Abstract
MicroRNAs are known as non-coding RNAs that regulate the expression of target mRNA. Accumulating evidence has indicated that microRNA expression in human malignancies can be utilized as a prognostic marker for patients. However, the prognostic value of miR-650 in human glioma has not been investigated yet. In the present investigation, we have recruited 168 cases glioma specimens and 21 normal control brain specimens. Quantitative real-time PCR was carried out to investigate the expression of miR-650. Kaplan–Meier analysis and Cox’s proportional hazards model was used to evaluate the association of miR-650 with prognosis of glioma patients. Results showed that miR-650 expression was increased in glioma compared with normal control specimens (P < 0.001). It was also found that miR-650 expression was related to World Health Organization grade and Karnofsky performance score (KPS) for high expression was more frequently detected in glioma of high grade or low KPS score (P < 0.001). The prognosis of glioma with high miR-650 expression was significantly worse compared with that of glioma with low miR-650 expression. These results proved that miR-650 expression was a significant prognostic indicator in glioma, which may suggest new management of human glioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most common primary malignant brain tumors, which comprise approximately one-third of intrinsic neoplasms of the central nervous system in both adults and children [1, 2]. It is a type of aggressive tumor with a tendency to invade the surrounding brain tissue. The WHO classification scheme divides gliomas into four stages, or grades in increasing level of malignancy by the expected clinical courses. Grades I and II are the least malignant phenotypes, whereas grade III (anaplastic astrocytoma, anaplastic oligoastrocytoma, and anaplastic oligodendroglioma) and grade IV (glioblastoma multiforme, GBM) constitute the most malignant as well as the most reported glioma histologies accounting for nearly half of all gliomas [3]. The survival of patients with gliomas is closely related to WHO tumor grade. Patients with glioblastoma, the most common histological type, have a poor prognosis with a median survival of approximately 15 months and a 2-year survival rate of 27 %, despite surgical resection, temozolomide chemotherapy and radiation therapy. The majority of patients would endure recurrence within 1 year and usually die of the disease within 6–8 months after recurrence [4–6]. Thus, efforts to better understand the biological basis for gliomas progression may provide important clinically relevant insights into disease management [7].
MicroRNAs (miRNAs) are a class of highly conserved, single-stranded, small noncoding RNA molecules, which are known as endogenous regulators of post-transcriptional gene expression regulating expression through translational repression and messenger RNA cleavage [8, 9]. It has been widely accepted that miRNAs play pivotal roles in various biological processes, including development, cell proliferation, differentiation, apoptosis and metabolism [10]. Accumulated evidence also suggests that miRNAs participate in the tumor angiogenesis, invasion and metastasis of human malignancy, acting as oncogenes or tumor suppressors depending on the their target, which may provide insight into the diagnosis and prognosis of human cancers [11, 12]. Recently, a number of studies have shown that expression of miRNAs is deregulated in in various types of human cancers including breast cancer, colon cancer, lung cancer, pancreas cancer and chronic lymphocytic leukemia [13–17]. In the human brain, a series of miRNAs have been found to play a crucial roles in both biological process and neurological disease, such as adaptive immune response, Parkinson’s disease and Alzheimer’s disease [18–20]. Moreover, previous studies proved that several miRNAs was deregulated in human glioma and could serve as prognostic markers [21–23]. miR-650, a recently described microRNA, has been proved to be associated with carcinogenesis and progression in various human malignancies such as gastric cancer, colorectal cancer and hepatocellular cancer [24–26]. Besides solid tumors, miR-650 was also proved to could influence cell proliferation and survival in chronic lymphocytic leukemia [27]. However, the expression pattern of miR-650 in glioma and its association with clinicopathologic characteristics have not been reported yet. Investigation on the function of miR-650 in glioma may contribute to molecular tumor characterization, which can help to make educated and state-of-the-art individualized treatment decision on glioma.
In the present study, we investigated the expression pattern of miR-650 in glioma specimens and normal control brain tissues, analyzed the relationship of miR-650 expression with glioma clinicopathologic characteristics and prognosis of patients.
Materials and methods
Patients and tissue specimens
The study was approved by the ethics committee of Beihua University and conducted according to the principles expressed in the Declaration of Helsinki. Both Department of Neurosurgery, Affiliated Hospital, Beihua University (Jilin, China) and Department of Neurosurgery, Xijing Hospital, the Fourth Military Medical University (Xi’an, China) supplied clinical specimens for this study. The study cohort has been described in our previous publication [28, 29]. Briefly, fresh clinical glioma specimens were obtained from 168 patients who underwent surgery between October 2000 and September 2003. None of the patients has received radiotherapy or chemotherapy prior to surgery. Twenty-one normal brain tissues samples were taken from patients who underwent surgery for reasons other than malignancy such as cerebral trauma served as the control. Histomorphology of all specimens were confirmed by the Department of Pathology, Affiliated Hospital, Beihua University and Department of Pathology, Xijing Hospital. Grading of the specimen was done based on WHO classification (Grade I–IV). Specimens were put immediately into liquid N2 after surgical resection for 10 min, then into a −70 °C ultra-freezer for mRNA isolation. In the follow-up period, overall survival was measured from diagnosis to death or last follow-up. Patients’ clinical information, such as age, sex, Karnofsky performance score (KPS) and WHO grade was collected and stored in a database. Follow-up information of all eligible patients was updated every 3 months by telephone visit and questionnaire letters. Overall survival was calculated from the date of the initial surgical operation to death. Patients who died of diseases not directly related to glioma had been excluded from this study. Death of participants was ascertained by reporting from the family and verified by review of public records.
Real-time PCR
Total RNA was purified from all the 168 glioma tissue and 21 control brain tissues and cells as recommended by the manufacturer using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Only those total RNA samples with OD A260/A280 ratio close to value of 2.0, which indicates that the RNA is pure, were subsequently analyzed. Real-time absolute quantification was utilized to insure the quality of samples. The miR-650 and RNU6B (as internal control) specific cDNA were synthesized from total RNA using gene-specific primers according to the TaqMan MicroRNA assays protocol (Applied Biosystems, Foster City, CA, USA). The reverse transcription products were then amplified and detected by real-time PCR using Taqman MicroRNA Assay (Applied Biosystems) specific for hsa-miR-650. Each sample was examined in triplicate and the raw data were presented as the relative quantification of miR-650 expression evaluated by the comparative cycle threshold (CT) method, normalized with respect to RNU6B. Relative expression of miR-650 was calculated by 2−∆∆Ct method. Mean normalized miR-650 expression ± standard deviation (SD) was calculated from triplicate analysis. Real-time PCR was performed using an ABI 7500 system (Applied Biosystems) and comparative 2−ΔΔCt analysis was performed using SDS 2.2.2 software (Applied Biosystems).
Statistical analysis
All statistic analyses were performed using SPSS® version 13.0 software (SPSS Inc., Chicago, USA). The measurement data were analyzed by one-way ANOVA. Randomized block design ANOVA was used to analyze the statistical difference among different tissue types. Associations between miR-650 expression and clinicopathological characteristics were analyzed by Mann–Whitney test or Kruskal–Wallis test. Survival curves were plotted using the Kaplan–Meier product-limit method, and differences between survival curves were tested using the log-rank test. Cox’s proportional hazards model was used to identify the factors that have significantly independent influence on survival. Statistical significance was set at P < 0.05.
Results
Quantitative analysis of miR-650 expression levels in glioma by real-time PCR
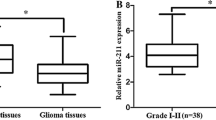
miR-650 expression was studied in a total of 168 glioma specimens of which 75 were low grade glioma (grade I and II) and 93 were high grade (grade III and IV). Twenty-one specimens taken from normal brain tissue served as control group. We firstly determined the mRNA expression of miR-650 normalized to RNU6B by real-time PCR. Results showed that the relative expression of miR-650 in glioma was 10.25 ± 2.16 (mean ± SD), which was significantly higher compared with 1.86 ± 0.42 in normal brain tissues (P < 0.001). To facilitate further analysis, we defined glioma specimens with miR-650 levels less than mean expression level (10.25) as low miR-650 expression group, while specimens with miR-650 levels no less than mean level were defined as high miR-650 expression group. Thus, among 168 cases glioma, 72 cases were assigned to low expression group while other 96 cases were assigned to high expression group.
Association between miR-650 expression levels and clinicopathological characteristics
Based on quantitative real-time PCR assay and subgroup definition, we further evaluated the association of miR-650 expression with clinicopathological characteristics of patients. Results were showed in Table 1. It is proved that miR-650 expression was not significantly associated with gender (P = 0.442) or age (P = 0.928) of the patients with glioma. In contrast, the most salient finding was the tight association between miR-650 expression and WHO grade for miR-650 expression tended to increase from grade I to grade IV glioma (P < 0.001). In addition, miR-650 expression was also found to be associated with KPS of patients for high miR-650 expression was more frequently detected in glioma with KPS lower than 80, suggesting miR-650 might potentially affect functional impairment of patients with glioma (P = 0.003).
Association between miR-650 expression levels and prognosis of glioma patients
As statistical analysis proved that miR-650 expression was associated with glioma WHO grade and KPS, which indicated that miR-650 might potentially affect prognosis of glioam patients. Therefore, we reviewed follow-up information of these glioma patients with high miR-650 or low miR-650 expression. During the follow-up period, 131 of the 168 glioma patients (77.98 %) had died. Kaplan–Meier analysis proved that high expression of miR-650 was related to worse overall survival of patients (Fig. 1). The survival rate of patients with high miR-650 expression, as determined by the log-rank test, was lower than those with low miR-650 expression (P < 0.001). The median survival time of patients with high miR-650 expression was 8 months (95 % CI 6.9–9.1), while median survival time of those with low miR-650 expression was 32 months (95 % CI 24.8–39.2). In addition, age at diagnosis, KPS and WHO grade were also found to be associated overall survival of patients with glioma. Unadjusted hazard ration (HR) for patients with glioma was shown in Table 2.
Cox’s proportional hazards model was adjusted for sex, age at diagnosis, WHO grade and KPS. By multivariate analysis, high miR-650 expression was a significant and independent indicator of poor prognosis for patients with glioma besides sex, age, WHO grade and KPS for survival curve obtained by Cox’s proportional hazards model showed that patients with glioma of high miR-650 expression had worse overall survival (log-rank test: P < 0.001, Fig. 2). Further analysis proved that patients with high miR-650 expression have 6.8-fold risk of death compared to those with low expression, independent of considering age, WHO grade and KPS (Table 2). However, there were no significant differences in sex. Cox proportional hazards model also showed that age at diagnosis, WHO grade and KPS were independent prognostic marker (Table 2).
Discussion
Despite recent research efforts, the prognosis for patients with glioma remains poor. The prognosis for patients with WHO grade IV glioma was extremely grave with a median survival of 8–15 months for newly diagnosed patients. The prognosis for newly diagnosed grade III glioma is only slightly better, with a median survival of 12–24 months. Nevertheless, patients with similar stages of glioma showed a marked discrepancy in survival. To understand the genetic background and molecular pathogenic processes is may contribute to better assessment of the survival probability and, consequently, the tailoring of treatment for each individual patient. Recently, increasing evidence has proved that that miRNAs may participate in tumor carcinogenesis and progression [13–17]. Aberrant miRNA expression was also proved to be highly correlated with prognosis in various cancers [30, 31]. Thus, identification of tumor-specific miRNAs might provide insight into tumor diagnosis and therapeutic management.
Prior to our present study, little was known about miR-650 expression pattern in human glioma or its correlation with the clinicopathologic characteristics and prognosis of these patients. In this study, we investigated the mRNA expression of miR-650 in 168 cases of human glioma and its association with tumor clinicopathologic characteristics and prognosis of patients. Results provided the first evidence that miR-650 expression was increased in glioma compared with that in normal brain tissues, suggesting it might serve as an oncogene during human glioma carcinogenesis. It is consistent with previous studies focused on miR-650 and other types of human malignancies, which have demonstrated that miR-650 expression was increased in hepatocellular and colorectal cancer, and had the effect to promote cancer cell proliferation in these malignancies [25, 26]. We also found an increase trend of miR-650 expression from WHO grade I to grade IV glioma, which suggested that miR-650 might play a promotive role in the progression of human glioma. Other studies also found the same trend of miR-650 in tumor progression that miR-650 was involved in the metastasis in human gastric cancer and ectopic expression of miR-650 might promote tumorigenesis and proliferation of gastric cancer cells [24]. In addition, our study also demonstrated that miR-650 expression was related to KPS for high miR-650 expression was more frequently detected in glioma with KPS lower than 80, which suggested that the oncogenic role of miR-650 might potentially affect functional impairment of patients with glioma. However, miR-650 expression was not found to be related to sex or age at diagnosis. These results reveal a consistent pattern of miR-650 with known data on other type of human malignancies.
Considering the association of miR-650 with these clinicopathological parameters might provide insight into prognosis diagnosis of patients with glioma, we further analyzed the association of miR-650 expression with overall survival of patients with glioma. Our results proved for the first time that the postoperative overall survival of patients with high miR-650 expression was significant worse than those with low miR-650 expression, indicating that high miR-650 level is a marker of poor prognosis of glioma. Moreover, multivariate analysis demonstrated that high miR-650 expression was a marker of worse outcome independent of known clinical prognostic indicators such as age at diagnosis, KPS and WHO grade. These data suggested that miR-650 might be an independent prognostic marker for glioma patients.
The mechanism lies behind the association of miR-650 with KPS, WHO grade and prognosis of patients may be diverse. It is now accepted that miRNAs may function as oncogenes or tumor suppressors according to the roles of their target genes [11, 12]. As far as miR-650 is concerned, previous studies have proved that it can function as an oncogene by suppressing tumor suppressor genes. For example, NDRG2, an established tumor suppressor gene, is reported to be suppressed by miR-650 at the transcriptional level through promoter methylation in colorectal cancer [25]. The samples of the present investigation have also been utilized to detect NDRG1 and NDRG2 expression pattern in our previous studies, both of which were found to be decreased in glioma and have opposite association with clinicopathological parameters as well as prognosis to miR-650 [28, 29]. Thus, it is possible that miR-650 might play its oncogenic role in glioma by suppressing both NDRG1 and NDRG2, which are considered to could suppress tumor carcinogenesis and progression [32]. In addition, it is also reported that miR-650 can recognize the conserved binding sites on ING4 and lead to a significant decrease of ING4, whose products could interact with p53 enhancing the gene transcription, apoptosis and DNA repair [24, 26]. So, miR-650 might also affect glioma carcinogenesis and progression at least partially through directly targeting ING4.
In conclusion, to the best of our knowledge, this is the first study to determine the expression pattern of miR-650 and its association with clinical significance in a large series of glioma patients. Our study provided convincing evidence that miR-650 was increased in human glioma and associated WHO grade as well as KPS. It was also proved that miR-650 expression was correlated with overall survival of patients with glioma and might be a novel prognostic marker.
References
Kleihues P, Burger PC, Scheithauer BW (1993) The new WHO classification of brain tumours. Brain Pathol 3(3):255–268
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30. doi:10.3322/caac.21166
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK (2002) The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61(3):215–225; discussion 226–219
Tsao-Wei DD, Hu J, Groshen SG, Chamberlain MC (2012) Conditional survival of high-grade glioma in Los Angeles County during the year 1990–2000. J Neuro Oncol 110(1):145–152. doi:10.1007/s11060-012-0949-6
Shirai K, Chakravarti A (2011) Towards personalized therapy for patients with glioblastoma. Expert Rev Anticancer Ther 11(12):1935–1944. doi:10.1586/era.11.103
Franceschi E, Stupp R, van den Bent MJ, van Herpen C, Laigle Donadey F, Gorlia T, Hegi M, Lhermitte B, Strauss LC, Allgeier A, Lacombe D, Brandes AA (2012) EORTC 26083 phase I/II trial of dasatinib in combination with CCNU in patients with recurrent glioblastoma. Neuro Oncol 14(12):1503–1510. doi:10.1093/neuonc/nos256
Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ (2010) Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60(3):166–193. doi:10.3322/caac.20069
Esteller M (2011) Non-coding RNAs in human disease. Nat Rev Genet 12(12):861–874. doi:10.1038/nrg3074
Wienholds E, Plasterk RH (2005) MicroRNA function in animal development. FEBS Lett 579(26):5911–5922. doi:10.1016/j.febslet.2005.07.070
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Dela Cruz F, Matushansky I (2011) MicroRNAs in chromosomal translocation-associated solid tumors: learning from sarcomas. Discov Med 12(65):307–317
Lovat F, Valeri N, Croce CM (2011) MicroRNAs in the pathogenesis of cancer. Semin Oncol 38(6):724–733. doi:10.1053/j.seminoncol.2011.08.006
Harquail J, Benzina S, Robichaud GA (2012) MicroRNAs and breast cancer malignancy: an overview of miRNA-regulated cancer processes leading to metastasis. Cancer Biomark 11(6):269–280. doi:10.3233/CBM-120291
Rossi S, Di Narzo AF, Mestdagh P, Jacobs B, Bosman FT, Gustavsson B, Majoie B, Roth A, Vandesompele J, Rigoutsos I, Delorenzi M, Tejpar S (2012) microRNAs in colon cancer: a roadmap for discovery. FEBS Lett 586(19):3000–3007
Solomides CC, Evans BJ, Navenot JM, Vadigepalli R, Peiper SC, Wang ZX (2012) MicroRNA profiling in lung cancer reveals new molecular markers for diagnosis. Acta Cytol 56(6):645–654. doi:10.1159/000343473
Ali S, Saleh H, Sethi S, Sarkar FH, Philip PA (2012) MicroRNA profiling of diagnostic needle aspirates from patients with pancreatic cancer. Br J Cancer 107(8):1354–1360. doi:10.1038/bjc.2012.383
Moussay E, Wang K, Cho JH, van Moer K, Pierson S, Paggetti J, Nazarov PV, Palissot V, Hood LE, Berchem G, Galas DJ (2011) MicroRNA as biomarkers and regulators in B-cell chronic lymphocytic leukemia. Proc Natl Acad Sci USA 108(16):6573–6578. doi:10.1073/pnas.1019557108
Guerau-de-Arellano M, Smith KM, Godlewski J, Liu Y, Winger R, Lawler SE, Whitacre CC, Racke MK, Lovett-Racke AE (2011) Micro-RNA dysregulation in multiple sclerosis favours pro-inflammatory T-cell-mediated autoimmunity. Brain J Neurol 134(Pt 12):3578–3589. doi:10.1093/brain/awr262
Mouradian MM (2012) MicroRNAs in Parkinson’s disease. Neurobiol Dis 46(2):279–284. doi:10.1016/j.nbd.2011.12.046
Delay C, Mandemakers W, Hebert SS (2012) MicroRNAs in Alzheimer’s disease. Neurobiol Dis 46(2):285–290. doi:10.1016/j.nbd.2012.01.003
Tan X, Wang S, Yang B, Zhu L, Yin B, Chao T, Zhao J, Yuan J, Qiang B, Peng X (2012) The CREB-miR-9 negative feedback minicircuitry coordinates the migration and proliferation of glioma cells. PLoS One 7(11):e49570. doi:10.1371/journal.pone.0049570
Hermansen SK, Dahlrot RH, Nielsen BS, Hansen S, Kristensen BW (2013) MiR-21 expression in the tumor cell compartment holds unfavorable prognostic value in gliomas. J Neuro Oncol 111(1):71–81. doi:10.1007/s11060-012-0992-3
Chang C, Shi H, Wang C, Wang J, Geng N, Jiang X, Wang X (2012) Correlation of microRNA-375 downregulation with unfavorable clinical outcome of patients with glioma. Neurosci Lett 531(2):204–208. doi:10.1016/j.neulet.2012.10.021
Zhang X, Zhu W, Zhang J, Huo S, Zhou L, Gu Z, Zhang M (2010) MicroRNA-650 targets ING4 to promote gastric cancer tumorigenicity. Biochem Biophys Res Commun 395(2):275–280. doi:10.1016/j.bbrc.2010.04.005
Feng L, Xie Y, Zhang H, Wu Y (2011) Down-regulation of NDRG2 gene expression in human colorectal cancer involves promoter methylation and microRNA-650. Biochem Biophys Res Commun 406(4):534–538. doi:10.1016/j.bbrc.2011.02.081
Zeng ZL, Li FJ, Gao F, Sun DS, Yao L (2013) Upregulation of miR-650 is correlated with down-regulation of ING4 and progression of hepatocellular carcinoma. J Surg Oncol 107(2):105–110. doi:10.1002/jso.23210
Mraz M, Dolezalova D, Plevova K, Stano Kozubik K, Mayerova V, Cerna K, Musilova K, Tichy B, Pavlova S, Borsky M, Verner J, Doubek M, Brychtova Y, Trbusek M, Hampl A, Mayer J, Pospisilova S (2012) MicroRNA-650 expression is influenced by immunoglobulin gene rearrangement and affects the biology of chronic lymphocytic leukemia. Blood 119(9):2110–2113. doi:10.1182/blood-2011-11-394874
Li W, Chu D, Chu X, Meng F, Wei D, Li H, Sun B (2011) Decreased expression of NDRG2 is related to poor overall survival in patients with glioma. J Clin Neurosci 18(11):1534–1537. doi:10.1016/j.jocn.2010.12.032
Sun B, Chu D, Li W, Chu X, Li Y, Wei D, Li H (2009) Decreased expression of NDRG1 in glioma is related to tumor progression and survival of patients. J Neuro Oncol 94(2):213–219. doi:10.1007/s11060-009-9859-7
Wang F, Sun GP, Zou YF, Hao JQ, Zhong F, Ren WJ (2012) MicroRNAs as promising biomarkers for gastric cancer. Cancer Biomark 11(6):259–267. doi:10.3233/CBM-2012-00284
Tang W, Zhu J, Su S, Wu W, Liu Q, Su F, Yu F (2012) MiR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS One 7(12):e51702. doi:10.1371/journal.pone.0051702
Melotte V, Qu X, Ongenaert M, van Criekinge W, de Bruine AP, Baldwin HS, van Engeland M (2010) The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J 24(11):4153–4166. doi:10.1096/fj.09-151464
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81172386, http://www.nsfc.gov.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Boqian Sun, Bo Pu, Dake Chu and Xiaodan Chu have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Sun, B., Pu, B., Chu, D. et al. MicroRNA-650 expression in glioma is associated with prognosis of patients. J Neurooncol 115, 375–380 (2013). https://doi.org/10.1007/s11060-013-1243-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1243-y