Abstract
Whether intermittent pneumatic compression (IPC) is a more effective form of thromboprophylaxis than anticoagulants in individuals undergoing neurosurgery remains controversial. Relevant studies are sparse and inconsistent. Therefore, direct comparisons are difficult to perform and impractical. Hence, we summarized and compared the efficacy and safety of IPC and anticoagulants for the prevention of venous thromboembolism (VTE) in adults undergoing cranial or spinal procedures. Several electronic databases were searched for randomized controlled trials on the use of IPC and anticoagulants for thromboembolism prevention in neurosurgical patients, from inception to August 6, 2019. Studies reporting the selected endpoints were included in direct and Bayesian network meta-analyses to estimate the relative effects of the interventions. Overall, our analysis included 18 trials comprising 2474 patients. Both IPC (RR, 0.41; 95% CrI, 0.26–0.60) and chemical prophylaxis (RR, 0.48; 95% CrI, 0.28–0.68) were found to be more efficacious than the placebo in reducing the risk of deep vein thrombosis (DVT). In addition, our analysis also demonstrated that both IPC (RR, 0.10; 95% CrI, 0.01–0.60) and chemical prophylaxis (RR, 0.31; 95% CrI, 0.05–1.00) reduced the risk of pulmonary embolism (PE) significantly more than the placebo. Based on the available evidence of moderate-to-good quality, IPC is equivalent to anticoagulants for thromboprophylaxis in terms of efficacy. Evidence to support or negate the use of pharmacological prophylaxis in terms of safety is lacking. The results of ongoing and future large randomized clinical trials are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is life-threatening but preventable a complication of surgery [1, 2]. It has been reported that each year in the USA and Europe, nearly 500,000 patients die from VTE [3]. Neurosurgery patients who are not expected to mobilize soon after operations are at a high risk of developing VTE. The risks for developing DVT and PE are estimated to be as high as 31% and 5%, respectively [4, 5], for patients undergoing craniotomy.
Evidence suggests that thromboprophylaxis is of pivotal importance in reducing the mortality and morbidity associated with venous thromboembolism [6, 7]. There are two major methods currently for thromboembolism prevention: chemical prophylaxis and mechanical prophylaxis. These methods differ widely and have been debated in the literature [8,9,10]. Anticoagulants such as low molecular weight heparin (LMWH) and unfractionated heparin have been suggested to be effective for DVT prevention in neurosurgical patients [4, 11]. However, extreme caution must be exercised with their use due to the risk of bleeding in the brain or spine, which can be fatal [11, 12]. Many clinicians perceive that the risk of bleeding due to pharmacological thromboprophylaxis is more important than the risk of venous thromboembolism. Therefore, mechanical methods of prophylaxis might be the optimal choice. Intermittent pneumatic compression (IPC) devices are one of the most common mechanical techniques used in clinical practice [13]. By reducing blood stasis and improving blood flow in the lower extremities, IPC seems to be efficacious and safe for DVT prevention [14, 15]. However, whether IPC is superior to anticoagulants remains controversial. The current controversy is mainly related to the safety of pharmacological prophylaxis [16, 17]. The latest ASH guidelines suggest not using pharmacological prophylaxis for patients undergoing neurosurgical procedures due to the risk of bleeding, but the evidence of the effects of pharmacological prophylaxis is of low quality [18, 19]. On the other hand, chemoprophylaxis is always warranted in comatose patients with persistent mobility restrictions following surgery, while IPC is suggested in ambulatory patients. In this context, a direct comparison of IPC and anticoagulants is difficult to perform and impractical.
Given the restrictions of direct meta-analyses, we performed a network meta-analysis of RCTs to investigate the efficacy and safety of chemical and mechanical thromboprophylaxis techniques performed in neurosurgical patients, with the aim of providing robust evidence regarding the value of the different treatment modalities in clinical applications.
Materials and methods
Protocol and guidance
This study was registered with OSF platform (DOI: https://doi.org/10.17605/OSF.IO/M7VG6). The systematic review and meta-analysis were conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) extension statement for network meta-analyses [20].
Selection criteria
The eligible studies met the following PICOS (participants, interventions, comparators, outcomes, and study design) criteria: (1) The population included adult patients (≥ 18 years of age) who underwent neurosurgeries, including craniotomy and spinal operations. (2) The interventions included chemical prophylaxis and intermittent pneumatic compression prophylaxis, regardless of the type of device used, dosage, and duration of the intervention. (3) The comparison interventions included a placebo, no treatment/routine physiotherapy, or compression stockings/graduated stockings as a control therapy. (4) The outcomes included efficacy endpoints, including the incidence rates of symptomatic or asymptomatic DVT and PE, and safety endpoints, including the incidence rates of major intracranial haemorrhage (ICH), major extracranial haemorrhage (ECH), minor bleeding complications, and all-cause mortality. The definitions and diagnostic criteria of the outcomes are presented in Appendix 1. (5) The study design was that of a randomized controlled trial.
Besides, we excluded (1) trials that include participants with hypercoagulable state, (2) trials that include patients with stroke and any other neurological conditions that have not been surgically treated, and (3) trials that aim to assess secondary VTE prevention (prophylaxis after a VTE event has already occurred).
Search strategy
We searched Ovid MEDLINE, Ovid EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception to August 6, 2019. To identify any additional studies, we also performed a recursive search of the bibliographies of the selected articles as well as published systematic reviews on this topic. The search strategy was designed and conducted by an experienced medical librarian. The search strategy is presented in Appendix 2.
Selection process
We first excluded duplicates, and then, we excluded publications that were not eligible for inclusion based on the titles and abstracts. Then, the full texts of the articles were reviewed for inclusion based on the aforementioned criteria.
Two reviewers independently completed this procedure. Conflicts regarding whether to include a study were resolved by consensus or by a third independent reviewer.
Data extraction
The following data were recorded in a standardized form: (1) study characteristics, including the primary author, year of publication, geographical location where study was conducted, and duration of the follow-up period; (2) patient characteristics, including the patient age and sex; (3) surgery characteristics, including cranial or/and spinal procedures; and (4) treatment characteristics, including the methods of the intervention.
Two reviewers independently extracted data from the included trials. When data were missing or could not be extracted, we contacted the study authors. Disagreements were resolved by consensus or by a third independent reviewer.
Assessment of the risk of bias and quality of the evidence
The risk of bias for all RCTs was assessed using the Cochrane Collaboration Risk of Bias tool across seven domains: random sequence generation, allocation concealment, blinding of the study participants, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias. Each domain was determined to have either a low, unclear, or high risk of bias. We contacted the original study investigators for more information if necessary.
The quality of evidence for the outcomes was determined using a framework developed by the Grading of Recommendations Assessment, Development, and Evaluation working group (GRADE) designed for rating the quality of effect estimates.
Sensitivity analyses
Sensitivity analyses were conducted for all of the outcomes by (1) excluding studies only reported as abstracts; (2) excluding trials with < 100 patients in total; and (3) excluding studies published before 2000.
Statistical analysis
Direct meta-analysis was performed using a random-effects model to estimate pooled relative risk (RR) and 95% confidence intervals (CIs). Statistical heterogeneity across the studies was evaluated by using the I2 statistic. I2 values exceeding 50% corresponded to substantial heterogeneity, and in these cases, we conducted further analysis to identify the source of heterogeneity. Potential publication bias was evaluated by a funnel plot. We assessed the asymmetry of the funnel plot by using Egger’s regression test and Begg’s adjusted rank correlation test. Significant publication bias was determined to be present when the p value was less than 0.05.
To perform indirect comparisons, we conducted Bayesian network meta-analyses using the consistency model in R software (gemtc package). We modelled the comparative efficacy of any two treatments as a function of each treatment relative to the reference treatment. We updated the Markov chain Monte Carlo model with 20,000 simulated draws after a burn-in of 5000 iterations. The point estimate (RR) and the corresponding 95% credible intervals (CrIs) were obtained using the 2.5th and 97.5th percentiles of the posterior distribution. The rankograms were estimated to rank the intervention hierarchy in the network meta-analysis.
All analyses were done in R (release version 3.6.3) and RevMan (5.3.3; the Cochrane collaboration). A two-sided p value of less than 0.05 was regarded as statistically significant.
Patients and public involvement
Neither patients nor the general public was involved in the development of the research question or selection of the outcome measures because the data included in the systematic review were derived from previously published studies.
Results
Eligible studies and study characteristics
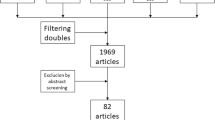
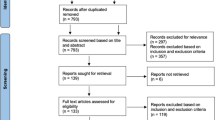
An extensive bibliographical search was conducted, and 1189 articles from the Ovid MEDLINE, Ovid EMBASE, and CENTER were identified; 449 of these articles were excluded because they were duplicates. Figure 1 shows that a total of 18 trials [4, 11, 14,15,16,17, 21,22,23,24,25,26,27,28,29,30,31,32] were deemed eligible and included in the systematic review and meta-analysis.
Table 1 summarizes the study characteristics. The studies were published from 1977 to 2018. The population sizes ranged from 14 to 485. Two trials were published as abstracts. Nine trials used IPC prophylaxis as the intervention and used a placebo for comparison. Seven trials used chemical prophylaxis as the intervention and a placebo for comparison, and only two trials compared chemical prophylaxis with IPC prophylaxis. The complete evidence network for the outcomes is shown in Fig. 2 and Appendix 3. The inclusion criteria of the trials are shown in Appendix 4.
Risk of bias and quality of evidence
The risks of bias results are shown in Appendices 5 and 6. None of the studies met all of the methodological criteria. Most trials were considered to have a high risk of bias because the participants were not blinded. Using the GRADE summary of the evidence, the quality of evidence of the efficacy outcomes was regarded as moderate to high, and the evidence of the safety outcomes was regarded as low (Appendix 7).
Efficacy outcomes
Deep venous thrombosis
According to the direct meta-analysis, both IPC and chemical prophylaxis were associated with a decreased risk of deep venous thrombosis (RR, 0.43; 95% CI, 0.30–0.62 and RR, 0.59; 95% CI, 0.44–0.80, respectively) compared with the placebo. However, no significant difference was detected between IPC and chemical prophylaxis (RR, 1.69; 95% CI, 0.50–5.65). There was no obvious asymmetry in the funnel plot (Appendix 8). Similar results were found in the network meta-analysis. Compared with the placebo, IPC (RR, 0.41; 95% CrI, 0.26–0.60) and chemical prophylaxis (RR, 0.48; 95% CrI, 0.28–0.68) reduced the risk of deep venous thrombosis. A difference between IPC and anticoagulants was not found (RR, 0.86; 95% CrI, 0.50–1.50) (Fig. 3a). IPC prophylaxis had the highest probability of being the most effective treatment in reducing the incidence of DVT, followed by chemical prophylaxis (Fig. 3b).
Results of direct and network meta-analysis comparing incidence of DVT. a Forest plots of incidence of DVT between different interventions. b Ranking positions of two interventions and placebo in DVT. From rank 1—worst to rank 3—best. DVT, deep vein thrombosis; IPC, intermittent pneumatic compression
Pulmonary embolism
According to the direct meta-analysis, neither IPC (RR, 0.37; 95% CI, 0.06–2.25) nor anticoagulants (RR, 0.61; 95% CI, 0.34–1.11) were associated with a decreased risk of pulmonary embolism, and no significant difference was found between IPC and chemical prophylaxis (RR, 0.50; 95% CI, 0.10–2.63). No obvious asymmetry was detected in the funnel plot (Appendix 9). However, network meta-analysis demonstrated that both IPC and anticoagulants can reduce the risk of PE (RR, 0.10; 95% CrI, 0.01–0.60 and RR, 0.31; 95% CrI, 0.05–1.00, respectively). There was no evidence suggesting that IPC is inferior to chemical prophylaxis in the prevention of PE (RR, 0.34; 95% CrI, 0.03–1.90) (Fig. 4a). IPC prophylaxis had the highest probability of being the most effective treatment in reducing the incidence of PE, followed by chemical prophylaxis (Fig. 4b).
Safety outcomes
Direct meta-analysis
According to the direct meta-analysis, there was no difference between IPC and chemical prophylaxis in terms of the effectiveness in reducing the risk of major intracranial haemorrhage (RR, 0.44; 95% CI, 0.06–3.22). There was no evidence that IPC is superior to anticoagulants in reducing the risk of major extracranial haemorrhage (RR, 0.33; 95% CI, 0.01–8.02) or minor bleeding complications (RR, 1.75; 95% CI, 0.54–5.67). In addition, there was no evidence suggesting that IPC is inferior to anticoagulants in reducing all-cause mortality (RR, 0.88; 95% CI, 0.36–2.16). The results of the direct meta-analysis are summarized in Table 2 and Appendices 10, 11, 12, and 13.
Network meta-analysis
In the network meta-analysis, no significant difference was found between IPC and chemical prophylaxes in terms of the risk of major intracranial haemorrhage (ICH), major extracranial haemorrhage (ECH), minor bleeding complications, and all-cause mortality. The network results of the safety outcomes are summarized in Table 2 and Appendix 14.
Sensitivity analysis and publication bias
Appendix 15 demonstrates that the results for the efficacy and safety outcomes were similar in all sensitivity analyses that were conducted. In addition, we did not find evidence of publication bias (p value for Egger’s regression test was > 0.05 for all comparisons).
Discussion
To the best of our knowledge, this is the first network meta-analysis comparing the relative efficacy and safety between IPC and anticoagulants in neurosurgical patients. In this systematic review and network meta-analysis, we combined direct and indirect evidence from 18 RCTs involving 2474 patients to estimate the relative efficacy and safety of different thromboprophylaxis interventions. We made several key observations: (1) IPC prophylaxis and chemical prophylaxis are superior to a placebo in reducing the risk of DVT; (2) IPC prophylaxis and chemical prophylaxis are superior to a placebo in reducing the risk of PE; and (3) there is no evidence suggesting that chemical prophylaxis is more likely to cause bleeding complications compared with IPC.
Comparison with other studies
Very few systematic reviews comparing the impact between IPC and drugs have been published. To the best of our knowledge, there is only one meta-analysis comparing IPC and drugs in terms of their efficacy and safety. In 2008, Collen et al. [5] concluded that there was no difference in the incidence of DVT and PE between IPC and drugs, with a RR of 0.79 (95% CI 0.30 to 2.12) and RR of 1.62 (95% CI 0.35 to 7.46), respectively. They also suggested that chemoprophylaxis among patients undergoing craniotomy might increase the risk for minor bleeding, with a RR of 2.06 (1.07 to 3.96). Existing studies have mainly compared either a placebo and IPC or drugs and a placebo among patients undergoing neurosurgery. In 2017 [33], Arnold et al. conducted a systematic review to investigate the efficacy, safety, and timing of anticoagulant thromboprophylaxis in patients with acute spinal cord injury. They concluded that anticoagulants can reduce the risk of VTE events without increasing the risk of bleeding and mortality and should be initiated as early as is safe after injury. A study in 2016 by Connell et al. [34] demonstrated that IPC is associated with a reduced risk of DVT compared with a placebo (RR 0.49, 95% CI 0.5 to 0.96). However, statistical heterogeneity was detected (I2 = 60%), and the conclusions were debatable because they included orthopaedic as well as neurosurgical patients. In 2011, Hamilton et al. [9] conducted a meta-analysis of 6 trials and found that heparin prophylaxis for patients undergoing elective cranial neurosurgery reduces the risk of VTE (RR 0.58, 95% CI 0.45 to 0.75) but might also increase the risk of bleeding, such as extracranial minor bleeding (RR 2.28, 95% CI 1.02 to 5.10). However, in 2000, Iorio et al. [10] found no significant increase in the occurrence of either major or minor bleeding complications with chemoprophylaxis compared with a placebo, but there was a significant decrease in the occurrence of VTE (OR 0.48, 95% CI 0.35 to 0.66). Their conclusions were proven by Khan et al. [35] in 2018.
Given that the decision to use IPC or anticoagulation in neurosurgical patients is made based on several considerations including their respective indications and contraindications, direct comparison is difficult to perform. The latest ASH guidelines suggest mechanical prophylaxis used in ordinary patients, while pharmacological prophylaxis may be warranted in high-risk patients experiencing prolonged immobility following surgery [19]. In this context, network meta-analyses can help assess the comparative effectiveness of multiple interventions and synthesize evidence across a network of RCTs. In our study, we used network analysis to calculate a mixed-effect estimate of the direct evidence and indirect evidence. Such a method may improve the precision of the estimate and allow the estimation of the comparative efficacy of IPC and anticoagulants, even though there are few studies that directly compare them.
Study implications
According to the latest American College of Chest Physicians evidence-based clinical practice guidelines on antithrombotic therapy and the prevention of thrombosis, IPC has been suggested to be a more favourable choice for patients undergoing craniotomy or spinal surgery compared with pharmacologic prophylaxis [7]. However, the grade 2C recommendations were mainly based on a consensus among experts with weak evidence. We suggest, based on the existing moderated to good evidence, that IPC is equivalent to anticoagulants in terms of efficacy for thromboprophylaxis.
Strength and limitations
The strengths of the present review include a comprehensive search strategy, explicit eligibility criteria that enhance generalizability, and rigorous use of the GRADE approach to rate the quality of the evidence. This meta-analysis included more than 2000 patients, which was larger than the sample sizes in previous studies on the same topic and was robust despite multiple subgroup and sensitivity analyses being conducted.
Our review has some limitations. First, given that IPC devices cannot be concealed, patients and care providers are aware of the treatment allocation results, leading to an increased likelihood of bias. Second, most trials have included a short-term follow-up period. Larger studies with longer follow-up periods need to be conducted to provide more definitive conclusions regarding whether the beneficial effect of IPC on the prevention of venous thromboembolism is sustained in the long term. Third, we regarded GCS and physical interventions as inactive interventions, which can decrease the effect of the interventions. On the other hand, the conclusions may be more convincing if positive results were obtained. Fourth, although the statistical heterogeneity was very low in the study, clinical heterogeneity may have inevitably occurred in trials considering different doses of anticoagulants used for pharmacologic prophylaxis. Thus, we conducted a meta-regression analysis and confirmed there is a positive interaction between the doses of drugs consumed and the occurrence of major bleeding complications (Appendix 16). Fifth, patients in this study were included in randomized controlled trials and therefore lacked representativeness since patients with severe conditions are likely to be excluded from these clinical trials. Additional studies in the “real world” setting need to be conducted to determine whether anticoagulants are as safe as IPC and whether IPC or/and anticoagulants can reduce the risk of PE in neurosurgical patients in the long term.
Conclusion
IPC and pharmacological prophylaxis are equally effective, but their comparative safety remains unclear due to limited sample size of this study. The results of ongoing and future large randomized clinical trials are needed.
References
Epley D (2000) Pulmonary emboli risk reduction. J Vasc Nurs 18:61–68 quiz 69-70
Heit JA (2012) Estimating the incidence of symptomatic postoperative venous thromboembolism: the importance of perspective. JAMA 307:306–307
Beckman MG, Hooper WC, Critchley SE, Ortel TL (2010) Venous thromboembolism: a public health concern. Am J Prev Med 38:S495–S501
Cerrato D, Ariano C, Fiacchino F (1978) Deep vein thrombosis and low-dose heparin prophylaxis in neurosurgical patients. J Neurosurg 49:378–381
Collen JF, Jackson JL, Shorr AF, Moores LK (2008) Prevention of venous thromboembolism in neurosurgery: a metaanalysis. Chest 134:237–249
Agnelli G (2004) Prevention of venous thromboembolism in surgical patients. Circulation 110:IV-4–IV-12
Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ (2012) Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141:7s–47s
Danish SF, Burnett MG, Stein SC (2004) Prophylaxis for deep venous thrombosis in patients with craniotomies: a review. Neurosurg Focus 17:E2
Hamilton MG, Yee WH, Hull RD, Ghali WA (2011) Venous thromboembolism prophylaxis in patients undergoing cranial neurosurgery: a systematic review and meta-analysis. Neurosurgery 68:571–581
Iorio A, Agnelli G (2000) Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 160:2327–2332
Giancarlo Agnelli MD, Franco Piovella MD, Pio Buoncristiani MD et al (1998) Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 110:Iv4–I12
Danish SF, Burnett MG, Ong JG, Sonnad SS, Maloney-Wilensky E, Stein SC (2005) Prophylaxis for deep venous thrombosis in craniotomy patients: a decision analysis. Neurosurgery 56:1286–1292 discussion 1292-1284
Shaikhouni A, Baum J, Lonser RR (2018) Deep vein thrombosis prophylaxis in the neurosurgical patient. Neurosurg Clin N Am 29:567–574
Prell J, Schenk G, Taute BM et al (2018) Reduced risk of venous thromboembolism with the use of intermittent pneumatic compression after craniotomy: a randomized controlled prospective study. J Neurosurg Mar 1:1–7
Sobieraj-Teague M, Hirsh J, Yip G et al (2012) Randomized controlled trial of a new portable calf compression device (Venowave) for prevention of venous thrombosis in high-risk neurosurgical patients. J Thromb Haemost 10:229–235
Kurtoglu M, Yanar H, Bilsel Y, Guloglu R, Kizilirmak S, Buyukkurt D, Granit V (2004) Venous thromboembolism prophylaxis after head and spinal trauma: intermittent pneumatic compression devices versus low molecular weight heparin. World J Surg 28:807–811
Dickinson LD, Miller LD, Patel CP, Gupta SK (1998) Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery 43:1074–1081
Schunemann HJ, Cushman M, Burnett AE et al (2018) American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv 2:3198–3225
Anderson DR, Morgano GP, Bennett C et al (2019) American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv 3:3898–3944
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162:777–784
Turpie AG, Gallus A, Beattie WS, Hirsh J (1977) Prevention of venous thrombosis in patients with intracranial disease by intermittent pneumatic compression of the calf. Neurology 27:435–438
Skillman JJ, Collins REC, Coe NP et al (1978) Prevention of deep vein thrombosis in neurosurgical patients: a controlled, randomized trial of external pneumatic compression boots. Surgery 83:354–358
Turpie AGG, Delmore T, Hirsh J, Hull R, Genton E, Hiscoe C, Gent M (1979) Prevention of venous thrombosis by intermittent sequential calf compression in patients with intracranial disease. Thromb Res 15:611–616
Gruber UF, Rem J, Meisner C, Gratzl O (1984) Prevention of thromboembolic complications with miniheparin-dihydroergotamine in patients undergoing lumbar disc operations. Eur Arch Psychiatry Neurol Sci 234:157–161
Weitz J, Michelsen J, Gold K, Owen J, Carpenter D (1986) Effects of intermittent pneumatic calf compression on postoperative thrombin and plasmin activity. Thromb Haemost 56:198–201
Bucci MN, Papadopoulos SM, Chen JC, Campbell JA, Hoff JT (1989) Mechanical prophylaxis of venous thrombosis in patients undergoing craniotomy: a randomized trial. Surg Neurol 32:285–288
Turpie AG, Hirsh J, Gent M, Julian D, Johnson J (1989) Prevention of deep vein thrombosis in potential neurosurgical patients: a randomized trial comparing graduated compression stockings alone or graduated compression stockings plus intermittent pneumatic compression with control. Arch Intern Med 149:679–681
Nurmohamed MT, van Riel AM, Henkens CM et al (1996) Low molecular weight heparin and compression stockings in the prevention of venous thromboembolism in neurosurgery. Thromb Haemost 75:233–238
Wautrecht JC, MacQuaire V, Vandesteene A et al (1996) Prevention of deep vein thrombosis in neurosurgical patients with brain tumors: a controlled, randomized study comparing graded compression stockings alone and with intermittent sequential compression. Correlation with pre- and postoperative fibrinolysis. Preliminary results. Int Angiol 15:5–10
Constantini S, Kanner A, Friedman A, Shoshan Y, Israel Z, Ashkenazi E, Gertel M, Even A, Shevach Y, Shalit M, Umansky F, Rappaport ZH (2001) Safety of perioperative minidose heparin in patients undergoing brain tumor surgery: a prospective, randomized, double-blind study. J Neurosurg 94:918–921
Hamidi S, Riazi M (2015) Incidence of venous thromboembolic complications in instrumental spinal surgeries with preoperative chemoprophylaxis. J Korean Neurosurg Soc 57:114–118
Melon E, Keravel Y, Gaston A (1991) Deep venous thrombosis prophylaxis by low molecular weight heparin in neurosurgical patients. Anesthesiology 75:A214
Arnold PM, Harrop JS, Merli G, Tetreault LG, Kwon BK, Casha S, Palmieri K, Wilson JR, Fehlings MG, Holmer HK, Norvell DC (2017) Efficacy, safety, and timing of anticoagulant thromboprophylaxis for the prevention of venous thromboembolism in patients with acute spinal cord injury: a systematic review. Global Spine J 7:138S–150S
O’Connell S, Bashar K, Broderick BJ, Sheehan J, Quondamatteo F, Walsh SR, ÓLaighin G, Quinlan LR (2016) The use of intermittent pneumatic compression in orthopedic and neurosurgical postoperative patients: a systematic review and meta-analysis. Ann Surg 263:888–889
Khan NR, Patel PG, Sharpe JP, Lee SL, Sorenson J (2018) Chemical venous thromboembolism prophylaxis in neurosurgical patients: an updated systematic review and meta-analysis. J Neurosurg 129:906–915
Funding
This work was funded by the 1.3.5 Project for Disciplines of Excellence of West China Hospital of Sichuan University (grant number: ZY2016102).
Author information
Authors and Affiliations
Contributions
Dr. Fang was the guarantor of the review. Dr. You received the funding.
Project design: Wang, Zhang, and Fang; data collection: Wang, Zhang, Fang, Jia, Faramand, You, and Xu; manuscript drafting: Wang, Zhang, Fang, Jia, Faramand, You, and Xu; statistical analysis: Wang, Zhang, Fang, Jia, Faramand, You, and Xu; manuscript revision: Wang, Zhang, Fang, Faramand, and You. Supervision: Wang, Zhang, and Fang.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not required.
Informed consent
Not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1453 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Zhang, Y., Fang, F. et al. Comparative efficacy and safety of pharmacological prophylaxis and intermittent pneumatic compression for prevention of venous thromboembolism in adult undergoing neurosurgery: a systematic review and network meta-analysis. Neurosurg Rev 44, 721–729 (2021). https://doi.org/10.1007/s10143-020-01297-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01297-0