Abstract
Pituitary tumor-transforming gene 1 (PTTG1) has been identified as an oncogene and is overexpressed in many tumor types. However, the role of PTTG1 in glioblastoma (GBM) has not been well characterized, especially in relation to angiogenesis, migration, and invasion. In the present study, our results showed that the expression of PTTG1 was significantly higher in patients with GBM. Bioinformatic analysis showed that angiogenesis and the cell migration-related process were increased in patients with high PTTG1 expression levels; meanwhile, PTTG1 was positively correlated with marker genes of angiogenesis, migration and the evasion of apoptosis. In vitro assays showed that PTTG1 knockdown dramatically suppressed angiogenesis, migration and invasion, and increased the apoptosis of GBM cells. Moreover, our results also showed that silencing PTTG1 suppressed the activity of the TGF-β/PI3K-AKT-mTOR pathway, which induced tumor deterioration in multiple organs. Overall, our findings indicate that PTTG1 is a glioma malignant factor that promotes angiogenesis, migration, invasion, and the evasion of apoptosis, and these roles may be related to the TGF-β/PI3K-AKT-mTOR pathway. Thus, the targeted inhibition of PTTG1 might be a novel therapeutic strategy and a potential diagnostic biomarker for GBM-targeted therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas, which are classified into four subtypes, are the most common primary brain tumors. Grade 4 glioma, or glioblastoma (GBM), is the most aggressive subtype and shows minimal response to current therapies [1,2]. Irrespective of surgical resection and radiotherapy/chemotherapy, the median survival time for this condition remains approximately 14.6 months [3]. Invasion and angiogenesis are major hallmarks of GBM growth. While invasive tumor cells grow adjacent to blood vessels in normal brain tissue, tumor cells within neovascularized regions exert hypoxic stress and promote angiogenesis [4]. The aggressiveness of GBM depends not only on the invasion of tumor cells and extracellular matrix remodeling but also on the strong vascular and nutrition supply to the tumor cells [5]. These dependencies remind us that there may be genes that specifically enable the angiogenic action of glioma cells, allowing the effective invasion and malignancy of GBM.
Pituitary tumor-transforming gene 1 (PTTG1) plays a pivotal role in cell transformation and is partly involved in angiogenesis; this gene is overexpressed in numerous cancer cell lines and tissues [6,7,8,9]. PTTG1 demonstrates a transforming activity and expresses at undetectable or low levels in normal tissues, but it is abundantly expressed in malignant cell lines and pituitary tumors, especially in highly invasive tumors [7,10,11,12]. As an anaphase inhibitor, PTTG1 is involved in the modulation of metastasis in human ovarian cancer and is implicated in the regulation of senescence and growth of pituitary tumors. Previous studies have indicated that PTTG1 promotes the resistance to gefitinib-induced apoptosis in human bladder cancer [12]. Notably, PTTG1 was also found to enhance the migration and invasion of breast cancer cells [13], similar to its angiogenic induction ability in hepatocellular carcinoma and gastric cancer, implying a possible role for PTTG1 in GBM cell migration and angiogenesis. With respect to patients with gliomas, it has been reported that PTTG1 protein is overexpressed in high-grade astrocytomas compared to low-grade astrocytomas [14]. It can be inferred that high PTTG1 levels are correlated with higher tumor grade, invasiveness, and tumor vascularity. Moreover, previous studies have indicated that PTTG1 is involved in the modulation of cell-cycle arrest and in the proliferation of primary GBM tumors [15]. Previous study and our latest results both showed that silencing PTTG1 gene-inhibited cell proliferation and invasion in glioma cell lines [16,17]. However, whether PTTG1 can directly or indirectly induce angiogenesis in GBMs is still unknown, and related molecule mechanisms of PTTG1 in gliomas have not been reported thus far. Therefore, this study aimed to investigate the effect of PTTG1 on the cellular behavior and signaling pathways in GBM cell lines, with the aim of identifying novel targets for GBM therapeutic strategies.
Materials and methods
Materials
U251 cells were obtained from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. PTTG1 siRNA, negative control siRNA, transfection reagent and buffer were purchased from Ruibo Inc. (China). Transwell inserts (6.5 mm diameter, 8 μm pore size, polycarbonate membrane sterile) and Matrigel basement membrane matrix were purchased from Corning Inc. (USA); the MTS assay (Cell Titer 96 Aqueous One Solution reagent) was purchased from Promega Corporation (USA); anti-TGF-β (56E4), anti-total-AKT, anti-phosphor-AKT (Ser473), and anti-mTOR were purchased from CST Inc. (USA); anti-PI3-kinase p85-alpha/gamma polyclonal and anti-mTOR (Ser9) were purchased from Omnimabs Inc. (USA); anti-GAPDH was obtained from Proteintech Group, Inc. (Wuhan, China); anti-PTTG1 was purchased from Sigma (USA). High-glucose Dulbecco’s modified Eagle’s medium (DMEM) (USA), and fetal bovine serum (Gibco, USA) were obtained from the indicated manufactures.
Bioinformation analysis
The GBM gene expression data contained in the GPL570 was obtained from the Gene Expression Omnibus database (GEO, https://www.ncbi.nlm.nih.gov/geo). The raw CEL files were downloaded and normalized using robust multichip average (RMA). Probe sequences downloaded from GEO or microarray manufacturers were re-annotated using BLAST + 2.2.30 on GENCODE release 21 sequence databases for mRNAs. The maximum normalized signal was selected when multiple probes corresponded to one gene. Two-sample or paired-sample t-tests were performed, followed by Benjamini and Hochberg (false discovery rate) adjustment. Pearson's correlation analysis was conducted to assess the relationship between two genes. Meanwhile, the mRNA expression of PTTG1 in primary STAD (TCGA Brain Cancer, n = 174) were analyzed the data from the Cancer Genome Atlas (TCGA).
siRNA transfection
siRNA was transfected according to the product instructions. The concentration of siRNA used in the study was 50 nM. The three PTTG1 siRNA target sequences were GACCTGCAATAATCCAGAA, GGGAGATCTCAAGTTTCAA and GGCTACTCTGATCTATGTT. The target sequence of negative control siRNA (NC siRNA) was a random sequence with no biological effect.
Quantitative PCR
Total RNA was extracted from U251 cells using Trizol reagent, and reverse transcriptions were performed in a 20-μl mixture with 1 μg of total RNA, according to the manufacturer's instructions. The sequences of the oligonucleotide primers used for PCR amplification that were acquired from target cellular RNA are listed below in Table 1. PCR amplification consisted of 30 cycles of denaturation at 95℃ for 2 min, annealing at 60℃ for 45 s, and extension at 72℃ for 2 min. Real-time PCR was carried out using Fast Start Universal SYBR Green Master (Roche Applied Science, Penzberg, Germany) on an ABI PRISM 7300 sequence detection system. The PTTG1 gene expression Ct values were normalized to the corresponding values for GAPDH gene expression. The primer sequence used in qPCR is shown in Table 1.
Western blotting
Protein samples were obtained from U251 cells treated with lysis buffer on ice for 15 min, followed by centrifugation at 12,000×g for 10 min. Aliquots of 30 μg protein per lane were separated on 10% polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween-20 and incubated with specific antibodies. The values were normalized to the GAPDH intensity levels. Immunoreactive proteins were observed with an enhanced chemiluminescence (ECL) kit (Millipore, Billerica). The relative density of the protein bands was assessed using an Image Station 4000R (Rochester, New York, USA) and analyzed by densitometric evaluation using Quantity-One software (Bio-Rad Hercules, CA, USA).
MTS assay
To assess the effect of PTTG1 on cell proliferation, an MTS assay was carried out according to the manufacturer's instructions. U251 cells were transfected with PTTG1 siRNA or negative control siRNA for 24 h and then seeded into 96-well plates at a concentration of approximately 5 × 103 cells/well. The cells were culture at 37 ℃ for 24 h in serum-free medium. Subsequently, 20 μL of MTS was added and the cells were incubated for another 3 h. The absorbance was measured at 490 nm.
Colony-formation assay
U251 cells were transfected with PTTG1 siRNA or NC siRNA for 24 h and then seeded into 6-well plates at a concentration of approximately 5 × 102 cells/well in media containing 10% FBS. The medium was replaced every 4 days. After 2 weeks, the cells were fixed with methanol and stained with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO). Visible colonies were manually counted. Triplicate wells were measured for each treatment group.
Public datasets
Microarray miRNA expression data for 324 gliomas were downloaded from the Chinese Glioma Genome Atlas (CGGA) data portal (https://www.cgga.org.cn.portal.phpg). Whole genome mRNA expression microarray data and clinical information of 158 glioma samples were obtained from the CGGA database (https://www.cgga.org.cn) and used as the discovery set [18]. The three validation sets were the Cancer Genome Atlas database (TCGA, https://tcga-data.nci.nih.gov/), and the Repository of Molecular Brain Neoplasia Data (REMBRANDT, https://caintegrator.nci.nih.gov/rembrandt/).
Vascular mimicry experiment
Corning Matrigel basement membrane matrix was thawed according to the product instructions. Using cooled pipets, the matrix was mixed to homogeneity. The culture plates were maintained on ice, and 150 μL of matrix was added to the wells as a growth surface. The plates were then incubated at 37 ℃ for 30 min. U251 cells were transfected with PTTG1 siRNA or negative siRNA and then seeded into the wells containing Matrigel basement membrane matrix. The cell morphology was photographed using a light microscope (Nikon, Tokyo, Japan) 24 h after the cells attached to the membrane.
Flow cytometry
Briefly, U251 cells transfected with PTTG1 siRNA or negative control siRNA were harvested after 48 h for apoptosis analysis. The cells were then treated with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI) in the dark at room temperature according to the manufacturer's protocol. Subsequently, the cells were analyzed by FACScan® and identified as viable, dead, early apoptotic, or late apoptotic cells.
Cell migration and invasion assays
Cell migration and invasion were tested using a Transwell chamber with 8 μm pore membranes (Corning, USA). U251 cells were transfected with PTTG1 siRNA or negative control siRNA for 24 h, and then the U251 cells were resuspended in serum-free medium. The cells were seeded on the top of the membrane that was precoated with Matrigel (BD) (without Matrigel for the cell migration assay), and 10% FBS DMEM was added in the lower chambers. After 24 h, cells on the upper surface of the membrane that had not migrated were scraped off with cotton swabs, and cells that had migrated to the lower surface were fixed by 4% paraformaldehyde and stained with crystal violet reagent. The cell numbers were counted under a microscope in three random fields per well (100 ×).
Scarification test
U251 cells were transfected with PTTG1 siRNA or negative control siRNA for 24 h and then seeded into 6-well cell culture plates containing serum-free DMEM. Once adherent, the cells were scratched with a 10 μm pipet tip, and the wounds were photographed 36 h later using a light microscope (Nikon, Tokyo, Japan). The data were expressed as the migrated ratio, which was the area that the migrated cells had covered divided by the initial area scratched by the pipet tip.
Kaplan–Meier survival analysis
The correlation between PTTG1 expression and prognosis was analyzed using Kaplan–Meier plotter (KM plotter) [19]. We chose ‘overall survival’ (OS), ‘auto-select best cut-off’ and ‘only JetSet best probe set’ to calculate and plot the Kaplan–Meier survival curves. Best cut-off values were generated through computing all possible cut-off values between the lower and upper quartiles, and the best performing threshold was used as a cut-off (Supplementary Table 1). Statistical significance was determined by log–rank p values and hazard ratios (HRs) with 95% CIs.
Statistical analysis
The results were expressed as the mean ± S.E.M. Cell viability and migration ratio were compared using two-way ANOVA. Other statistical analyses were performed using one-way ANOVA with Tukey’s post hoc test for comparisons between two groups, followed by the Student–Newman–Keuls test (Prism 7 for Windows, GraphPad Software Inc., USA). Significance was established at P < 0.05.
Results
PTTG1 was overexpressed in human GBM tissues
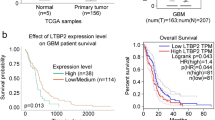
To identify whether PTTG1 was involved in glioma tumorigenesis, we performed an integrative analysis of glioblastoma multiforme (GBM) microarray profile comprising GSE4290, GSE7696, GSE15824 and GSE21354 from GEO datasets. To avoid the limitation of small sample sizes in individual studies, we performed a meta-analysis on these three datasets using a RankProd approach. Interestingly, PTTG1 was found to be overexpressed in the meta-analysis of four microarray datasets (Fig. 1a and b). Meanwhile, PTTG1 was also overexpressed in GBM tissues by integrative analysis of TCGA data (Fig. 1c and d).
Relative PTTG1 expression in GBM tissues and its clinical parameters. a, b Relative expression of PTTG1 in GBM tissues compared with normal tissue was analyzed using GEO datasets. c, d Relative expression of PTTG1 in pancreatic cancer tissues compared with normal tissue was analyzed using TCGA datasets. (Fold change ≥ 2, P < 0.05). The values are compared to a normal group and are represented as the means ± S.E.M., **P < 0.01; ***P < 0.001
PTTG1 silencing suppressed the proliferation ability of U251 cells
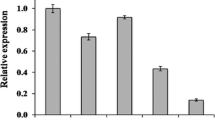
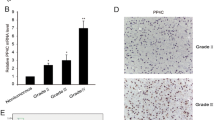
To explore the biological function of PTTG1 on GBM, NC siRNA and PTTG1 siRNA were transfected into U251 cells. Both mRNA and protein expression levels of PTTG1 were detected 48 h post transfection. As shown in Fig. 2a and b, PTTG1 expression was significantly reduced by three PTTG1 siRNA sequence, and the second PTTG1 siRNA produced the best interference in U251 cells. Next, the MTT and colony-formation assay was used to evaluate the effect of PTTG1 on cell viability. The results shown that cell growth (Fig. 2c) and clonogenic survival (Fig. 2d) were repressed following the downregulation of PTTG1 in U251 cells. The second PTTG1 siRNA produced the best interference in cell viability. Therefore, the second PTTG1 siRNA sequence was selected for future study.
Correlations between PTTG1 levels and the mRNA levels of HIF-α, PDGFβ, VEGF, MMP2, MMP9, MMP14, p53, p21 and cyclin D1
Pearson correlation analysis was implemented using MEV software to identify target genes that were positively associated with PTTG1 expression in the TCGA, CGGA, and Rembrandt databases (r > 0.4). In total, 324 related genes were identified (Fig. 3a). To clarify the associations between these genes, the DAVID web tool (https://david.ncifcrf.gov/tools.jsp) was used for gene oncology enrichment analysis. The upregulated genes were mainly enriched in the positive regulation of cytoskeleton, epithelial to mesenchymal transition (EMT) and DNA damage response (Fig. 3b). These suggested us that the roles of PTTG1 might be involved in angiogenesis and migration in glioma cells. To further clarify this view, we further explored the correlations between PTTG1 levels and marker genes of angiogenesis, apoptosis-suppressing and migration. The results shown that the positive correlations were found between PTTG1 and angiogenesis genes, including HIF-α, VEGF and PDGF-B (Fig. 3c–e). Positive correlations were also found between PTTG1 and apoptosis-suppressing genes, including p53 and p21 (Fig. 3f and h), but there were negative correlations with the apoptosis gene caspase 10 (Fig. 3g). Furthermore, there were also positive correlations between PTTG1 and migration-related MMP genes, including MMP2, MMP9 and MMP14 (Fig. 3i–k). Therefore, these findings support a role for PTTG1 in GBM that is related to tumor-sustaining angiogenesis, cell migration and apoptosis evasion.
Correlation between PTTG1 and angiogenesis-, cell migration- and apoptosis-related gene expression in GBM patient. a PTTG1 positively-associated genes from correlation analysis performed in the TCGA, CGGA, and Rembrandt. b Biological processes enrichment results from GO database were shown. The orders of different biological processes were based on their enriched number. c–k Correlations between PTTG1 levels and the mRNA levels of HIF-α, PDGF-B, VEGF, MMP2, MMP9, MMP14, p53, p21 and cyclin D1. The r values and P values are from Pearson's correlation analysis
Effects of PTTG1 on angiogenesis, apoptosis, invasion and migration in U251 GBM cells
It has been reported that PTTG1 can regulate the proliferation and the cell cycle of glioma cells [10,14,15]. Additionally, as stated above, our data imply that the roles of PTTG1 in GBM promotion may be dependent on tumor-sustaining angiogenesis, cell migration and apoptosis evasion. Next, we investigated the effects of PTTG1 on the angiogenesis, apoptosis, invasion and migration of glioma cells using the human astroglioma U251 cell line.
PTTG1 knockdown represses U251 cell angiogenesis
To test our hypothesis that PTTG1 inhibition could suppress the ability of GBM cells to develop vascular-like channels. We transfected U251 cells with NC or PTTG1 siRNA and then performed a vascular mimicry experiment that recapitulated the ability of vascular endothelial cells to develop vasculature in vitro. After transfection with PTTG1 siRNA, the U251 cells developed a much sparser network compared with those transfected with NC siRNA (Fig. 4). This result supports our hypothesis that PTTG1 promotes vasculogenic mimicry formation in GBM cells.
Knockdown of PTTG1 induced U251 cell apoptosis
To further determine whether the effect of PTTG1 on U251 cell proliferation is reflected in cell apoptosis, we performed flow cytometry assays. The results showed that U251 cells transfected with PTTG1 siRNA had a higher apoptotic rate compared with PTTG1 siRNA-transfected cells (Figs. 5). These findings indicate that PTTG1 suppresses the apoptosis of glioma cells.
PTTG1 knockdown represses U251 cell invasion and migration in vitro
We further evaluated the role of PTTG1 in cell invasion and migration. First, a transwell experiment was conducted to test the invasion and migration ability of U251 cells. The results showed that knockdown of PTTG1 significantly repressed the invasion ability of U251 cells (Fig. 6a). Subsequently, we designed a scarification test to ascertain the time effect of PTTG1 silencing on the cell migration ability. The results showed that PTTG1 siRNA could significantly inhibit U251 migration ability at 36 h after transfection (Fig. 6b). These results indicate that PTTG1 may be closely associated with the invasion and migration of glioma cells.
Inhibition of PTTG1 in U251 cells can suppress migration and invasion. NC siRNA and PTTG1 siRNA (50 nM) were transfected into U251 cells for 48 h. a Cell invasion was measured by transwell assay. b Cell migration was measured by wound-healing assay. Values are shown as means ± S.E.M (n = 3 per group). ***P < 0.001 vs. negative control groups
PTTG1 exerts an oncogene function through the TGF-β1/PI3K/AKT/mTOR-signaling pathway
Given the roles of PTTG1 in angiogenesis promotion, apoptosis evasion, invasion and migration in U251 glioma cells, we tried to find a PTTG1-related signaling pathway that could control angiogenesis, apoptosis, invasion and migration in tumors. According to bioinformatics analytical tools, we found that PTTG1 had significant positive correlations with TGF-β, PI3K, AKT and mTOR expression levels (Fig. 7a–d). AKT is a downstream gene of PI3K and exerts oncogenic functions, such as tumor invasion, apoptosis inhibition, proliferation and angiogenesis, and TGF-β is one of the regulatory signals of the PI3K pathway. Interestingly, as a recognized downstream effector of PI3K/AKT signaling, mTOR regulates the tumorigenesis of glioma, while inhibiting mTOR signaling with rapamycin can attenuate the migration and invasion of glioma. TGF-β can mediate the functions of colorectal, breast and liver cancer cells through the activation of the PI3K/AKT/mTOR signaling pathway. Nonetheless, the role of PTTG1 in the TGF-β/PI3K-AKT-mTOR signaling pathway remains unclear. Next, to identify whether the TGF-β/PI3K/AKT/mTOR pathway is involved in the roles of PTTG1 in glioma tumorigenesis, U251 cells were transfected with NC or PTTG1 siRNA. We found that the levels of TGF-β and PI3K were significantly downregulated by PTTG1 suppression in U251 cells (Fig. 7e). Meanwhile, transfection of PTTG1 siRNA repressed AKT phosphorylation at Ser-473 and enhanced total AKT protein levels (Fig. 7e). PTTG1 inhibition suppressed the mTOR level in U251 cells (Fig. 7e). Collectively, these findings suggest that PTTG1 acts as an upstream effector of the TGF-β/PI3K/AKT/mTOR signaling pathway in the regulation of glioma cell behavior in vitro.
PTTG1 exerts an oncogene function through the TGF-β1/PI3K/AKT/mTOR signaling pathway. a–d Correlations between PTTG1 levels and the mRNA levels of TGFβ, PI3K, AKT and mTOR; NC siRNA and PTTG1 siRNA (50 nM) were transfected into U251 cells for 48 h. The r values and P values are from Pearson's correlation analysis. e Representative western blot images and quantitative analysis of TGF-β, PI3K-P85, p-AKT, AKT and mTOR. The values are expressed as percentages compared to a normal group (set to 100%) and are represented as the means ± S.E.M. (n = 3 per group), *P < 0.05; **P < 0.01
Prognostic values of PTTG1 in glioma patients with different cancer grades
To further examine the prognostic value of PTTG1, we assessed the prognostic value of PTTG1 in brain glioblastoma multiforme (GBM) of glioma samples using KM plotter. As shown in Fig. 8, the levels of PTTG1 were significantly associated with prognosis in GBM, the high expression of PTTG1 leads to poor prognosis. Given that PTTG1 is overexpressed in GBM tissues and is associated with tumorigenic behavior of glioma cells, such as angiogenesis, invasion and migration, we further confirmed that high levels of PTTG1 might induce glioma malignancy and worsen GBM prognosis.
Prognostic values of PTTG1 for OS in GBM patients. The Kaplan–Meier plots were generated by analyzing GBM patients. Effect of PTTG1 expression levels on GBM patients (n = 329 for high expression levels; n = 330 for low/medium expression levels). The p values were calculated by log–rank test. HRs with 95% CIs are displayed
Discussion
Gliomas are malignant tumors that originate from neural cells. Enhanced angiogenesis and diffuse cell invasion are prominent hallmarks of GBM, which increase its malignant and lethal character. Therefore, determining the specific genes related to GBM potency may facilitate the identification of therapeutic targets and prognostic biomarkers for GBM patients. In this study, we evaluated the effects of PTTG1 on the malignant phenotypes of GBM. We found that inhibition of PTTG1 represses angiogenesis and reverses the invasion and migration of glioma cells, and these effects may arise through the TGF-β/PI3K-AKT-mTOR signaling pathway. These findings provide novel evidence of the tumor-promoting action of PTTG1 in GBM cell lines by activating the TGF-β/PI3K-AKT-mTOR pathway, suggesting that PTTG1 suppression may be utilized as a promising therapeutic target for GBM.
PTTG1 is upregulated in many human cancers and functions as an oncogene. As a potent tumor-transforming gene, PTTG1 has been shown to induce oncogenesis by facilitating cell proliferation and independent tumorigenesis in in vivo and in vitro studies [20,21,22]. In this study, we analyzed PTTG1 expression and the associated clinical prognosis of glioma using 2 databases: The Cancer Genome Atlas (TCGA) and GEO (GSE15471, GSE15932, and GSE16515). The results showed that PTTG1 protein was overexpressed in GBM patients. To further validate the function of PTTG1 in affecting glioma cell proliferation, we performed MTT assays and colony-formation assays in U251 cells. Consistent with previous studies, knockdown of PTTG1 inhibited the cell proliferation of glioma cells. Indeed, several studies have reported that PTTG1 is highly expressed in glioma and is positively correlated with pathological grades. Su et al. found that the inhibition of PTTG1 by microRNA suppressed the proliferation of malignant glioma cells [10]. PTTG1 was demonstrated to increase the proliferation of glioma through stabilizing E2F [15]. PTTG1 was reported to promote human glioma cell proliferation and inhibit cell cycle arrest by microRNA-520d-5p [14]. These studies indicate that PTTG1 is associated with cell proliferation and cell cycle arrest. PTTG1 acted in a microRNA function to regulate tumor cell proliferation, apoptosis, metastasis, and the cell cycle.
The major reasons for inefficient treatment are the proliferative and highly invasive nature of glioma cells and the occurrence of increased angiogenesis [23,24]. PTTG1 overexpression induces chromosome instability through binding to separase and regulating sister chromosome separation. Previous studies have indicated that PTTG1 is involved in modulating the migration and invasion of breast cancer and oophoroma cells [25]. PTTG1 was also found to induce VEGF activity, which plays an important role in tumor micro-angiogenesis and cancer metastasis [20,26]. In this study, we sought to explore the effect of PTTG1 on the angiogenesis and invasion ability of glioma cells because its related molecular mechanisms are unclear and require additional investigation. In our bioinformation analysis, three datasets were overlapped to mine PTTG1-associated biological pathways. GO analyses indicated that indicated that genes that were positively correlated to PTTG1 were potentially associated with both the angiogenesis and invasion pathways. Based on this, we also verified that high PTTG1 expression was associated with marker genes of angiogenesis, apoptosis evasion, invasion and migration. We then verified that PTTG1 knockdown represses U251 cell angiogenic ability. By flow cytometry, we confirmed that the rate of apoptotic cells was indeed increased after the downregulation of PTTG1. Moreover, we demonstrated that knockdown of PTTG1 significantly suppressed the migration and invasion ability of glioma cells.
Glioma cell invasion and increased angiogenesis share mechanisms of the degradation of the extracellular matrix (ECM) through upregulated ECM-degrading proteases and the activation of aberrant signaling pathways. TGF-β has been shown to induce the degradation of various extracellular matrix components and induced angiogenesis, which contributes to tumor invasion and metastasis [27]. Multifunctional cellular responses evoked by TGF-β are mediated by the ECM-related pathways, including mitogen-activated protein kinase (MAPK) pathways and the PI3K–AKT pathway [28,29]. Furthermore, mTOR is a direct substrate for AKT kinase, which induces cell growth and represses tumor cell apoptosis. It has been reported that TGF-β can mediate the response to angiogenesis through the activation of the PI3K/Akt/mTOR signaling pathway [30]. In the present study, it was observed that the protein expressions of TGF-β, mTOR, and phosphorylated AKT were decreased in the PTTG1-silenced group. Downregulated PTTG1 has been associated with restraining tumor cell migration and suppressing cancer angiogenesis. Therefore, our results suggested that PTTG1 knock-down decreases glioma cell angiogenesis and migration via inhibiting the TGFβ-PI3K/AKT/mTOR pathway.
In conclusion, our results demonstrate that PTTG1 acts as an oncogenic factor in the progression of GBM. Our study clarified the expression of PTTG1 in glioma, its influence on the prognosis of patients, and especially its effect on the angiogenesis and cell invasion of glioma cells and the related mechanisms. Our results provide a theoretical basis for the development of novel anti-malignant cancer therapeutic drugs by targeting PTTG1 and the related TGFβ-PI3K/AKT/mTOR pathway. However, many research avenues remain, such as exploring the specific mechanism by which PTTG1 affects the angiogenesis and cell invasion of glioma cells in vivo.
Data availability
All data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Herrlinger U, Tzaridis T, Mack F, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet (London, England). 2019;393:678–88.
Lee JH, Lee JE, Kahng JY, et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018;560:243–7.
Gusyatiner O, Hegi ME. Glioma epigenetics: From subclassification to novel treatment options. Semin Cancer Biol. 2018;51:50–8.
Zeng W, Liu P, Pan W, Singh SR, Wei Y. Hypoxia and hypoxia inducible factors in tumor metabolism. Cancer Lett. 2015;356:263–7.
Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501.
Heaney AP, Singson R, McCabe CJ, Nelson V, Nakashima M, Melmed S. Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet (London, England). 2000;355:716–9.
Tong Y, Eigler T. Transcriptional targets for pituitary tumor-transforming gene-1. J Mol Endocrinol. 2009;43:179–85.
Lin X, Yang Y, Guo Y, et al. PTTG1 is involved in TNF-alpha-related hepatocellular carcinoma via the induction of c-myc. Cancer Med. 2019;8:5702–15.
Hu ZG, Zheng CW, Su HZ, et al. MicroRNA-329-mediated PTTG1 downregulation inactivates the MAPK signaling pathway to suppress cell proliferation and tumor growth in cholangiocarcinoma. J Cell Biochem. 2019;120:9964–78.
Su X, Chen J, Ni L, et al. Inhibition of PTTG1 expression by microRNA suppresses proliferation and induces apoptosis of malignant glioma cells. Oncol Lett. 2016;12:3463–71.
Fujii T, Nomoto S, Koshikawa K, et al. Overexpression of pituitary tumor transforming gene 1 in HCC is associated with angiogenesis and poor prognosis. Hepatology (Baltimore, MD). 2006;43:1267–75.
Xiang W, Wu X, Huang C, et al. PTTG1 regulated by miR-146a-3p promotes bladder cancer migration, invasion, metastasis and growth. Oncotarget. 2017;8:664–78.
Yoon CH, Kim MJ, Lee H, et al. PTTG1 oncogene promotes tumor malignancy via epithelial to mesenchymal transition and expansion of cancer stem cell population. J Biol Chem. 2012;287:19516–27.
Zhi T, Jiang K, Xu X, et al. MicroRNA-520d-5p inhibits human glioma cell proliferation and induces cell cycle arrest by directly targeting PTTG1. Am J Transl Res. 2017;9:4872–87.
Zhi T, Jiang K, Xu X, et al. ECT2/PSMD14/PTTG1 axis promotes the proliferation of glioma through stabilizing E2F1. Neuro-Oncology. 2019;21:462–73.
Cui LLT, Xu L, Wang G, Lin J, Feng S, Cao Y, Cao Y, Song Z, Jin X. The effect of down-regulated gene PTTG1 on proliferation, apoptosis, migration and invasion of human glioma cell SHG44. China Oncol. 2019;29:338–44.
Genkai N, Homma J, Sano M, Tanaka R, Yamanaka R. Increased expression of pituitary tumor-transforming gene (PTTG)-1 is correlated with poor prognosis in glioma patients. Oncol Rep. 2006;15:1569–74.
Schwartzbaum JA, Fisher JL, Aldape KD and Wrensch M. Epidemiology and molecular pathology of glioma. Nature clinical practice. Neurology. 2006: 2: 494–503; quiz 491 p following 516.
Wang X, Chen D, Gao J, et al. Centromere protein U expression promotes non-small-cell lung cancer cell proliferation through FOXM1 and predicts poor survival. Cancer Manag Res. 2018;10:6971–84.
Hamid T, Malik MT, Kakar SS. Ectopic expression of PTTG1/securin promotes tumorigenesis in human embryonic kidney cells. Mol Cancer. 2005;4:3.
Huang JL, Cao SW, Ou QS, et al. The long non-coding RNA PTTG3P promotes cell growth and metastasis via up-regulating PTTG1 and activating PI3K/AKT signaling in hepatocellular carcinoma. Mol Cancer. 2018;17:93.
Ren Q, Jin B. The clinical value and biological function of PTTG1 in colorectal cancer. Biomed Pharmacother. 2017;89:108–15.
Shono T, Mizoguchi M, Sasaki T. Latest treatment strategies for malignant gliomas. Fukuoka Igaku Zasshi. 2007;98:333–6.
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507.
Zhang G, Zhao Q, Yu S, Lin R, Yi X. Pttg1 inhibits TGFbeta signaling in breast cancer cells to promote their growth. Tumour Biol. 2015;36:199–203.
Demin DE, Bogolyubova AV, Zlenko DV, et al. The novel short isoform of securin stimulates the expression of cyclin D3 and angiogenesis factors VEGFA and FGF2, but does not affect the expression of MYC transcription factor. Mol Biol. 2018;52:508–18.
LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updates. 2008;11:32–50.
Chen XF, Zhang HJ, Wang HB, et al. Transforming growth factor-beta1 induces epithelial-to-mesenchymal transition in human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling pathways. Mol Biol Rep. 2012;39:3549–56.
Dong F, Liu T, Jin H, Wang W. Chimaphilin inhibits human osteosarcoma cell invasion and metastasis through suppressing the TGF-beta1-induced epithelial-to-mesenchymal transition markers via PI-3K/Akt, ERK1/2, and Smad signaling pathways. Can J Physiol Pharmacol. 2018;96:1–7.
Jiang X, Zhang Z, Song C, et al. Glaucocalyxin A reverses EMT and TGF-beta1-induced EMT by inhibiting TGF-beta1/Smad2/3 signaling pathway in osteosarcoma. Chem Biol Interact. 2019;307:158–66.
Funding
This study was supported by Grants from the Fundamental Research Funds for the Central Universities (No. 20720180042), the Health Science Research Personnel Training Program of Fujian Province (2016-CXB-12), the Natural Science Foundation of Fujian, China (No. 2016D019).
Author information
Authors and Affiliations
Contributions
YLC and JX designed the experiments and drafted the manuscript. CLS, RT, SZH, GXM and FDR performed the study, and ZHT, CSD, ZMS performed the statistical analysis. ZRG revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, L., Ren, T., Zhao, H. et al. Suppression of PTTG1 inhibits cell angiogenesis, migration and invasion in glioma cells. Med Oncol 37, 73 (2020). https://doi.org/10.1007/s12032-020-01398-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01398-2