Abstract
Venous thromboembolism (VTE) events are frequent in neurooncological patients in perioperative period thus increasing mortality and morbidity. The role of prophylaxis has not yet been established with certainty, and in various neurosurgery and intensive care units the practice is inconsistent. A better definition of the risk/cost/benefit ratio of the various methods, both mechanical (intermittent pneumatic compression-IPC, graduated compression stockings-GCS) and pharmacological (unfractionated heparin-UFH or low molecular weight heparin-LMWH), is warranted. We aim to define the optimal prophylactic treatment in the perioperative period in neurooncological patients. A systematic review of the literature was performed in Medline, Embase and Cochrane Library. Thirteen randomized controlled trials (RCTs) were identified, in which physical methods (IPC or GCS) and/or drugs (UFH or LMWHs) were evaluated in perioperative prophylaxis of neurological patients, mostly with brain cancer not treated with anticoagulants for other diseases. The analysis was conducted on a total of 1,932 randomized patients of whom 1,558 had brain tumours. Overall data show a trend of reduction of VTE in patients treated with mechanical methods (IPC or GCS) that should be initiated preoperatively and continued until discharge or longer in case of persistence of risk factors. The addition of enoxaparin starting the day after surgery, significantly reduces clinically manifest VTE, despite an increase in major bleeding events. Further studies are needed to delineate the types of patients with an increase of VTE risk and risk/benefits ratio of physical and pharmacological treatments in the perioperative period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE) is a common complication in patients with cancer and its frequency is particularly high in malignant glioma, occurring in approximately 20–30 % of such patients [1]. The risk of symptomatic deep venous thrombosis (DVT) in patients undergoing craniotomy for brain tumour has been reported to be as high as 31 % [2]. The risk of pulmonary embolism (PE) is estimated to be 5 % with a mortality ranging from 9 to 50 % [3].
The mechanism of VTE development is unclear, but risk factors include histologic diagnosis of glioblastoma multiforme (GBM), larger tumour size (tumours produce pro-coagulation factors, then larger tumours release it in larger quantities), presence of leg paresis (the absence of the muscle pump effect favours venous stagnation), older age (pro-coagulant factors increase with age, but anticoagulant proteins remain stable), more lengthy surgery, chemotherapy (it reduces fibrinolytic activity) and steroids [4–6]. Both mechanical and pharmacological methods are used in the prevention of DVT. Mechanical methods include intermittent pneumatic compression (IPC) and graduated compression stockings (GCS). IPC uses a pump periodically inflating and deflating air bladders incorporated into sleeves which envelop the limb. There are different ways of applying IPC, using different compression techniques and variation of inflation and deflation times, but all the major types of IPC systems seem to be successful in emptying lower limb deep veins and preventing stasis in a variety of subject groups [7]. The exact mechanism by which GCSs function is unknown, however they appear to function more by preventing distension of veins. Pharmacological treatments include the use of unfractionated heparin (UFH) or low molecular weight heparins (LMWHs) that are administered parenterally. Through their anticoagulant effect, these agents reduce the incidence of VTE and its associated mortality [8] but, at the same time, they increase the risk of haemorrhagic complications that are much feared in neurosurgery [9–13].
The role of perioperative VTE prophylaxis in brain tumour surgery has not yet been established with certainty, and in various neurosurgery and intensive care units the practice is highly variable. It is unclear when treatment should start, which treatment is the most beneficial for these patients and the risk/benefit ratio of prophylactic treatment. We therefore systematically reviewed the literature to determine the optimal perioperative prophylaxis in patients with brain tumour, in terms of safety and efficacy.
Materials and methods
Search strategy and selection criteria
We assessed randomized controlled trials (RCTs) that compared (1) UFH or LMWHs with placebo; (2) mechanical prophylaxis (IPC or GCS) with no treatment or with UFH or LMWHs; and (3) combined pharmacological prophylaxis (UFH or LMWHs) and mechanical prophylaxis (IPC or GCS) with mechanical prophylaxis or pharmacological prophylaxis alone, in patients who underwent craniotomy for brain tumour and were not treated with anticoagulants for other diseases.
We searched for trials in MEDLINE (1970–2011), EMBASE (1988–2011) and the Cochrane controlled trials register, and hand-searched references in identified trials and symposia reports (1990–2011) from the major neurooncology and neurosurgery associations. The search strategy combined terms for brain tumour or thromboembolism, anticoagulant and was limited to RCTs publication type.
Data extraction and quality assessment
Two of the authors scrutinised titles and abstracts retrieved by the searches and decided whether or not a trial met the criteria for inclusion in the review. Any disagreement about trial status was resolved by discussion with a third author.
The primary endpoint measures were the proportion of patients who had DVT or PE, both symptomatic or identified by a diagnostic test during the follow-up periods, and major bleeding.
The two authors independently extracted trial data onto a standard form that focused on seven criteria to measure the risk of bias in RCTs according to Cochrane criteria [14]: (1) random sequence generation (defined as adequate, unclear, or inadequate); (2) allocation concealment (defined as adequate, unclear, or inadequate); (3) blinding of patients and personnel; (4) blinding of endpoint assessors; (5) incomplete outcome data; (6) selective reporting; and (7) other bias (e.g. major baseline imbalance). We also considered external validity, i.e. applicability and transferability of the results to clinical practice. Extracted trial data included also description of treatment groups, type and period duration of treatment, type and time frame of endpoints. If necessary, additional information was sought from the trial investigators. Disagreements on extracted data were resolved by discussion with a third author.
Statistical analysis
We performed separate analysis for all DVTs, PE and major bleeding. We also performed subgroup analysis for intervention type. Comparison of endpoints was assessed, within each of these groups, estimating odds ratios (ORs) with 95 % confidence intervals (CIs), using the Peto fixed method. The analysis always adhered to the original randomisation, as presented in trial publications. Between study heterogeneity of findings was assessed using the I2 statistic. If I2 exceeded 50 %, we considered the heterogeneity to be substantial and used a random effects approach [15] to assess its effect on treatment efficacy. The meta-analysis was done using Review Manager (RevMan) software, V. 5.1 (Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) [16].
Results
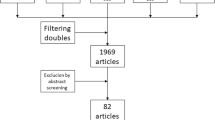
Figure 1 shows the flow diagram of study selection for inclusion in the review. Thirteen trials [1–6, 8, 17–22] were eligible in which 1,932 patients had been randomised. Table 1 provides details on the characteristics of the included studies. Three trials [4, 8, 21] included patients who underwent cranial or spinal cord neurosurgery; all other studies included patients who had craniotomy. In three studies [2, 5, 22] all patients had craniotomy for brain tumour; in the other ten studies the proportion of patients with brain tumour ranged from 48 % [21] to 93 % [1] and other reasons for craniotomy were haemorrhage or hematoma, aneurysm or arteriovenous malformation. One of these trials was published as an abstract and characteristics of patients were not reported [18]. Mean age of participants varied from 47 to 64 years across included trials. The start of treatment in the included studies ranged from the day before surgery to 24 h after surgery and follow-up ranged from 5 to 60 days. Treatment comparisons included:
-
Pharmacological prophylaxis with UFH [3, 5] or LMWH [18] versus placebo.
-
Mechanical prophylaxis with IPC versus no treatment [19, 20] GCS versus GCS and IPC versus no treatment [21] GCS versus IPC [17] GCS versus GCS and IPC [22].
-
Combined modalities with enoxaparin and GCS versus placebo and GCS [4] nadroparin and GCS versus placebo and GCS [8] enoxaparin, IPC and GCS versus heparin, IPC and GCS [1, 6], enoxaparin versus IPC versus enoxaparin and IPC [2].
Ultrasound was used to diagnose asymptomatic DVT by four studies [1, 2, 6, 8] the I-labeled fibrinogen uptake test by four [3, 19–21] venography by three [4, 18, 22] and plethysmography by one study [17]. Symptomatic DVT was reported by one trial [5]. Eight studies reported the number of patients who had developed symptomatic PE, diagnosed mainly with scintigraphy scanning, a pulmonary angiogram or CT scanning. Major bleeding was reported by all studies of pharmacological prophylaxis.
The methodological quality of the included studies is summarised in Fig. 2 Concealment of treatment allocation had been adequate in three trials [4–6] and unclear in the other ten. Patients were blinded in one study [1], unblinded in eight [2, 4, 6, 17, 19–22] and not reported in four trials [3, 5, 8, 18]. Blinding of outcome assessment was reported in six studies [4, 8, 18, 19, 21, 22], unclear in six [1, 3, 5, 6, 17, 20] and in one trial [2] the assessors were unblinded. Incomplete outcome data were found in three studies [8, 20, 22] and unclear in one [8].
Effects of interventions
Anticoagulant prophylaxis (UFH or LMWH)
Two trials [5, 18] reported on symptomatic DVT, assessing 225 patients did not conclusively rule out a symptomatic DVT reduction or increase with UFH [5] or LMWH [18] compared to placebo (OR = 0.63; 95 % CI 0.28–1.41) (Fig. 3a). There was not heterogeneity between the two studies (I2 = 0 %). One trial [3] assessing 100 patients showed a reduction in asymptomatic DVT with UFH compared to placebo (OR = 0.18; 95 % CI 0.07–0.47). In two trials [1, 6] assessing 245 patients, all included patients had mechanical prophylaxis before being randomised to LMWH or UFH. The results showed a non-significant reduction in asymptomatic DVT from 9 % (11/123) in the LMWH group to 4 % (5/122) in the UFH group. The OR was 0.44 (95 % CI 0.16–1.22) (Fig. 3a). Results did not demonstrate heterogeneity (I2 = 0 %). Two [1, 6] of these trials reported that none of the participants had symptomatic PE. The other three trials [3, 5, 18] did not report the number of patients who had developed PE. The two trials [3, 5] evaluating UFH to placebo did not find an increase in major bleeding in the UFH group (OR = 0.93; 95 % CI 0.18–4.71) and there was not heterogeneity between the two trials (I2 = 0 %). The two studies [1, 6] comparing UFH to LMWH did not conclusively rule out a reduction or increase of major bleeding with UFH compared to LMWH (OR = 0.52; 95 % CI 0.10–2.60) and there was not heterogeneity between the two studies (I2 = 0 %).
a Anticoagulant prophylaxis. Patients who had DVT. UFH unfractionated heparin, LMWH low molecular weight heparin. DVT deep venous thrombosis. b Mechanical prophylaxis. Patients who had asymptomatic DVT. GCS graduated compression stockings, ICP intermittent pneumatic compression, DVT deep venous thrombosis. c Mechanical plus anticoagulant prophylaxis versus mechanical prophylaxis. Patients who had asymptomatic DVT. DVT deep venous thrombosis
Mechanical prophylaxis
Four small trials assessed asymptomatic DVT for mechanical prophylaxis comparing GCS versus no treatment [21], IPC versus no treatment [19, 20], IPC versus GCS [17], GCS + IPC versus no treatment [21], and GCS + IPC versus GCS [21] (Fig. 3b). In the GCS group, 7 (9 %) of the 80 patients developed asymptomatic DVT in comparison to 16 (20 %) of the 81 in the control group (no treatment) (OR 0.41; 95 % CI 0.17–0.99). In the ICP group, 2 (5 %) of the 36 patients developed asymptomatic DVT in comparison to 13 (28 %) of the 47 in the control group (no treatment) (OR 0.24; 95 % CI 0.08–0.75) and there was not heterogeneity between the two trials (I2 = 0 %). One trial [17] assessing 70 patients found no difference in asymptomatic DVT with ICP compared to GCS (OR 1.19; 95 % CI 0.07–19.64). In the Turpie trial [21] there was a reduction in asymptomatic DVT with combined GCS and ICP compared to no treatment (OR 0.42; 95 % CI 0.17–1.02), but no difference with combined GCS and ICP compared to GCS alone (OR 1.03; 95 % CI 0.34–3.07). Wautrecht’s small trial [22] reported that none of the 18 patients in the treatment group (GCS plus IPC prophylaxis) developed symptomatic DVT in comparison to two of the five in the control group (GCS prophylaxis alone).
Bucci et al. [17] reported that one patient treated with GCS suffered symptomatic PE. Turpie et al. [21] reported that one patient was diagnosed with PE at autopsy but did not state which group this patient belonged to. One further trial [19], reported that none of the participants who underwent craniotomy for tumour suffered fatal PE.
Mechanical plus anticoagulant prophylaxis versus mechanical prophylaxis
Three [2, 4, 7] of the included studies evaluated the role of combined modalities on the incidence of asymptomatic DVT. Enoxaparin was used in two trials [2, 4] and nadroparin in the other one [8]. These studies showed a reduction in DVT from 28 % (92/330) in the control group (mechanical prophylaxis) to 18 % (57/319) in the treatment group (mechanical plus anticoagulant prophylaxis). The OR was 0.57 (95 % CI 0.39–0.82) and there was not heterogeneity (I2 = 11 %) (Fig. 3c). A non-significant decrease in symptomatic PE with combined modalities was also observed (OR 0.27; 95 % CI 0.05–1.35). However, the incidence of major bleeding was higher with LMWH compared to mechanical prophylaxis (OR 2.09; 95 % CI 0.87–5.05) and one trial [2] stopped early because of the increased incidence of adverse effects in the enoxaparin-treated groups.
Discussion
The prevention of VTE in neurosurgical patients is extremely various. There are mechanical methods such as IPC devices (by providing a wavelike compression on the leg it is possible to evacuate leg veins), and GCS (with a continuous and graduated pressure on the whole lower extremity it is possible to improve venous clearance and prevent venous stasis). In clinical practice, compression methods, especially IPC, are not universally used in prophylaxis routine because application of the devices is not very practical and also because of relatively high costs of leg cuff and machine. On the other hand, the use of anticoagulant therapy in the perioperative period has been limited for fear of intracranial bleeding.
The review aimed to define the optimal prophylactic treatment in the perioperative period in neurooncological patients. This systematic review showed a clear reduction of VTE in patients treated with either IPC or GCS; concerning IPC, Turpie et al. [20] showed how 5 days IPC application reduced the rate of VTE from 12 of 63 control patients to one of 65 patients treated with prophylaxis. In patients affected by brain tumours, calf compression decreased the incidence of venous thrombosis from six of 27 control patients, to one of 25 patients given prophylaxis. However the development of late thrombi was not prevented by 5 days of prophylaxis, showing that some patients were still at risk after the treatment. Therefore, it seems reasonable to conclude that IPC should be initiated preoperatively and continued until discharge or longer in case of persistence of risk factors (such as paresis). Very similar results were reported by Skillman et al. [19].
In the trials [1, 6] comparing LMWH to UFH in addition to mechanical methods, a trend was observed to reduce subclinical DVT in patients treated with UFH, but this paralleled an increase in major bleeding of borderline statistical significance. Turpie [21] reported a clear reduction in the frequency of DVT with the use of GCS versus no treatment (16 events in 81 patients in the control group vs 7 events in 80 patients in the treated group). In the single trial [17] comparing the effect of IPC to that of GCS no significant differences were detected in the frequency of DVT, although a small trial by Wautrecht et al. [22] seems to suggest that addition of IPC to GCS offers further protection versus GCS alone. On the other hand, Turpie et al. [21] showed no further reduction of DVT after IPC + GCS versus GCS alone.
According to the authors of a Cochrane review [23] on the use of physical methods in the prevention of post stroke DVT, no significant protective effect emerged for GCS, whereas the role of IPC, deserves further investigation.
Moreover, the CLOTS trial 1 showed that no significant effect was seen on the prevention of DVT after thigh-length compression stockings in stroke patients [24].
The difficulties in evaluating the impact of GCS on the prevention of DVT/PE in neurosurgical patients are highlighted by striking differences in the frequency of these events in the different trials; for instance, in Agnelli et al. [4] the frequency of clinically overt DVT achieved 33 % in the group given GCS and placebo, while in Turpie et al. [21] the frequency of clinically occult DVT in patients treated with GCS was of only 8.75 %. Differences in the clinical features of patients (nearly 100 % of patients had CNS tumours in Agnelli et al., whereas in Turpie et al. this percentage was of about 50 %, with a significant amount of stroke patients) and in the timing of GCS application (i.e. at hospital admission versus the morning of surgery) may partly explain these discrepancies.
In the group [2, 4, 8] comparing the addition of LMWH to mechanical methods (IPC or GCS) versus mechanical methods, a clear reduction of clinical DVT was detected in those patient receiving LMWH, whereas only trends were observed for reduction of PE and increase in major bleeding in these same patients.
On the other hand, in Dickinson’s trial, the addition of enoxaparin dose of 6000 IU/die, starting at anaesthesia induction, induced an increase of major intracranial bleeding events in patients operated on for brain tumours.
Results show no difference from the statistical point of view in Constantini et al. [5] and Melon et al. [18] studies, regarding the use of UFH or LMWH versus placebo for symptomatic DVT, while in Cerrato et al. [3] the use of UFH versus no treatment is relevant for asymptomatic DVT.
A recently published systematic review and meta-analysis on VTE prophylaxis in patients undergoing cranial neurosurgery [25] has shown in six RCTs (also analysed in the present work), a reduction in the frequency of VTE in patients treated with heparin prophylaxis, together with an increase in the risk of major bleeding. In that review, the authors very rightly state, in the discussion, that although the number of prevented VTE outweighs that of intracranial haemorrhages, a more balanced view must be taken into consideration due to the fact that many of the trials have occult VTE as outcome. When the outcome is limited to clinically overt VTE, only one trial provides data [4], in favour of enoxaparin 4000 IU/day.
Due to the lack of side effects of mechanical methods, there is little doubt that at the present time they should be used in “at risk” neurooncological surgical patients.
The timing of application of these devices seems critical (the earlier, the better, starting before surgery), as well as the duration of their application in relationship to persisting risk factors for VTE events.
Differences in effectiveness between GCS and IPC should be evaluated in face-to-face trials.
Ease in application and costs are very relevant factors influencing clinical practice outside the setting of clinical trials; for this reason it is not surprising that IPC is less widely used than GCS.
Whether mechanical methods should routinely be integrated by pharmacological prevention remains undecided.
As a matter of fact, the addition of LMWH to mechanical methods, seems to significantly reduce clinical DVT with an increase in major bleeding. However, this incidence is contributed to mostly by a single, well-designed trial [4].
Unfractionated heparin also seems able to decrease (non significantly) the frequency of DVT with a borderline increase in major bleedings; again, the diffusion of LMWH treatment is explained by its ease of administration and lack of haematological monitoring.
Recommendations of scientific societies on the prophylaxis of VTE in neurooncological patients undergoing tumour surgery are scarce, with the European Society for Medical Oncology (ESMO) mentioning LMWH use.
In the National Institute for Health and Clinical Excellence (NICE) guidelines [26], prevention of DVT/EP is recommended in all neurosurgical patients with mechanical devices, but addition of pharmacological prophylaxis is recommended only in those patients deemed at “low risk” for major bleeding. The definition of this threshold is very complex in neurosurgery, and the risk likely to be increased in neurooncological surgery, due to pathological neovascularisation in high grade glial tumours and to rich vascular supply in brain metastases and meningiomas.
The American College of Chest Physicians (ACCP) guidelines [27] identify neurooncological patients undergoing central nervous system surgery as very high risk subjects for VTE, and they recommend addition of LMWH, but the exact dose and timing is not specified. The data analyzed in our revision suggest that if additional protection from VTE is wished by the physician, a maximum dose of 4000 IU/day of enoxaparin, starting the day after surgery, may be combined. However the risk-benefit ratio is still not clearly defined, and appropriate physical prophylaxis with IPC started at hospital admission and continued until full ambulatory status is recovered might reduce the frequency of clinically overt VTE to very low levels even without addition of LMWH. Further studies should focus on specific subset of well-defined neurooncological patients.
In the future oral thrombin inhibitors, a very promising new class of anticoagulants drugs already used for thromboembolism prophylaxis in hip and knee surgery, may be investigated also in the neurooncological field.
Conclusion
In conclusion, despite the limited numbers, our systematic review suggests that the use of mechanical methods, in particular IPC, is not only safe but also protective for neurooncological patients undergoing surgery: using them preoperatively and continuing until discharge, reduces VTE without risk of haemorrhage. The addition of pharmacological treatment (LMWH) is even more protective in terms of reduction of VTE with a modest increase in the frequency of major bleeding: the dose should not exceed 4000 IU/die and the administration should begin the day after surgery. However, the evidence in favour of the addition of enoxaparin to physical prophylaxis is provided by a single (although adequately powered, well-designed and assessing clinically overt VTE) trial in which GCS were used and in which a higher frequency of VTE than in other trials was reported in the arm of patients treated with GCS + placebo. The assessment of the value of addition of LMWH to mechanical methods is further complicated by the fact that no trial investigated this putative additive effect in patients undergoing IPC and by the fact that head to head comparison of IPC and GCS provides data of limited quality.
References
Goldhaber SZ, Dunn K, Gerhard-Herman M, Park JK, Black PM (2002) Low rate of venous thromboembolism after craniotomy for brain tumor using multimodality prophylaxis. Chest 122:1933–1937
Dickinson LD, Miller LD, Patel CP, Gupta SK (1998) Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery 43:1074–1081
Cerrato D, Ariano C, Fiacchino F (1978) Deep vein thrombosis and low-dose heparin prophylaxis in neurosurgical patients. J Neurosurg 49:378–381
Agnelli G, Piovella F, Buoncristiani P et al (1998) Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 339:80–85
Constantini S, Kanner A, Friedman A et al (2001) Safety of perioperative minidose heparin in patients undergoing brain tumor surgery: a prospective, randomized, double-blind study. J Neurosurg 94:918–921
Macdonald RL, Amidei C, Baron J et al (2003) Randomized, pilot study of intermittent pneumatic compression devices plus dalteparin versus intermittent pneumatic compression devices plus heparin for prevention of venous thromboembolism in patients undergoing craniotomy. Surg Neurol 59:363–372
Auguste KI, Quinones-Hinojosa A, Berger MS (2004) Efficacy of mechanical prophylaxis for venous thromboembolism in patients with brain tumors. Neurosurg Focus 17:E3
Nurmohamed MT, van Riel AM, Henkens CM et al (1996) Low molecular weight heparin and compression stockings in the prevention of venous thromboembolism in neurosurgery. Thromb Haemost 75:233–238
Kakkar AK, Haas S, Wolf H, Encke A (2005) Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost 94:867–871
Simanek R, Vormittag R, Hassler M et al (2007) Venous thromboembolism and survival in patients with high-grade glioma. Neuro Oncol 9:89–95
Agnelli G, Verso M (2007) Thromboprophylaxis during chemotherapy after advanced cancer. Thromb Res 120(Suppl 2):S128–S132
Marras LC, Geerts WH, Perry JR (2000) The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer 89:640–646
Dennis M, Cranswick G (2010) Thigh-length versus below-knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med 153:553–562
Higgins JPT, Altman DG (2009) Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of interventions, version 5.0.2. The cochrane collaboration. http://www.cochrane-handbook.org
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Bucci MN, Papadopoulos SM, Chen JC et al (1989) Mechanical prophylaxis of venous thrombosis in patients undergoing craniotomy: a randomized trial. Surg Neurol 32:285–288
Melon E, Keravel Y, Gaston A, Huet Y, Combes S (1991) Deep venous thrombosis prophylaxis by low molecular weight heparin in neurosurgical patients. [abstract]. Anesthesiology 75:A214
Skillman JJ, Collins RE, Coe NP, Goldstein BS, Shapiro RM, Zervas NT, Bettmann MA, Salzman EW (1978) Prevention of deep vein thrombosis in neurosurgical patients: a controlled, randomized trial of external pneumatic compression boots. Surgery 83:354–358
Turpie AG, Gallus A, Beattie WS, Turpie HJ (1977) Prevention of venous thrombosis in patients with intracranial disease by intermittent pneumatic compression of the calf. Neurology 27:435–438
Turpie AG, Hirsh J, Gent M, Julian D, Johnson J (1989) Prevention of deep vein thrombosis in potential neurosurgical patients. A randomized trial comparing graduated compression stockings alone or graduated compression stockings plus intermittent pneumatic compression with control. Arch Intern Med 149:679–681
Wautrecht JC, Macquaire V, Vandesteene A et al (1996) Prevention of deep vein thrombosis in neurosurgical patients with brain tumors: a controlled, randomized study comparing graded compression stockings alone and with intermittent sequential compression: correlation with pre- and post-operative fibrinolysis; preliminary results. Int Angiol 15(suppl 1):5–10
Naccarato M, Grandi FC, Dennis M, Sandercock PA (2010) Physical methods for preventing deep vein thrombosis in stroke. Cochrane Database Syst Rev. (8):CD001922. doi:10.1002/14651858.CD001922.pub3
CLOTS Trials Collaboration, Dennis M, Sandercock PA, Reid J, Graham C, Murray G, Venables G, Rudd A, Bowler G (2009) Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 373:1958–1965
Hamilton MG, Yee WH, Hull RD et al (2011) Venous thromboembolism prophylaxis in patients undergoing cranial neurosurgery: a systematic review and meta-analysis. Neurosurgery 68:571–581
Treasure T, Hill J (2010) NICE guidance on reducing the risk of venous thromboembolism in patients admitted to hospital. J R Soc Med 103:210–212
Guyatt GH, Norris SL, Schulman S, et al. (2012) American college of chest physicians. Methodology for the development of antithrombotic therapy and prevention of thrombosis guidelines: antithrombotic therapy and prevention of thrombosis, 9th edn: American college of chest physicians evidence-based clinical practice guidelines. Chest. 141(2 Suppl):53S–70S. doi:10.1378/chest.11-2288
Conflict of interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salmaggi, A., Simonetti, G., Trevisan, E. et al. Perioperative thromboprophylaxis in patients with craniotomy for brain tumours: a systematic review. J Neurooncol 113, 293–303 (2013). https://doi.org/10.1007/s11060-013-1115-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1115-5