Abstract
Pentraxin 3 (PTX3) is an inflammatory molecule that is involved in immune responses, inflammation, and cancer. Recent evidence suggests that PTX3 plays a critical role in tumor progression; however, its impact on the biological function of gliomas remains unknown. In the present study, immunohistochemical staining showed that patients with high-grade gliomas exhibited increased expression levels of PTX3 compared to those with low-grade gliomas (P < 0.001). Furthermore, knockdown of PTX3 in GBM8401 cells inhibits proliferation, increases p21 protein levels, and decreases cyclin D1 protein levels, resulting in cell cycle arrest at the G0/G1 phase. In addition, knockdown of PTX3 significantly decreases GBM8401 cell migration and invasion through the downregulation of matrix metalloproteinase-1 and -2 (MMP-1 and MMP-2) expression. In a GBM8401 xenograft animal model, PTX3 knockdown decreases tumor growth in vivo. In conclusion, PTX3 plays an important role in glioma cell proliferation and invasion, and may thus serve as a novel potential therapeutic target in the treatment of gliomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most common type of malignant brain tumor [1]. Gliomas exhibit various pathological classifications, including ependymomas, astrocytomas, oligodendrogliomas, and choroid plexus papillomas [2]. However, the standard treatment for malignant glioma consists primarily of a combination of surgery, postoperative radiotherapy, and chemotherapy [3]. Enhanced understanding of the molecular mechanisms involved in glioma formation and progression could help in the identification of novel therapeutic targets and promising therapeutic approaches.
Pentraxin 3 (PTX3; also known as TSG-14) belongs to the pentraxin superfamily [4], the members of which are characterized by a cyclic multimeric structure. Previous studies have reported that PTX3 not only interacts with several extracellular matrix (ECM) proteins, various growth factors, and certain pathogens, but is also involved in the activation of the complement system [5], in addition to accelerating pathogen recognition by phagocytes [6]. Moreover, PTX3 is overexpressed in different cancer types and may represent a useful diagnostic and prognostic marker of various cancers, including breast cancer [7], small-cell lung cancer [8], pancreatic cancer [9], and gliomas [10]. These findings suggest that downregulation of PTX3 may be an important means of promoting anti-tumour or antimetastatic activity; however, its precise role in the development of gliomas remains highly unclear.
By immunohistochemistry, Locatelli et al. [10] suggested that PTX3 was positively correlated with glioma tumor grade and severity. Based on this finding, the present study was conducted in order to determine the biological function and molecular mechanism of PTX3 in glioma cells, information which has previously proven elusive. The results of this study, however, showed that the up-regulation of PTX3 in human gliomas is closely related to tumor grade, and it significantly correlated with the patients’ survival time. Knockdown of PTX3 reduces the proliferation, migration, and invasion of glioma cells in vitro and in vivo. These results should provide new insights into the biological functions of PTX3, as well as its regulatory mechanisms in gliomas.
Materials and methods
Reagents and antibodies
Various antibodies were used for Western blotting, including antibodies against PTX3, which were obtained from R&D Technology (Beverly, MA, USA). Antibodies against p21, cyclin D1, MMP-1, MMP-9, MMP-2, and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against Ki-67 were obtained from Abcam (Cambridge, MA, USA). Dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma (St Louis, MO, USA). PVDF (polyvinylidenedifluoride) membranes, goat anti-rabbit IgG, and horseradish peroxidase-conjugated IgG were obtained from Millipore (Billerica, MA, USA). Chemiluminescent HRP substrate was purchased from Pierce (Rockford, IL, USA). Penicillin/streptomycin and sodium pyruvate were purchased from Invitrogen (Gaithersburg, MO, USA).
Immunohistochemistry analysis
A human brain tissue array was purchased from US Biomax (GL804; Rockville, MD, USA), and immunohistochemistry analysis was performed consistent with the procedure described in a previous study [11]. Sections were cut from paraffin-embedded tissue blocks. Samples were dewaxed in xylene and rehydrated in alcohol and distilled water. Antigen retrieval was then performed by heating samples for 15 min at 95 °C in citrate buffer (pH 6.0). Samples were cooled to room temperature and incubated in 3 % hydrogen peroxide to quench peroxidase activity. After incubating at 4 °C overnight in anti-PTX3 (1:500) or anti-Ki-67 (1:300) antibodies and washing with Tris buffer, a biotin-labeled secondary antibody was added for 15 min, followed by streptavidin peroxidase for 15 min. After eluting with PBS, diaminobenzidine and haematoxylin counterstaining was performed. The staining results were scored by two investigators blinded to the clinical data.
Cell lines
The normal human astrocyte (NHA) cell line (Gibco. K1884) from the American Type Culture Collection (Rockville, MD, USA) was grown in Gibco® Astrocyte Medium supplemented with N-2 Supplement, One Shot™ Fetal Bovine Serum (FBS) and 100 mg/mL penicillin–streptomycin (Gibco, Grand Island, NY, USA). The human glioma cell lines, GBM8401, U251, MO59J, U87-MG, and Hs-683, were used. GBM8401, MO59J, U87-MG, and Hs-683 cell lines were obtained from the Bioresource Collection and Research Center, Inc. (Hsinchu, Taiwan). The U-251 cell line was a gift from Professor Dah-Yuu Lu in China Medical University (Taichung, Taiwan). GBM8401 cells were maintained in RPMI medium. U251 and Hs683 cells were maintained in DMEM medium with high glucose. U87-MG and MO59J cells were maintained in MEM medium with 1 mM sodium pyruvate. All media were supplemented with 10 % FBS and 1 % antibiotic mix (penicillin/streptomycin). All the cell lines were cultured at 37 °C in a humidified incubator with 5 % carbon dioxide.
Lentiviral particle production and PTX3 transduction
The shLuc (TRCN0000072244), shPTX3#1 (TRCN0000146265), shPTX3#2 (TRCN0000149220) and shPTX3#3 (TRCN0000149744) shRNA were obtained from the National RNA Interference Core Facility (Institute of Molecular Biology, Academia Sinica, Taipei, Taiwan). Lentiviral particles were produced by triple transfection of HEK 293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Packaging 293T cells were plated in 6 cm plates at a cell density of 5 × 105 cells/well 1 day prior to transfection. Transfection of the packaging cells and infection of the GBM8401 cells were completed using standard protocols with some modifications [12].
Cell proliferation assay
Cells were plated in 24-well culture plates (2 × 104 cells per well) for 24 h. The cells were then infected with shRNA-PTX3#1∼#3 or shRNA-shLuc and further incubated for 24, 48 and 72 h. Then 100 μL of MTT solution (5 mg/mL) was transferred to each well to yield a final assay volume of 1 mL/well. Plates were incubated for 4 h at 37 °C and 5 % CO2. After incubation, supernatants were removed, and 1 mL of isopropanol was added to ensure the total solubility of formazan crystals. The number of viable cells was quantified using the absorbance was recorded at 570 nm with a microplate reader.
Cell cycle distribution by flow cytometry
The shLuc, shPTX3#2, and shPTX3#3 cells were seeded in 6 cm culture plates at a density of 2 × 105 cells/well. After 24 h, the cells were collected and washed once with PBS, then fixed overnight with 75 % ethanol. After washing twice with PBS, the cells were incubated with propidium iodide (PI; 2 mg/mL) for 20 min in the dark at room temperature, and flow cytometry (FACSCalibur; BD Biosciences; Franklin Lakes, NJ, USA) was performed to determine the DNA content and cell cycle distribution of the cells. Data were analyzed with Cell Quest software (BD Biosciences).
In vitro migration and invasion assay
Cell migration was determined using Boyden chambers (8 μm pore size; Costar, NY) according to the manufacturer’s instructions. In brief, 3 × 105 cells suspended in 35 μL of serum-free medium were seeded onto the upper chamber, and 50 μL of the same medium containing 20 % FBS was placed in the lower chamber. The invasive abilities of the cells were assessed using a Matrigel-coated chamber (BD Biosciences, San Jose, CA, USA). After an overnight incubation, cells were washed with PBS, fixed with 4 % paraformaldehyde, and stained with 0.5 % Gimema. Cells remaining on the upper surface of filter membrane were completely removed by wiping with a cotton swab. Migrated cells were then counted from six random fields under a light microscope.
RNA extraction and RT-PCR analysis
Total RNA was isolated using Trizol (Invitrogen) according to the manufacturer’s instructions, and 2 µg of total RNA from each cell line was then transcribed into cDNA using the first-strand cDNA synthesis kit for ImProm-II™ Reverse Transcriptase system (Promega, Madison, WI, USA). PCR amplification was carried out with the Ex Taq DNA polymerase reaction system according to the manufacturer’s instruction. The following primers were used: sense PTX3-primer, 5′-AACGGGACCATGCACGGCTT-3′; antisense PTX3-primer, 5′-TGTTGTGGTGGTTGCACGGC-3′; sense β-actin-primer, 5′-AACGGGACCATGCACGGCTT-3′; and antisense β-actin-primer, 5′-TGTTGTGGTGGTTGCACGGC-3′. The PCR products were electrophoresed through agarose gels and analyzed by computerized densitometric scanning of the images with β-actin serving as an internal control to normalize the expression data.
Immunofluorescence assay
The shLuc, shPTX3#2, and shPTX3#3 cells were grown on glass coverslips, fixed with 4 % paraformaldehyde, and blocked to prevent non-specific binding. The coverslips were probed with primary antibodies followed by Alexa Flour®-conjugated secondary antibodies in 5 % bovine serum albumin. DAPI reagent was used as a mounting and counterstaining media. After mounting, the coverslips were visualized under a confocal microscope.
Western blot analysis
Cell lysates from the shLuc, shPTX3#2, and shPTX3#3 cells were separated by 10 % SDS/PAGE and transferred to PVDF membranes. Western blot analyses were performed using mouse anti-PTX3, anti-MMP-1, anti-MMP-2, anti-MMP-9, anti-cyclin D1, anti-p21, and anti-β-actin antibodies. After washing, the blots were incubated with secondary anti-rabbit (for MMP-1, cyclin D1, and p21), anti-goat (for MMP-9 and PTX3), and anti-mouse (for MMP-2 and β-actin) antibodies conjugated to horseradish peroxidase (1:10,000) for 1 h at room temperature and visualized using an enhanced chemiluminescence kit (Amersham, Little Chalfont, UK).
In vivo anti-tumor activity analysis
Fifteen BALB/c female nude mice (5–6 weeks-old; 18–20 g) provided by the animal center of Chung Shan Medical University were randomly divided into three groups receiving a subcutaneous injection of 6 × 106 shLuc-GBM8401 cells, GBM8401-shPTX3#2 cells, or GBM8401-shPTX3#3 cells (n = 5/group) into their right flanks. Treatments were initiated when the tumors reached a mean group size of approximately 80 mm3. The volume of each tumor was measured with calipers every 7 days and was calculated using the following: (W2/L)/2, where w is the width and L is the length of tumor. After 5 weeks, the animals were sacrificed, and the transplanted tumor specimens were immediately excised, fixed with 10 % formalin, and embedded in paraffin. These experiments were conducted according to institutional IACUC guidelines.
Statistical analysis
Data are presented as mean ± SD of at least three independent experiments performed in triplicate. Significant differences were determined using SPSS 10.0 software (SPSS, Chicago, IL, USA). Two-tailed Student’s t tests were used for comparisons between groups. Survival curves were plotted using the Kaplan–Meier method and compared by log-rank test. Differences were considered statistically significant when P < 0.05 or P < 0.01.
Results
Expression of PTX3 positively correlates with glioma grade and is associated with poor prognosis
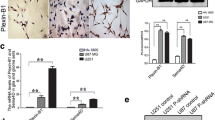
The expression PTX3 protein was analyzed in a brain tissues microarray consisting of 74 brain tumors and normal brain tissue by immunohistochemistry. As shown Fig. 1a, PTX3 protein expression was significantly higher in glioma tissues than in normal brain tissues (P < 0.01). In addition, PTX3 expression was increased in all gliomas analyzed, and its expression was significantly higher in high-grade glioma tissues (Grades III and IV) than in low-grade glioma tissues (Grades I and II) (Fig. 1b). Furthermore, PTX3 level was positively correlated with tumor grade (P < 0.001) in glioma patients. However, PTX3 expression level was not correlated with sex (P = 0.375) or age (P = 0.271) (Table 1). Analysis of high-throughput RNA sequencing data from the glioma cohort of The Cancer Genome Atlas (TCGA) revealed that PTX3 expression was upregulated in glioma tissues compared with normal brain tissue (Fig. 1c). Importantly, Kaplan–Meier analysis showed that the expression level of PTX3 in gliomas was significantly correlated with patients’ survival time (Fig. 1d).
PTX3 expression in the glioma tissues and glioma cell lines. a Immunohistochemical staining revealed higher levels of PTX3 protein expression in high-grade glioma tissues (Grades III and IV) than in low-grade glioma tissues (Grades I and II) and normal brain tissue. b Bar graph summarizes the scored signal intensities of different grade glioma tissues and normal brain tissues. Low PTX3 low expression of PTX3; high PTX3 high expression of PTX3. c PTX3 expression was significantly increased in glioma tissues compared with normal tissues of patients from the TCGA GBM dataset. d Survival analysis of patients from the TCGA GBM dataset (P < 0.002). e, f PTX3 expression levels in five glioma cell lines (GBM8401, U-251, Hs683, M059J, and U87-MG) and the normal human astrocyte cell line (NHA) were analyzed by western blot and RT-PCR analyses. β-Actin was used as an internal loading control. Relative densities of PTX3 protein and mRNA expression were estimated by densitometry. Original magnification 200×
We next examined the expression of PTX3 in five glioma cell lines and the NHA cell line using western blotting and RT-PCR analyses. Up-regulation of PTX3 expression was observed in the GBM8401, U251, and M059J glioma cells compared to the NHA cells (Fig. 1e) and other glioma cell lines (Fig. 1f). Taken together, these results imply that PTX3 might have an important role in the malignant progression of gliomas.
Infection efficiency of lentiviral vectors expressing shPTX3
We selected GBM8401 cells to investigate the biological function of PTX3 in the malignant progression of gliomas because they exhibit high endogenous PTX3 expression. Stable inhibition of PTX3 expression in human GBM8401 cells was achieved using lentiviral vectors expressing control shRNA (shLuc) or shPTX3 (shPTX3#1, shPTX3#2, or shPTX3#3). After 24 h following transduction, the cells were incubated with puropomylin (3 µg/mL) for 5 days. Western blot and RT-PCR analyses revealed that the protein and mRNA expression levels of PTX3 in the shPTX3#2- and shPTX3#3-GBM8401 cells were significantly decreased in comparison to those of the shLuc- and shPTX3#1-GBM8401 cells (Fig. 2a, b). Therefore, shPTX3#2- and shPTX3#3-GBM8401 cells were used for all subsequent experiments.
The efficiency of PTX3 knockdown in GBM8401 cells. a Western blots, b RT-PCR assay demonstrating decreased PTX3 protein and mRNA levels in GBM8401 cells after infection of lentiviral-based PTX3-targeting shRNA constructs (shPTX3#1, shPTX3#2 and shPTX3#3), respectively. β-Actin was used as an internal loading control, and all data were normalized to β-actin. Relative densities of PTX3 protein and mRNA expression were estimated by densitometry. Results are shown as the mean ± SE of three independent experiments. **P < 0.01, compared with shLuc cells
Knockdown of PTX3 expression inhibits glioma cell proliferation and induces cell cycle arrest at the G0/G1 phase
Previous studies have indicated that PTX3 impacts cancer cell proliferation [13, 14], therefore, we investigated whether the knockdown of PTX3 affects GBM8401 cell proliferation. MTT analysis revealed that the proliferation of shPTX3#2- and shPTX3#3-GBM8401 cells was significantly decreased in comparison with that in the shLuc cells at 48 and 72 h (Fig. 3a). To further confirm the tumorigenicity of PTX3, we transfected it into U87-MG cells, which exhibit relatively low PTX3 expression. Western blotting confirmed that PTX3 protein was overexpressed in U87-MG cells compared with Neo-U87-MG cells (Supporting Information Fig. S1a). Moreover, the MTT assay showed that proliferative capacity of PTX3-overexpressing U87-MG cells was significantly higher than observed in Neo-U87-MG cells (Supporting Information Fig. S1b). The impact of PTX3 expression on cell cycle distribution was next analyzed using flow cytometry. Analysis of shLuc-GBM8401 cells revealed that 43.4 % were in the G0/G1 phase while 60.4 and 62.1 % of the shPTX3#2- and shPTX3#3-GBM8401 cells were in the G0/G1 phase, respectively (Fig. 3b). Furthermore, western blot analysis revealed that the expression of p21 was significantly increased, and the expression of cyclin D1 was significantly decreased in the shPTX3#2 and shPTX3#3-GBM8401 cells compared to the shLuc-GBM8401 cells (Fig. 3c). Furthermore, immunofluorescence analysis showed that the expression levels of PTX3 and cyclin D1 were significantly decreased in the shPTX3#2 and shPTX3#3-GBM8401 cells compared to the shLuc-GBM8401 cells (Fig. 3d). These results suggest that the knockdown of PTX3 could induce cell arrest at the G0/G1 phase and ultimately inhibit glioma cell proliferation.
Down-regulation of PTX3 inhibited cell proliferation and cell cycle acceleration in GBM8401 cells. a The percentages of cell proliferation of shLuc, shPTX3#2, and shPTX3#3 stable GBM8401 cells were estimated by MTT assay at 24, 48, and 72 h. b The shLuc, shPTX3#2, and shPTX3#3 stable GBM8401 cells were harvested, fixed, and stained with PI for cell cycle analysis; the percentages of cells in each phase of the cell cycle are shown. c Western blotting was used to analyze the protein levels of PTX3, cyclin D1, and p21. β-Actin was used as an internal loading control. d All cells were fixed, permeabilized, and immunostained with anti-PTX3 antibody (green) and anti-Cyclin D1 (red), and cell nuclei were counter-stained with DAPI reagent (blue), and staining was visualized by confocal microscopy. Results are shown as the mean ± SE of three independent experiments. **P < 0.01, compared with shLuc cells
Inhibition of PTX3 suppresses glioma cell invasion and migration
Glioma cells are highly invasive, and MMP genes have been suggested to contribute to metastasis. To explore the influence of PTX3 on GBM8401 cell migration and invasion, we performed a Boyden chamber assay without Matrigel to detect cell migration and with Matrigel to examine invasion. As compared with the shLuc-GBM8401 cells, the shPTX3#2- and shPTX3#3-GBM8401 cells had significantly slower rates of both migration (Fig. 4a, upper panel) and invasion (Fig. 4a, lower panel). In contrast, the PTX3-overexpressing U87-MG cells displayed significantly greater migratory and invasive properties compared to the Neo-U87-MG cells (Supporting Information Fig. S1c). Because MMP-1, MMP-2, and MMP-9 play critical roles in glioma cell migration and invasion by degrading ECM, and their increased expression is associated with disease progression [15–17], we assessed whether PTX3 knockdown in GBM8401 cells altered MMP-1, MMP2, and MMP9 protein expression. Western blot analysis revealed that the protein expression levels of MMP-1 and MMP-2 in the shPTX3#2- and shPTX3#3-GBM8401 cells were significantly lower than those in the shLuc cells, but the knockdown did not changes in MMP-9 protein expression were detected (Fig. 4b). These results indicate that PTX3 knockdown suppresses the migration and invasion potential of GBM8401 cells through down-regulation of MMP-1 and MMP-2 expression.
Down-regulation of PTX3 inhibited cell migration and invasion. a Representative images showing invasive shLuc-, shPTX3#2- and shPTX3#3-GBM8401 cells in the absence (−) or presence ( +) of Matrigel. The numbers of cells that migrated and invaded are quantified in the lower panel. b Western blotting was used to analyze the protein levels of PTX3, MMP-1, MMP-2, and MMP-9. β-Actin was used as an internal loading control. Results are shown as the mean ± SE of three independent experiments. **P < 0.01, compared with shLuc cells
Inhibition of PTX3 suppressed the growth of glioma cells in vivo
We next investigated the efficacy of PTX3 knockdown against tumor growth in vivo. The tumor volume of shPTX3#2 and shPTX3#3-GBM8401 tumor xenografts in mice was significantly smaller than that of the shLuc-GBM8401 tumor xenografts (Fig. 5a, b, respectively), and the average tumor xenograft weights in the shPTX3#2 and shPTX3#3-GBM8401 groups were significantly decreased as compared to that of the shLuc-GBM8401 group (Fig. 5d), suggesting that inhibition of PTX3 could suppress the growth of glioma xenografts in nude mice (Fig. 5c). Ultimately, there was no difference in average body weight between the shPTX3#2 and shPTX3#3-GBM8401 or shLuc-GBM8401 groups, which indicated that there were no adverse effects from PTX3 inhibition in mice (Fig. 5e). The cell proliferation of tumors was indicated by the expression of the proliferation marker, Ki-67. As shown in Fig. 5f, the shPTX3#2 and shPTX3#3-GBM8401 groups had decreased Ki-67 expression within the tumors. Taken together, these results suggested that the inhibition of PTX3 could suppress xenograft glioma cancer cell growth.
Effect of PTX3 knockdown on GBM8401 tumor xenografts in vivo. a, b Representative images of the tumor formation from respective groups are shown (n = 5). c Tumor growth curves were measured after the injection of GBM8401 cells infected with shLuc, shPTX3#2, or shPTX3#3. Tumor volume was calculated every 7 days. d Average tumor weights and e body weights of mice 35 days after injection are shown (n = 5). f Immunohistochemistry was performed using Ki-67 to determine the percentage of Ki-67-positive proliferating cells in the GBM8401 tumor xenografts. Results are shown as the mean ± SE of three independent experiments. **P < 0.01, compared with shLuc group
Discussion
Previous in vitro and in vivo studies have suggested that PTX3 can serve as a diagnostic and prognostic marker of cancers, including pancreatic cancer [18], breast cancer [19], lung cancer [13], and head and neck cancer [20]. In this study, we found that PTX3 was upregulated in glioma tissues, and that it was significantly associated with glioma pathology grade. Knockdown of PTX3 inhibited the proliferation of glioma cancer cells and induced glioma cell cycle arrest at the G0/G1 phase by modulating cell-cycle related proteins. Knockdown of PTX3 suppressed the migration and invasion of glioma cells via down-regulation of MMP-1, MMP-2, and MMP-9 expression. Finally, knockdown of PTX3 inhibited the growth of xenograft tumors in vivo.
Identification of useful biomarkers is important in the diagnosis of glioma cancer. Although PTX3 has been shown to be a helpful clinical biomarker for the prediction and prognostic assessment of small-cell lung cancer [8], prostate cancer [13] and endometrioid ovarian cancer [21], but its biological function in glioma cancer is less well-understood. In the present study, high expression of PTX3 was observed in glioma tissues as compared to adjacent non-tumor tissues, and a positive correlation between PTX3 expression and glioma grade was detected. These findings are in agreement with the past gene expression analyses and immunohistochemistry assays performed for gliomas of different grades, which showed that glioblastoma cells exhibit higher levels of PTX3 than astrocytomas, oligodendrogliomas, and normal cells [10, 22]. PTX3 regulates tumor proliferation via modulation of leukocyte recruitment [23], complement activation [24], angiogenesis [4], and tissue remodeling [25]. Xiao et al. [14] found that knockdown of PTX3 decreased lung adenocarcinoma cell proliferation in vitro as well as down-regulation of p-Akt, p-NF-kappa B, PCNA, and MMP-9 expression. To further investigate the biological function of PTX3 in gliomas, we suppressed its expression in GBM8401 cells and found that it repressed cell proliferation by inducing cell cycle arrest at the G0/G1 phase, suggesting that PTX3 expression influences the proliferation rates of glioma cells.
Previous studies have shown that glioma cells are highly invasive by destroying intercellular and ECM components, allowing the cells to diffuse into normal brain tissue and resulting in distant metastasis [26]. MMP-1, MMP-2, and MMP-9 play critical roles in tumor invasion and metastasis [27]. Up-regulation of the expression of MMP-1 and MMP-2 plays a key role in increasing the WHO grading of the glioma [28, 29]. Epidermal growth factor (EGF)-induced PTX3 expression enhanced the invasion of head and neck squamous cell carcinoma (HNSCC), which then increased the expression of fibronectin and MMP-9 and the activation of the PI3K/Akt and NF-κB pathways, resulting in enhanced tumor metastasis [20]. Alternatively, other studies have shown that PTX3 may inhibit tumorigenesis and metastasis. Presta et al. showed that PTX3 inhibits fibroblast growth factor (FGF)/FGFR-driven epithelial-mesenchymal transition (EMT) in human BRAF-mutated A2058 and A375 melanoma cells [30]. Furthermore, a recent report showed that PTX3 deficiency was associated with increased macrophage infiltration, cytokine production, angiogenesis, DNA damage in knockout-PTX3 mice [31]. Moreover, overexpression of PTX3 inhibited tumor cell growth in FGF-dependent human PTX3 transgenic mouse models [32]. Therefore, PTX3 may be have a suppressive function in tumor progression. In our study, knockdown of PTX3 significantly inhibited the migration and invasion of GBM8401 cells and decreased the expression of MMP-1 and MMP-2. In contrast, overexpression of PTX3 increased the proliferation and invasion of U87-MG cells. It is possible that we detected an oncogenic role for PTX3 in glioma cancer in the present study, which may cell line-, tumor type- or species-specific.
To the best of our knowledge, the results of the present study are the first to demonstrate the biological function and possible mechanism of PTX3 in the proliferation, migration, and invasion of glioma cells. The results highlight the importance of PTX3 in regulating glioma tumorigenesis, suggesting that PTX3 is a promising therapeutic target in the treatment of gliomas.
References
Wei J, Gabrusiewicz K, Heimberger A (2013) The controversial role of microglia in malignant gliomas. Clin Dev Immunol 2013:285246
Jovcevska I, Kocevar N, Komel R (2013) Glioma and glioblastoma—how much do we (not) know? Mol Clin Oncol 1:935–941
Hochberg FH, Atai NA, Gonda D et al (2014) Glioma diagnostics and biomarkers: an ongoing challenge in the field of medicine and science. Expert Rev Mol Diagn 14:439–452
Presta M, Camozzi M, Salvatori G, Rusnati M (2007) Role of the soluble pattern recognition receptor PTX3 in vascular biology. J Cell Mol Med 11:723–738
Inforzato A, Peri G, Doni A et al (2006) Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry 45:11540–11551
Popovici RM, Krause MS, Jauckus J et al (2008) The long pentraxin PTX3 in human endometrium: regulation by steroids and trophoblast products. Endocrinology 149:1136–1143
Margheri F, Serrati S, Lapucci A et al (2009) Systemic sclerosis-endothelial cell antiangiogenic pentraxin 3 and matrix metalloprotease 12 control human breast cancer tumor vascularization and development in mice. Neoplasia 11:1106–1115
Liu C, Yao Y, Wang W (2014) Pentraxin-3 as a prognostic marker in patients with small-cell lung cancer. Med Oncol 31:207
Liu J, Li J, Li H, Li A, Liu B, Han L (2015) A comprehensive analysis of candidate genes and pathways in pancreatic cancer. Tumour Biol 36:1849–1857
Locatelli M, Ferrero S, Martinelli Boneschi F et al (2013) The long pentraxin PTX3 as a correlate of cancer-related inflammation and prognosis of malignancy in gliomas. J Neuroimmunol 260:99–106
Cheng CY, Chou YE, Ko CP et al (2014) Metastasis tumor-associated protein-2 knockdown suppresses the proliferation and invasion of human glioma cells in vitro and in vivo. J Neurooncol 120:273–281
Chen JC, Chen YJ, Lin CY et al (2015) Amphiregulin enhances alpha6beta1 integrin expression and cell motility in human chondrosarcoma cells through Ras/Raf/MEK/ERK/AP-1 pathway. Oncotarget 6:11434–11446
Stallone G, Cormio L, Netti GS et al (2014) Pentraxin 3: a novel biomarker for predicting progression from prostatic inflammation to prostate cancer. Cancer Res 74:4230–4238
Hu FQ, Qiao T, Xie X, Hu R, Xiao HB (2014) Knockdown of the inflammatory factor pentraxin-3 suppresses growth and invasion of lung adenocarcinoma through the AKT and NF-kappa B pathways. J Biol Regul Homeost Agents 28:649–657
Chao R, Chow JM, Hsieh YH et al (2015) Tricetin suppresses the migration/invasion of human glioblastoma multiforme cells by inhibiting matrix metalloproteinase-2 through modulation of the expression and transcriptional activity of specificity protein 1. Expert Opin Ther Targets 19:1293–1306
Jiang Y, Zhang Q, Bao J et al (2015) Schisandrin B suppresses glioma cell metastasis mediated by inhibition of mTOR/MMP-9 signal pathway. Biomed Pharmacother 74:77–82
McCready J, Broaddus WC, Sykes V, Fillmore HL (2005) Association of a single nucleotide polymorphism in the matrix metalloproteinase-1 promoter with glioblastoma. Int J Cancer 117:781–785
Kondo S, Ueno H, Hosoi H et al (2013) Clinical impact of pentraxin family expression on prognosis of pancreatic carcinoma. Br J Cancer 109:739–746
Choi B, Lee EJ, Song DH et al (2014) Elevated Pentraxin 3 in bone metastatic breast cancer is correlated with osteolytic function. Oncotarget 5:481–492
Chang WC, Wu SL, Huang WC et al (2015) PTX3 gene activation in EGF-induced head and neck cancer cell metastasis. Oncotarget 6:7741–7757
Tothill RW, Tinker AV, George J et al (2008) Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res 14:5198–5208
Sun L, Hui AM, Su Q et al (2006) Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 9:287–300
Deban L, Russo RC, Sironi M et al (2010) Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol 11:328–334
Doni A, Garlanda C, Bottazzi B, Meri S, Garred P, Mantovani A (2012) Interactions of the humoral pattern recognition molecule PTX3 with the complement system. Immunobiology 217:1122–1128
Inforzato A, Jaillon S, Moalli F et al (2011) The long pentraxin PTX3 at the crossroads between innate immunity and tissue remodelling. Tissue Antigens 77:271–282
Giese A, Westphal M (1996) Glioma invasion in the central nervous system. Neurosurgery 39:235–250
Maruiwa H, Sasaguri Y, Shigemori M, Hirohata M, Morimatsu M (1993) A role of matrix metalloproteinases produced by glioma-cells. Int J Oncol 3:1083–1088
Stojic J, Hagemann C, Haas S et al (2008) Expression of matrix metalloproteinases MMP-1, MMP-11 and MMP-19 is correlated with the WHO-grading of human malignant gliomas. Neurosci Res 60:40–49
Thorns V, Walter GF, Thorns C (2003) Expression of MMP-2, MMP-7, MMP-9, MMP-10 and MMP-11 in human astrocytic and oligodendroglial gliomas. Anticancer Res 23:3937–3944
Ronca R, Di Salle E, Giacomini A et al (2013) Long pentraxin-3 inhibits epithelial-mesenchymal transition in melanoma cells. Mol Cancer Ther 12:2760–2771
Bonavita E, Gentile S, Rubino M, Maina V, Papait R et al (2015) PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell 160:700–714
Ronca R, Giacomini A, Di Salle E, Coltrini D, Pagano K et al (2015) Long-Pentraxin 3 derivative as a small-molecule FGF trap for cancer therapy. Cancer Cell 28:225–239
Acknowledgments
This work was supported by grants from Chung Shan Medical University and Tungs’ Taichung Metro Harbor Hospital Taichung, Taiwan (CSMU-TTM-103-02), Chung Shan Medical Hospital, Taichung, Taiwan (CSH-2015-C-007).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Yi-Hsien Hsieh and Kun-Chung Chen contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tung, JN., Ko, CP., Yang, SF. et al. Inhibition of pentraxin 3 in glioma cells impairs proliferation and invasion in vitro and in vivo. J Neurooncol 129, 201–209 (2016). https://doi.org/10.1007/s11060-016-2168-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2168-z