 (21 %) patients had triple negative breast cancer (ER-negative, PR-negative, and HER2-negative). Thirty-four (90 %) patients had multiple sites of metastatic disease, and 26 (68 %) patients had parenchymal brain metastasis. Of the 38 patients, 17 patients were positive for malignant cells in their first CSF cytology exam, and 1 patient was positive in her third CSF cytology. 20 patients were negative for malignant cells in all the CSF cytological exams performed. The survival rate after study enrollment was 55.3 % at 24 weeks and 34.2 % at 48 weeks.
CSFTC assay configuration and optimization
The CellSearch™ method was originally designed to enumerate epithelial cells in 7.5 mLs of blood. To adapt this semiautomated assay for CSF, we used the “control mode” in the CellTracks Autoprep system (see "Methods"). The “control mode” is used to calibrate the CellSearch™ system using a clear suspension of pre-stained cultured cells and therefore does not require the separation line between the plasma and blood cell phase. In blood, extensive studies using CellSearch™ have confirmed that nonmalignant epithelial cells (defined as EPCAM-positive, cytokeratin-positive, and CD45 negative) are extremely rare [18]. However, we anticipated that CSF might contain a background of normal epithelial cells detectable by this assay. We therefore assayed CSF samples from 14 control individuals without a solid tumor diagnosis, including patients with unrelated hematologic malignancies. Mean and median cell counts in the control group were 0.3 and 0 cells/mL, respectively. Eight (8) of the 14 control samples exhibited 0 CSFTCs. We chose 1.9 cells/mL, representing the mean plus three standard deviations in the control group, as the cutoff for positivity. CSF samples with >1.9 cells/mL were considered positive.
CSFTC detection in breast cancer patients
A total of 102 CSF samples from 38 patients were analyzed via the modified CellSearch™ assay. The mean and median CSFTC counts by modified CellSearch™ were 407.1 and 14.9 cells/mL (range: 0–9323.0 cells/mL), respectively. 70 samples from 22 patients were CSFTC-positive. While most CSFTCs appeared as single cells, some were observed as clusters. Examples of CSFTCs detected by modified CellSearch™ are shown in Fig. 1a.
Conventional CSF cytology was obtained concurrently for 81 of the 102 samples (Table 2). This includes initial samples from 38 patients, and 43 follow-up samples. Thirty (30) of the 81 (37.0 %) samples were positive by CSF cytology, of which 28 samples were also positive by modified CellSearch™. Twelve (12) of the 17 samples that were interpreted to contain atypical cells by CSF cytology were CSFTC-positive. Of the 34 samples negative by CSF cytology, 21 samples were CSFTC-negative. The agreement of these two methods was 76.6 % (kappa = 0.54, moderate agreement).
In a subset of CSF samples, an alternative EPCAM-based method, IE/FC, was performed in parallel with the modified CellSearch™ assay to detect and isolate CSFTCs. Similar to modified CellSearch™, IE/FC uses magnetic beads coated with anti-EPCAM monoclonal antibodies to capture epithelial cells. However, instead of fluorescence microscopy detection, IE/FC uses flow cytometry to count CSFTCs (Fig. 1b). An advantage of the IE/FC approach is that it is readily amenable to FACS isolation of tumor cells for detailed molecular analysis [32]. Thirty-two (32) of 102 samples were analyzed by both assays. The mean and median of CSFTCs detected by the IE/FC method were 411.5 and 3.5 cells/mL, respectively (0–11,634.4 cells/mL). The CSFTC counts by modified CellSearch™ and IE/FC were highly correlated (Pearson’s r = 0.94, Fig. 1c).
CSFTC detection during diagnostic evaluation of LM
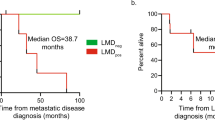
In 34 patients, baseline CSF samples were obtained in patients not yet diagnosed with LM and prior to any LM-directed therapy. The mean and median CSFTC counts in these baseline samples were 683.4 and 11.3 cells/mL (range: 0.0–5557.5), respectively. Fourteen (14) of the 34 patients showed clinically positive CSF cytology in their baseline CSF samples, and this subgroup showed significantly higher CSFTC counts (mean, 1649.5; median, 858.0) as compared to the control group (mean, 0.3: median, 0; p = 0.0025) (Fig. 2a). Patients with atypical CSF cytology results also showed higher CSFTC numbers as compared to the control group (p = 0.0488). No significant difference was observed between CSFTC counts in patients with negative CSF cytology results as compared to the control group (p = 0.0914).
CSFTC numbers by the modified CellSearch™ assay for baseline samples and comparison with the control group. a Patients with positive CSF cytology had significantly higher CSFTC counts as compared to those in the control group (p = 0.0025), but a difference was not observed in the patients with CSF cytology negative (p = 0.0914). b The patients diagnosed with leptomeningeal metastasis (LM) exhibited significantly higher CSFTCs than the control group (p = 0.0202), but there was no difference in CSFTCs between the patients without LM and the control group (p = 0.2299)
Twenty-one (21) out of 34 patients were formally diagnosed with LM during their clinical course, as follows: (i) all 14 patients with positive CSF cytology at baseline; (ii) one additional patient, who showed atypical cells in her baseline CSF cytology but was positive for CSFTCs by modified CellSearch™, was subsequently diagnosed with LM based on positive CSF cytology on repeat sampling; and (iii) 6 patients with negative baseline CSF cytology showed unequivocal findings on their neuroaxial MRIs and/or progression of their neurologic symptoms and clinical course, ultimately leading to death. Figure 2b summarizes the CSFTC counts in baseline CSF samples and the corresponding LM status. The patients who were ultimately diagnosed with LM exhibited significantly higher baseline CSFTC counts than the control group (p = 0.0202). Conversely, there was no difference in CSFTC counts between the patients never diagnosed with LM and the control group (p = 0.2299).
Table 3 summarizes the accuracy of initial CSFTC detection in diagnosing LM. The sensitivity and specificity of initial CSFTC assay to diagnose LM was 80.95% (95 % CI 58.08–94.44 %) and 84.62 % (95 % CI 54.54–97.63 %), respectively. In this series, the sensitivity of initial CSF cytology to diagnose LM was 66.67 % (95 % CI 43.04–85.35 %), while specificity was considered 100 % since detection of malignant cells by standard CSF cytology is regarded as pathognomonic for LM and accepted as the gold standard for diagnosis.
CSFTC monitoring
Serial CSF samples were collected in 7 patients to monitor changes in CSFTC counts throughout treatment for LM (range: 3–21 time points; Fig. 3). Six of these 7 patients had positive CSF cytology at least once (patient 6 did not). The CSFTC counts in all seven patients changed during LM-directed therapy, including intrathecal (IT) chemotherapy and/or radiotherapy.
Monitoring CSFTC counts by the modified CellSearch™ assay in serial samples collected during treatment for leptomeningeal metastasis. Patient clinical information is described in the main text and Supplementary Data. a Patients whose CSFTC counts decreased during treatment with at least one time point showing negative for CSFTCs (≤1.9 cells/mL). b Patients whose CSFTC counts remained positive (> 1.9 cells/mL) during treatment
Patients 1–4 exhibited decreased CSFTC counts during treatment with at least one time point showing negative for CSFTCs (≤1.9 cells/mL, Fig. 3a). The other group, which included patients 5–7, remained positive for CSFTCs at all time points (Fig. 3b). With the exception of patient 4 (who suffered sudden death due to unclear etiology), the first group had the longest survival in this cohort, i.e., patients 1, 2, and 3 with survival times of, 115.5, 47.6, and 142.0 weeks, respectively. In contrast, patients who failed to clear CSFTCs had shorter survival, e.g., patients 5, 6, and 7 with survival times of 24.9, 18.0, and 19.7 weeks, respectively.
Three of these cases illustrate the feasibility and potential clinical relevance of monitoring CSFTC counts:
(1) Patient 1 was a 54-year-old woman with estrogen receptor (ER)-positive, progesterone receptor (PR)-positive, and HER2-negative breast cancer with bone, liver, and pleural metastases (Fig. 3a). She presented with altered mental status, confusion, and memory loss, and was found to have leptomeningeal enhancement on brain MRI. Her baseline CSF cytology was positive for malignant cells, and her baseline CSFTC level was extremely high at 2927 cells/mL. IT methotrexate was initiated; subsequently, her CSF cytology remained positive but her CSFTC level declined significantly. The patient continued to have neurologic symptoms, but the patient and family desired continuation of treatment. However, treatment was changed to IT liposomal cytarabine on Day 15, followed by rapid further decline in the CSFTC level to nearly undetectable. The patient’s neurologic and performance status began to improve between 4 and 6 weeks into treatment, suggesting the decrease in CSFTCs was an early marker of response. Over time, the patient did experience worsening memory and cognitive dysfunction without evidence of progression of leptomeningeal enhancement or increase in CSFTCs. Subsequent treatment included intravenous (I.V.) thiotepa, nab-paclitaxel, and temozolomide. The patient survived for 115.5 weeks, and her death was attributed to systemic progression and possible infection.
(2) Patient 3 was a 38 year-old ER-positive, PR-positive, and HER2-negative breast cancer patient with bone, brain, and liver metastases (Fig. 3a). She began experiencing leg weakness, and her brain and spine MRI demonstrated leptomeningeal enhancement. Her first CSF cytology contained only atypical cells, while her CSFTC assay was clearly positive (45.0 cells/mL). She was initially treated with craniospinal irradiation alone. Her follow-up CSF sample demonstrated only atypical cells on standard cytology, but her CSFTCs were decreased (5.0 cells/mL). Six months later, repeat CSF sampling revealed a positive CSF cytology and increase in CSFTCs (38.0 cells/mL). IT methotrexate was initiated at that time. Her CSF cytology reverted to negative and CSFTC count progressively decreased and remained undetectable. Because the patient’s neurological symptoms improved, IT chemotherapy was discontinued. Nearly 2 years after discontinuation of IT methotrexate, the patient experienced worsening headaches and was found to have radiographic evidence of progression in parenchymal brain metastases with increasing leptomeningeal enhancement. CSFTC numbers were increased markedly to 111.6 cells/mL as well at day 966. She participated in a clinical trial of irinotecan and temozolomide, but deteriorated clinically and died of disease progression 1 week after the final CSF sampling.
(3) Patient 4 was a 53 year-old-patient with ER-positive, PR-positive, and HER2-negative breast cancer with metastases involving lung, liver, bone, and cerebellum (Fig. 3a). This patient presented with neurologic deterioration (confusion and altered mental status) despite stable parenchymal brain metastases. Her initial CSF cytology showed atypical cells, and 5 subsequent cytology tests were negative. Her baseline CSFTC result was positive at 8.3 cells/mL but became negative on subsequent testing after treatment with IT cytarabine. She experienced short survival time (15.3 weeks) despite never having a positive CSF cytology, and her death was attributed to progression of CNS disease based on neurologic deterioration.
Discussion
LM is a dismal complication of metastatic cancer, which usually leads to neurologic deterioration and death. Over the past two decades, little progress has been made in the diagnosis or treatment of this disease. Clinical management of LM is challenging owing to vague neurologic presentation, which often leads to delayed diagnosis and difficulty in assessing response to treatment. In addition, management is hampered by the dearth of efficacious treatment options as well as LM-specific biomarkers to guide therapy [33]. Neurologic symptoms and clinical condition frequently decline early in the course of treatment, and failure to “clear” the CSF by standard cytology may lead to early discontinuation of treatment for presumed futility. In the current study, we employed both IE/FC and modified CellSearch as adjuncts to LM diagnosis and for quantitative monitoring of CSFTC over time. IE/FC expands upon our previous molecular profiling results, while CellSearch is an established, widely available system which has amassed extensive clinical data when used for CTC testing.
The ability to quantify tumor cells, in contrast to conventional CSF cytology or imaging, can be an important advantage of new LM detection strategies. Recent studies have reported versions of the CellSearch™ assay to detect and quantify CSFTCs. For example, a pilot study involving 5 breast cancer patients showed a trend towards higher CSFTC counts in patients with positive CSF cytology [34]. Le Rhun et al. described counting of CSFTCs in 16 samples from 8 breast cancer patients with LM [35]. Finally, Nayak et al. reported high sensitivity and high specificity of a modified CellSearch™ system to diagnose LM among 51 patients with solid tumors and 9 patients without solid tumor [36]. Modifications to the CellSearch™ system made by Nayak and colleagues were similar to the ones implemented in this study. These include the elimination of the initial centrifugation step and the use of the “control mode”, which allows for enumeration of tumor cells in clear fluids like the CSF.
Here, we demonstrate the feasibility of detecting CSFTCs using two different techniques, i.e., modified CellSearch™ and IE/FC assays. We analyzed a total of 102 CSF samples, which to our knowledge is the largest study to date. The modified CellSearch™ system demonstrated high sensitivity for detecting tumor cells in CSF samples and was highly correlated with corresponding IE/FC results. Twenty-eight out of 30 samples in which malignant cells were identified by CSF cytology were CSFTC-positive by modified CellSearch™. Patients who were ultimately determined to have LM based on established clinicopathologic criteria had significantly higher CSFTC counts compared to those without LM or to the control group. In addition, we observed the presence of CSFTC clusters using the modified CellSearch™ method. A recent study demonstrated the increased metastatic potential of CTC clusters using mouse models [37]. The clinical significance of both CTC and CSFTC clusters, however, has yet to be determined.
We have coupled the IE/FC assay to fluorescence-activated cells sorting to fully isolate CTCs from blood using for downstream molecular characterization [32, 38, 39]. In addition, we have previously reported on the molecular profiling of IE/FC-isolated CSFTCs from 13 of the 38 patients in this cohort [40–42]. Genome-wide copy number analysis revealed chromosome aberrations typical of breast cancer. Interestingly, the 8q24 locus containing the MYC oncogene was preferentially amplified in most samples. Comparison of genomic profiles of CSFTCs with matched primary tumors demonstrated a clear clonal relationship. Taken together, our molecular data strongly suggests that CSFTCs detected by IE/FC are of breast cancer origin and are malignant in nature.
Although a positive CSF cytology is the current gold standard for LM diagnosis, this test is notoriously insensitive [17, 43]. For example, one patient who was negative by CSF cytology but CSFTC-positive by modified CellSearch™ on her initial examination was later found to have malignant cells by CSF cytology on repeat CSF sampling. However, one limitation of our study is the absence of consensus on how best to diagnose LM. In this study, we retrospectively assigned 21 patients as having LM based on a positive CSF cytology, radiographic findings, and/or their clinical course. However, it is possible that the remaining 13 patients who presented with symptoms or radiographic findings suspicious for LM, but who were not ultimately diagnosed or classified as having LM, may nevertheless have harbored LM. In the absence of a dedicated autopsy study, it is impossible to know with certainty which of the patients with suspicion for LM did or did not have the condition.
It was, however, possible to monitor CSFTC levels over time as a potential biomarker of LM disease status. Interestingly, changes in CSFTC counts anticipated treatment response and/or progression in several of these patients. Therefore, new approaches for CSFTC detection may not only increase the sensitivity and accuracy of LM diagnosis, but may also serve as strategy to assess treatment response or as an early marker of LM progression.
Despite recent advances in cancer treatment including new targeted therapies, patients with LM still experience extremely short survival times. New CSFTC detection methods, such as the modified CellSearch™ or the IE/FC assay, may facilitate earlier diagnosis. This approach may also allow quantitative monitoring of treatment response to improve the dismal outcomes typically associated with LM. Finally, CSFTC enumeration can be extended to include CSFTC isolation and molecular profiling to assist in diagnosis and monitoring of disease progression and treatment response, and to advance our understanding of the underlying biology of LM.
References
Chamberlain MC (2004) Neoplastic meningitis. Semin Neurol 24(4):363–374. doi:10.1055/s-2004-861531
Scott BJ, Kesari S (2013) Leptomeningeal metastases in breast cancer. Am J Cancer Res 3(2):117–126
Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM (2010) Leptomeningeal metastases in the MRI era. Neurology 74(18):1449–1454. doi:10.1212/WNL.0b013e3181dc1a69
Frisk G, Svensson T, Backlund LM, Lidbrink E, Blomqvist P, Smedby KE (2012) Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer 106(11):1850–1853. doi:10.1038/bjc.2012.163
Lin NU, Winer EP (2007) Brain metastases: the HER2 paradigm. Clin Cancer Res 13(6):1648–1655. doi:10.1158/1078-0432.CCR-06-2478
Altundag K, Bondy ML, Mirza NQ, Kau SW, Broglio K, Hortobagyi GN, Rivera E (2007) Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer 110(12):2640–2647. doi:10.1002/cncr.23088
Kim HJ, Im SA, Keam B, Kim YJ, Han SW, Kim TM, Oh DY, Kim JH, Lee SH, Chie EK, Han W, Kim DW, Kim TY, Noh DY, Heo DS, Park IA, Bang YJ, Ha SW (2012) Clinical outcome of central nervous system metastases from breast cancer: differences in survival depending on systemic treatment. J Neuro-oncol 106(2):303–313. doi:10.1007/s11060-011-0664-8
Gauthier H, Guilhaume MN, Bidard FC, Pierga JY, Girre V, Cottu PH, Laurence V, Livartowski A, Mignot L, Dieras V (2010) Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol 21(11):2183–2187. doi:10.1093/annonc/mdq232
de Azevedo CR, Cruz MR, Chinen LT, Peres SV, Peterlevitz MA, de Azevedo Pereira AE, Fanelli MF, Gimenes DL (2011) Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neuro-oncol 104(2):565–572. doi:10.1007/s11060-010-0524-y
Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, Ettinger DS (1993) Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol 11(3):561–569
Chamberlain MC (1998) Leptomeningeal metastases: a review of evaluation and treatment. J Neurooncol 37(3):271–284
Kaplan JG, DeSouza TG, Farkash A, Shafran B, Pack D, Rehman F, Fuks J, Portenoy R (1990) Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neuro-oncol 9(3):225–229
Balm M, Hammack J (1996) Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol 53(7):626–632
Glantz MJ, Cole BF, Glantz LK, Cobb J, Mills P, Lekos A, Walters BC, Recht LD (1998) Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer 82(4):733–739
Straathof CS, de Bruin HG, Dippel DW, Vecht CJ (1999) The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol 246(9):810–814
Deisenhammer F, Bartos A, Egg R, Gilhus NE, Giovannoni G, Rauer S, Sellebjerg F, Force ET (2006) Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol 13(9):913–922. doi:10.1111/j.1468-1331.2006.01493.x
Chamberlain MC, Glantz M, Groves MD, Wilson WH (2009) Diagnostic tools for neoplastic meningitis: detecting disease, identifying patient risk, and determining benefit of treatment. Semin Oncol 36(4 Suppl 2):S35–S45. doi:10.1053/j.seminoncol.2009.05.005
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10(20):6897–6904
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M (2007) Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450(7173):1235–1239. doi:10.1038/nature06385
Todenhofer T, Hennenlotter J, Feyerabend S, Aufderklamm S, Mischinger J, Kuhs U, Gerber V, Fetisch J, Schilling D, Hauch S, Stenzl A, Schwentner C (2012) Preliminary experience on the use of the Adnatest(R) system for detection of circulating tumor cells in prostate cancer patients. Anticancer Res 32(8):3507–3513
Tanaka F, Yoneda K, Kondo N, Hashimoto M, Takuwa T, Matsumoto S, Okumura Y, Rahman S, Tsubota N, Tsujimura T, Kuribayashi K, Fukuoka K, Nakano T, Hasegawa S (2009) Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 15(22):6980–6986. doi:10.1158/1078-0432.CCR-09-1095
Gallagher DJ, Milowsky MI, Ishill N, Trout A, Boyle MG, Riches J, Fleisher M, Bajorin DF (2009) Detection of circulating tumor cells in patients with urothelial cancer. Ann Oncol 20(2):305–308. doi:10.1093/annonc/mdn627
Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher HI (2007) Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 13(23):7053–7058. doi:10.1158/1078-0432.CCR-07-1506
Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ (2008) Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26(19):3213–3221. doi:10.1200/JCO.2007.15.8923
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791
Ignatiadis M, Xenidis N, Perraki M, Apostolaki S, Politaki E, Kafousi M, Stathopoulos EN, Stathopoulou A, Lianidou E, Chlouverakis G, Sotiriou C, Georgoulias V, Mavroudis D (2007) Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol 25(33):5194–5202. doi:10.1200/JCO.2007.11.7762
Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S (2012) Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 13(7):688–695. doi:10.1016/S1470-2045(12)70209-7
Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz JA, Stebbing J, Caldas C, Gazzaniga P, Manso L, Zamarchi R, de Lascoiti AF, De Mattos-Arruda L, Ignatiadis M, Lebofsky R, van Laere SJ, Meier-Stiegen F, Sandri MT, Vidal-Martinez J, Politaki E, Consoli F, Bottini A, Diaz-Rubio E, Krell J, Dawson SJ, Raimondi C, Rutten A, Janni W, Munzone E, Caranana V, Agelaki S, Almici C, Dirix L, Solomayer EF, Zorzino L, Johannes H, Reis-Filho JS, Pantel K, Pierga JY, Michiels S (2014) Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 15(4):406–414. doi:10.1016/S1470-2045(14)70069-5
de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14(19):6302–6309
Garcia JA, Rosenberg JE, Weinberg V, Scott J, Frohlich M, Park JW, Small EJ (2007) Evaluation and significance of circulating epithelial cells in patients with hormone-refractory prostate cancer. BJU Int 99(3):519–524. doi:10.1111/j.1464-410X.2007.06659.x
Magbanua MJ, Carey LA, DeLuca A, Hwang J, Scott JH, Rimawi MF, Mayer EL, Marcom PK, Liu MC, Esteva FJ, Park JW, Rugo HS, Translational Breast Cancer Research C (2015) Circulating tumor cell analysis in metastatic triple-negative breast cancers. Clin Cancer Res 21(5):1098–1105. doi:10.1158/1078-0432.CCR-14-1948
Magbanua MJ, Sosa EV, Roy R, Eisenbud LE, Scott JH, Olshen A, Pinkel D, Rugo HS, Park JW (2013) Genomic profiling of isolated circulating tumor cells from metastatic breast cancer patients. Cancer Res 73(1):30–40. doi:10.1158/0008-5472.CAN-11-3017
Chamberlain M, Soffietti R, Raizer J, Ruda R, Brandsma D, Boogerd W, Taillibert S, Groves MD, Le Rhun E, Junck L, van den Bent M, Wen PY, Jaeckle KA (2014) Leptomeningeal metastasis: a response assessment in neuro-oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro-oncology 16(9):1176–1185. doi:10.1093/neuonc/nou089
Patel AS, Allen JE, Dicker DT, Peters KL, Sheehan JM, Glantz MJ, El-Deiry WS (2011) Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget 2(10):752–760
Le Rhun E, Massin F, Tu Q, Bonneterre J, Bittencourt Mde C, Faure GC (2012) Development of a new method for identification and quantification in cerebrospinal fluid of malignant cells from breast carcinoma leptomeningeal metastasis. BMC Clin Pathol 12:21. doi:10.1186/1472-6890-12-21
Nayak L, Fleisher M, Gonzalez-Espinoza R, Lin O, Panageas K, Reiner A, Liu CM, Deangelis LM, Omuro A (2013) Rare cell capture technology for the diagnosis of leptomeningeal metastasis in solid tumors. Neurology 80(17):1598–1605. doi:10.1212/WNL.0b013e31828f183f discussion 1603
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158(5):1110–1122. doi:10.1016/j.cell.2014.07.013
Magbanua MJ, Park JW (2013) Isolation of circulating tumor cells by immunomagnetic enrichment and fluorescence-activated cell sorting (IE/FACS) for molecular profiling. Methods 64(2):114–118. doi:10.1016/j.ymeth.2013.07.029
Magbanua MJ, Sosa EV, Scott JH, Simko J, Collins C, Pinkel D, Ryan CJ, Park JW (2012) Isolation and genomic analysis of circulating tumor cells from castration resistant metastatic prostate cancer. BMC Cancer 12:78. doi:10.1186/1471-2407-12-78
Magbanua MJ, Melisko M, Roy R, Sosa EV, Hauranieh L, Kablanian A, Eisenbud LE, Ryazantsev A, Au A, Scott JH, Park JW (2013) Molecular profiling of tumor cells in cerebrospinal fluid and matched primary tumors from metastatic breast cancer patients with leptomeningeal carcinomatosis. Cancer Res 73(23):7134–7143. doi:10.1158/0008-5472.CAN-13-2051
Magbanua MJ, Melisko M, Park JW (2014) Molecular characterization of cerebrospinal fluid tumor cells associated with leptomeningeal carcinomatosis. Cancer Cell Microenviron 1:109–113. doi:10.14800/ccm.167
Magbanua MJ, Roy R, Sosa EV, Hauranieh L, Kablanian A, Eisenbud LE, Ryazantsev A, Au A, Scott JH, Melisko M, Park JW (2014) Genome-wide copy number analysis of cerebrospinal fluid tumor cells and their corresponding archival primary tumors. Genom Data 2:60–62. doi:10.1016/j.gdata.2014.04.003
Palma JA, Fernandez-Torron R, Esteve-Belloch P, Fontes-Villalba A, Hernandez A, Fernandez-Hidalgo O, Gallego Perez-Larraya J, Martinez-Vila E (2013) Leptomeningeal carcinomatosis: prognostic value of clinical, cerebrospinal fluid, and neuroimaging features. Clin Neurol Neurosurg 115(1):19–25. doi:10.1016/j.clineuro.2012.03.048
Acknowledgments
We thank Venkata Sai Vemula, Louai Hauranieh, and Eduardo Sosa for technical assistance.
Grant support
UCSF Research Allocation Program (M.E. Melisko). The Breast Cancer Research Foundation (M.J.M. Magbanua). Bay Area Breast Cancer Translational (SPORE) P50 CA58207 (J.W. Park).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethics and consent
The study protocol was approved by the Committee on Human Research at the University of California San Francisco. All patients gave informed consent for participation in this study.
Additional information
Jin Sun Lee, Michelle E. Melisko, and Mark Jesus M. Magbanua have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, J.S., Melisko, M.E., Magbanua, M.J.M. et al. Detection of cerebrospinal fluid tumor cells and its clinical relevance in leptomeningeal metastasis of breast cancer. Breast Cancer Res Treat 154, 339–349 (2015). https://doi.org/10.1007/s10549-015-3610-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3610-1